A Biodegradable Antifungal-Loaded Sol–Gel Coating for the Prevention and Local Treatment of Yeast Prosthetic-Joint Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sol–Gel Coatings and Substrates
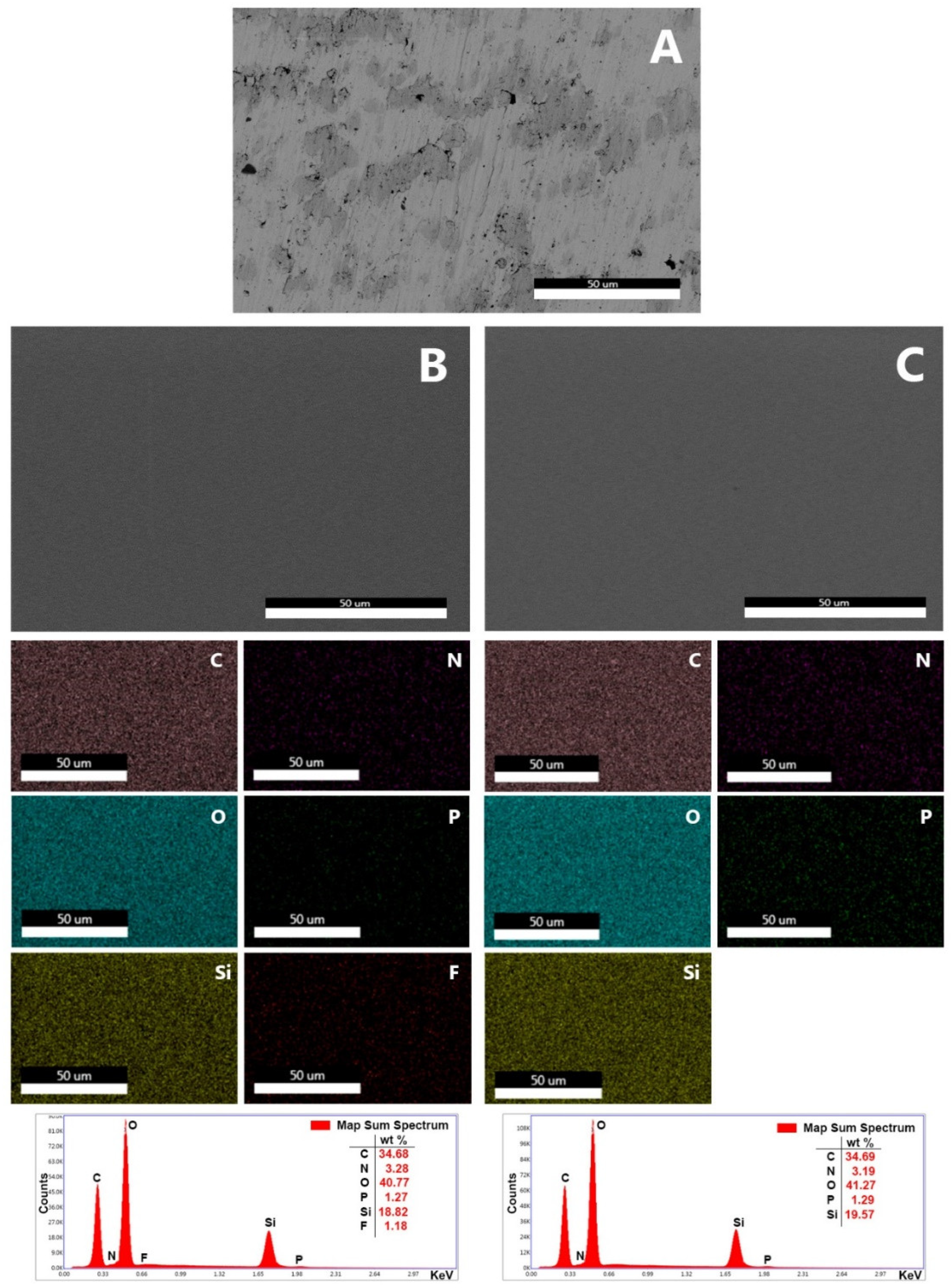
2.2. Surface Characterization
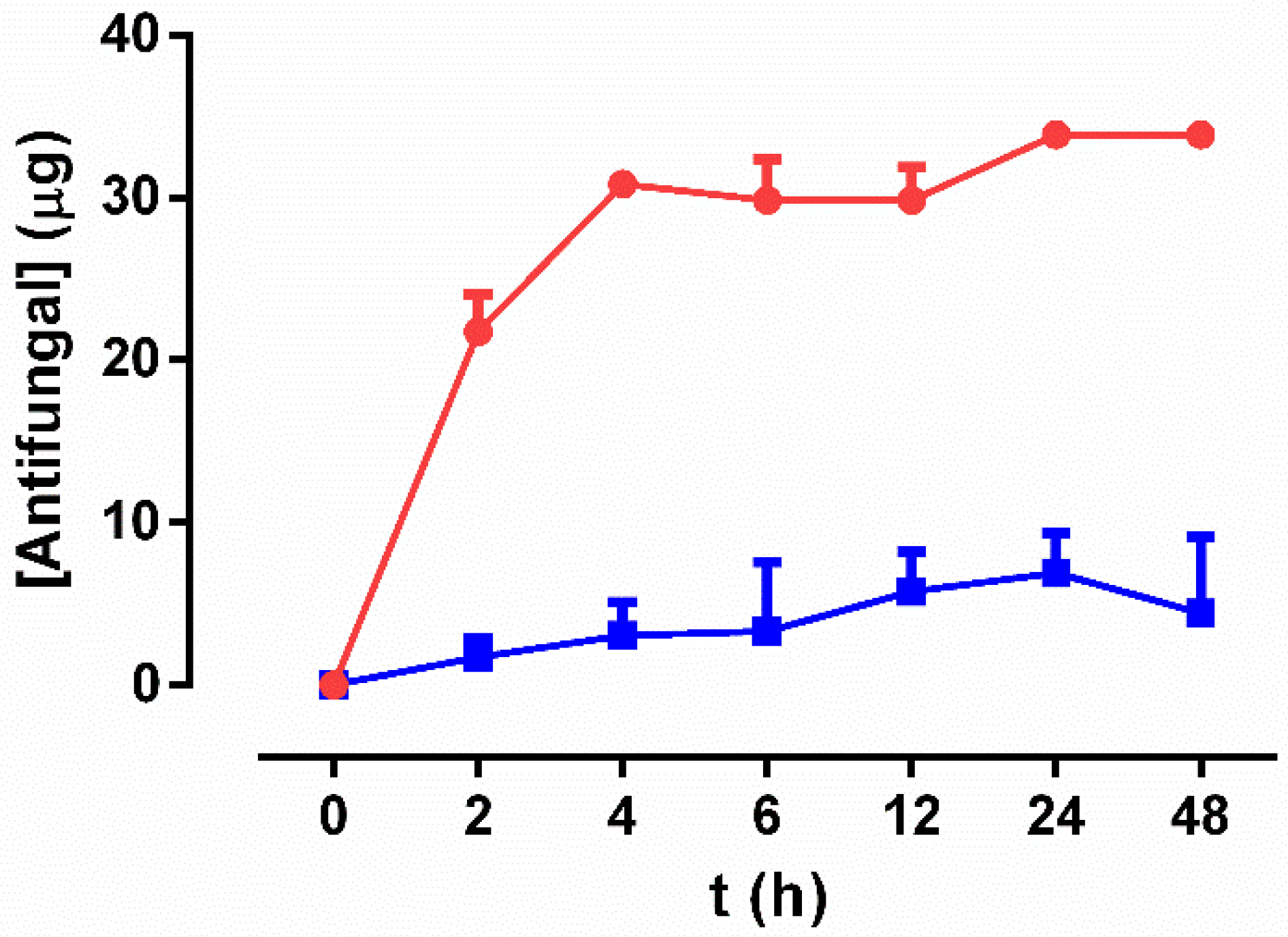
2.3. Kinetics Study of Antifungal Release
2.4. Selection and Maintenance of Strains for Microbiological Study
2.5. Adherence Study
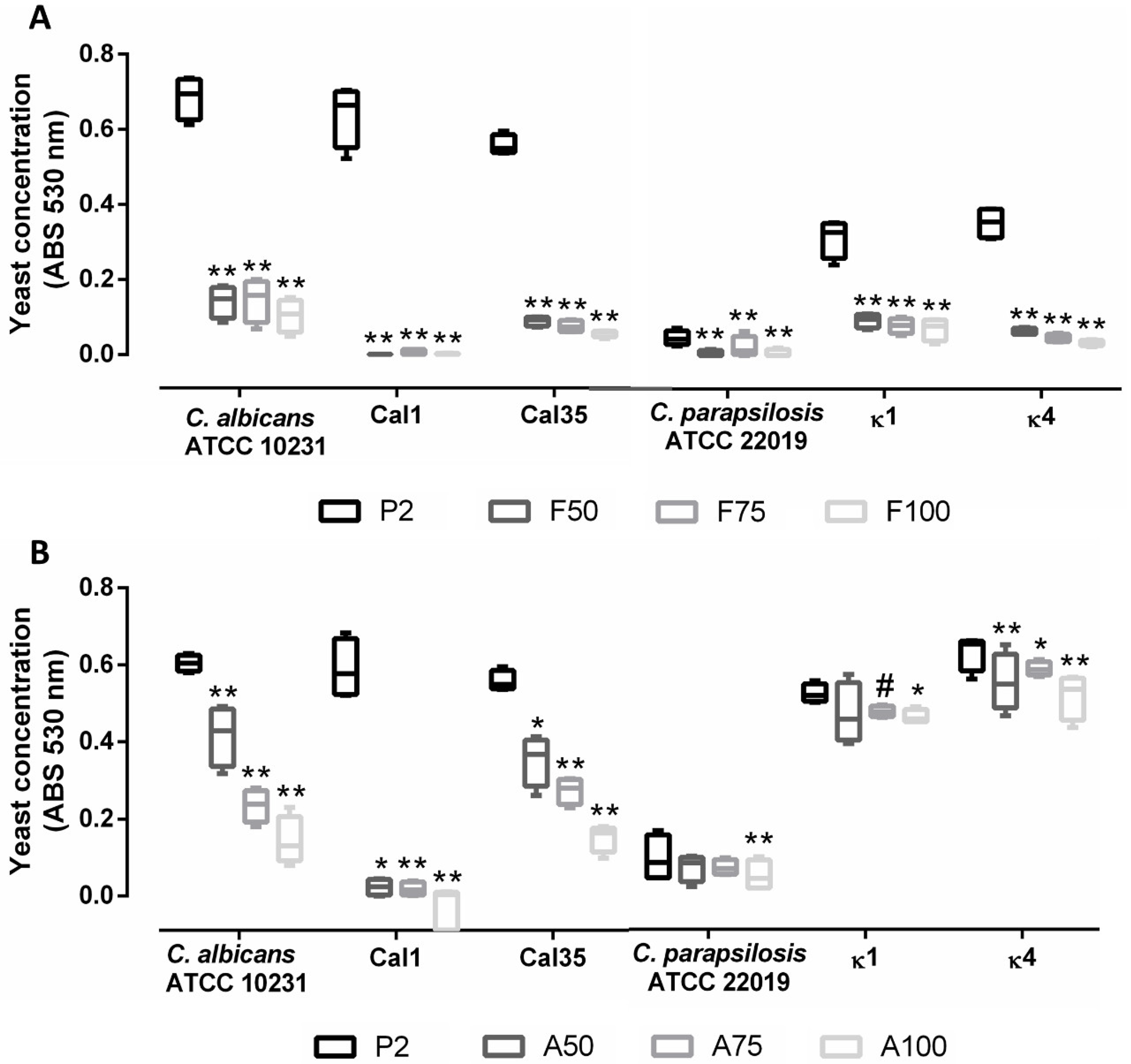
2.6. Evaluation of Biofilm Formation Inhibition
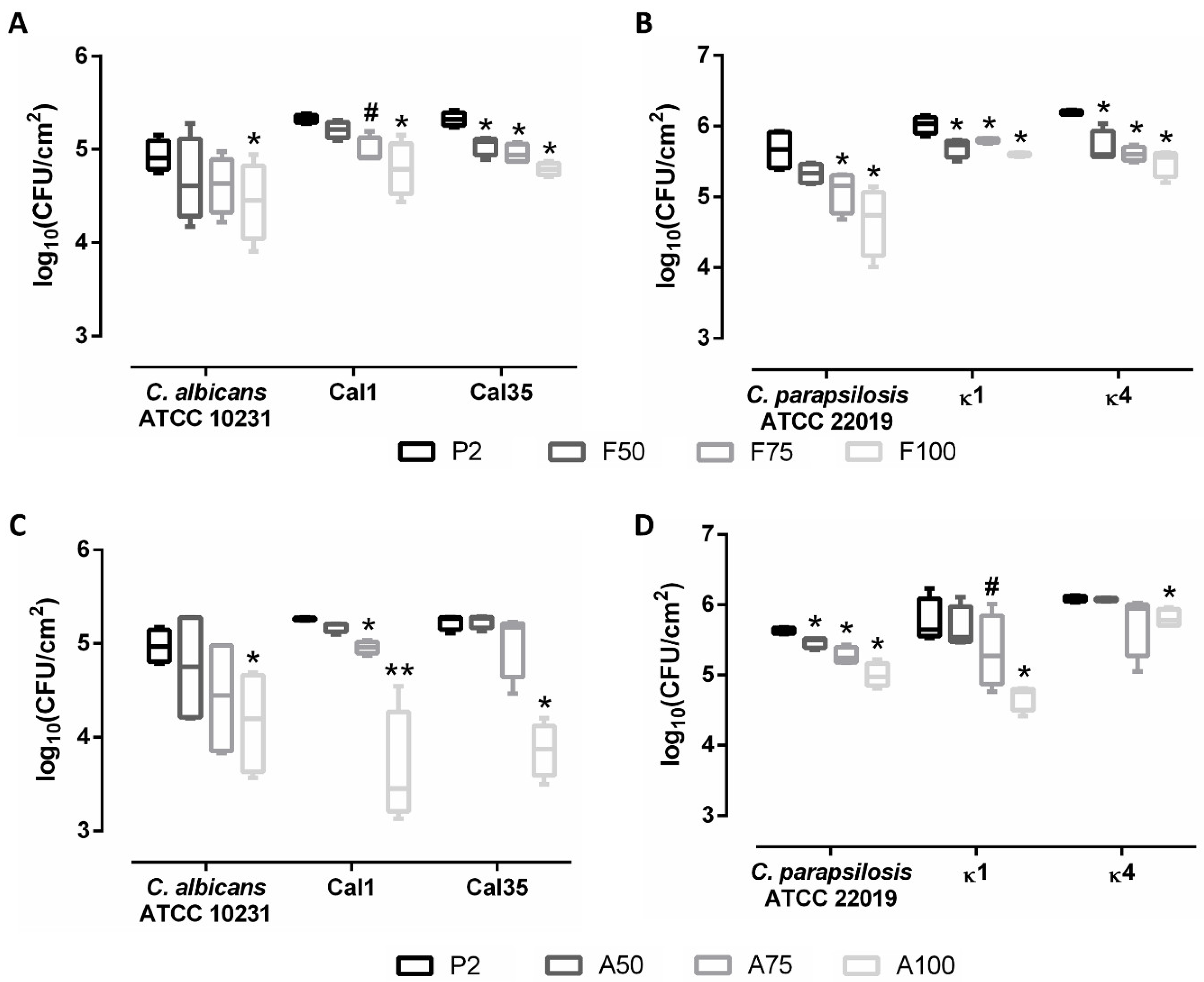
2.7. Evaluation of the Treatment of Mature Biofilms
2.8. Cellular Study
2.9. Statistical Analysis
3. Results
3.1. Synthesis of the Coatings and Surface Characterization
3.2. Drug-Release Study
3.3. Adherence Study
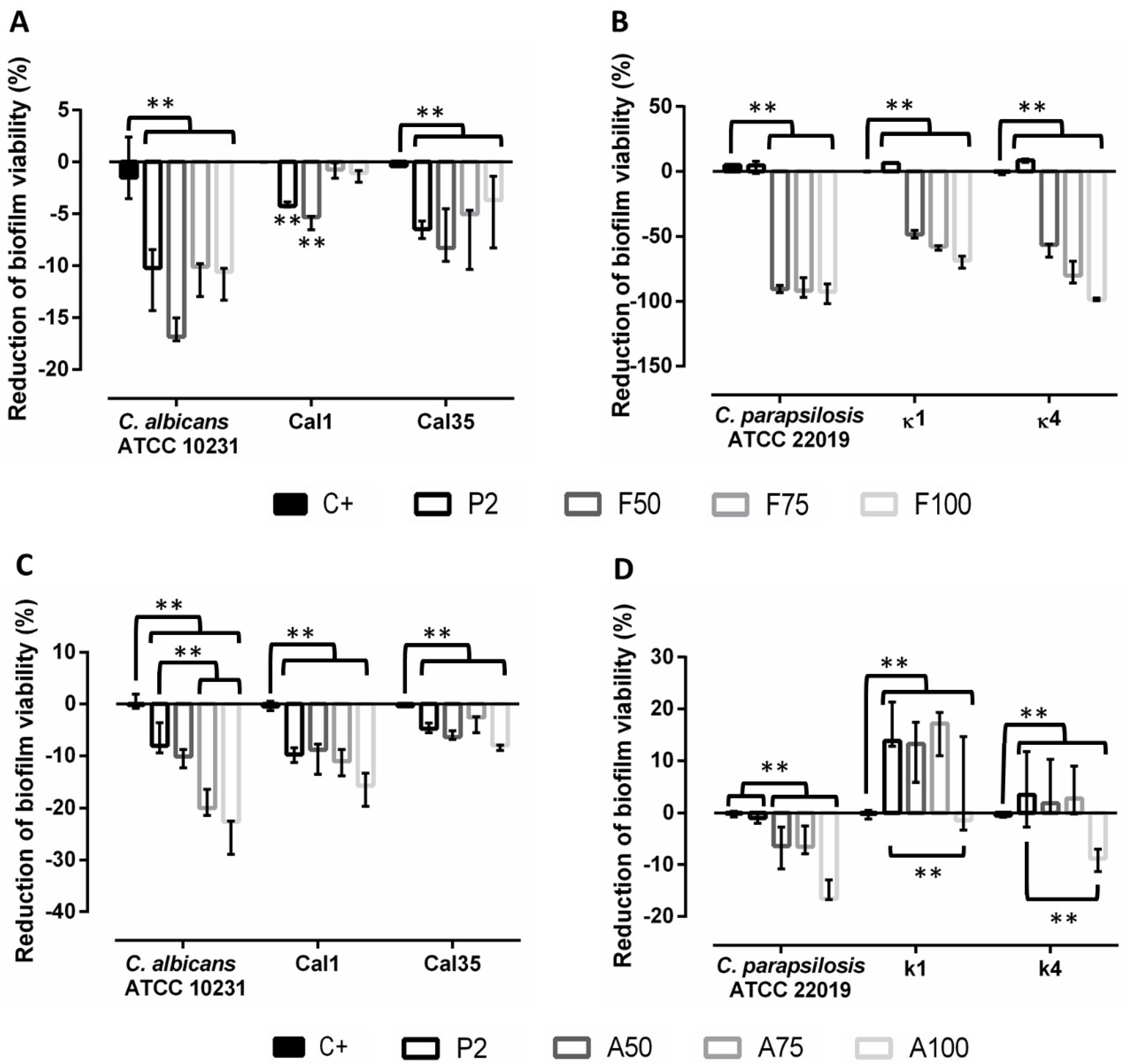
3.4. Prevention of Biofilm Formation
3.5. Evaluation of Treatment of Mature Biofilms
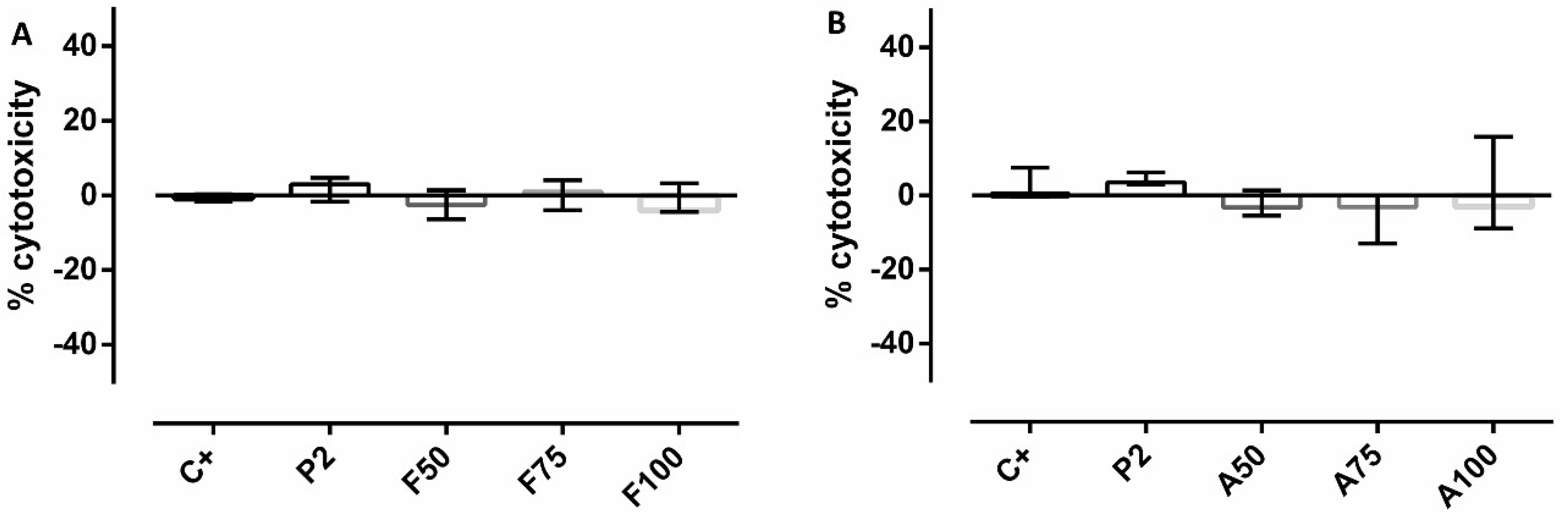
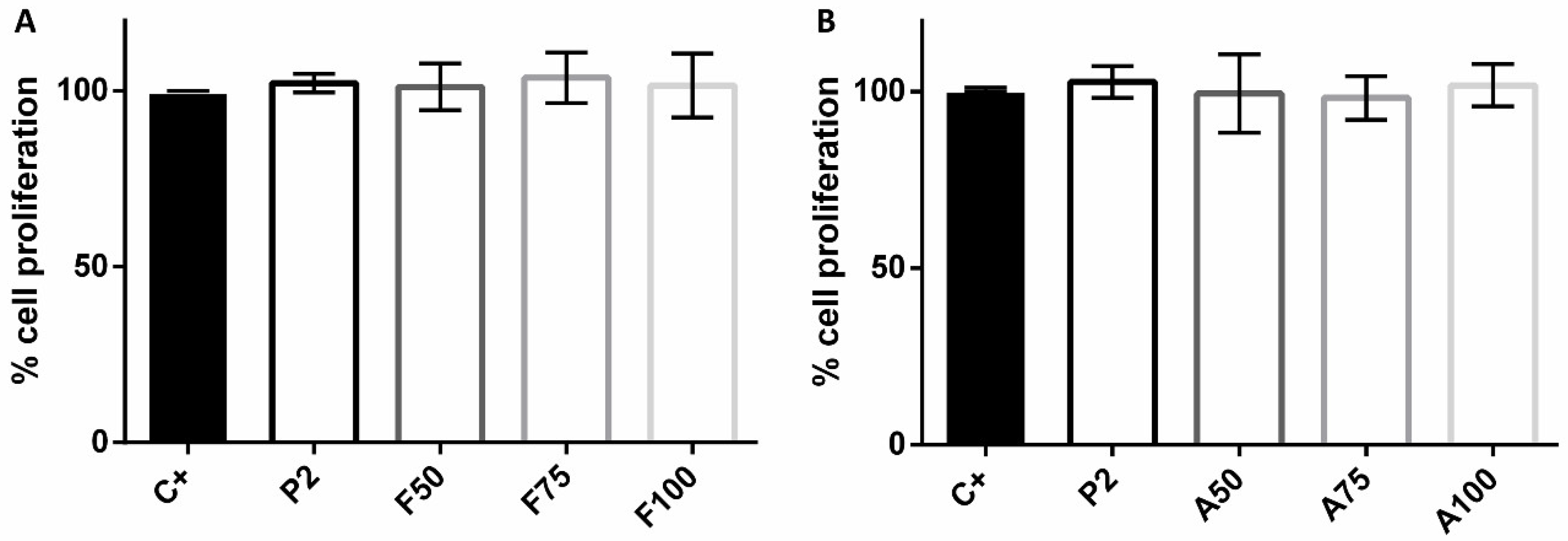
3.6. Cytotoxicity and Proliferation Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorli, L.; Fresco, G.; Fernandez-Sampedro, M.; Dolores Del Toro, M.; Guio, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e1–732.e8. [Google Scholar] [CrossRef] [PubMed]
- Azzam, K.; Parvizi, J.; Jungkind, D.; Hanssen, A.; Fehring, T.; Springer, B.; Bozic, K.; Valle, C.D.; Pulido, L.; Barrack, R. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: A multi-institutional experience. J. Bone Jt. Surg. Am. 2009, 91, 142–149. [Google Scholar] [CrossRef]
- Hirano, R.; Sakamoto, Y.; Kudo, K.; Ohnishi, M. Retrospective analysis of mortality and Candida isolates of 75 patients with candidemia: A single hospital experience. Infect. Drug Resist. 2015, 8, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Coad, B.R.; Griesser, H.J.; Peleg, A.Y.; Traven, A. Anti-infective surface coatings: Design and therapeutic promise against device-associated infections. PLoS Pathog. 2016, 12, e1005598. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Petis, S.M.; Osmon, D.R.; Mabry, T.M.; Berry, D.J.; Hanssen, A.D.; Abdel, M.P. Periprosthetic joint infection with fungal pathogens. J. Arthroplast. 2018, 33, 2605–2612. [Google Scholar] [CrossRef]
- Bartash, R.; Guo, Y.; Pope, J.B.; Levi, M.H.; Szymczak, W.; Saraiya, N.; Nori, P. Periprosthetic hip joint infection with Aspergillus terreus: A clinical case and a review of the literature. Med. Mycol. Case Rep. 2017, 18, 24–27. [Google Scholar] [CrossRef]
- Borghi, E.; Borgo, F.; Morace, G. Fungal biofilms: Update on resistance. Adv. Exp. Med. Biol. 2016, 931, 37–47. [Google Scholar]
- Schoof, B.; Jakobs, O.; Schmidl, S.; Klatte, T.O.; Frommelt, L.; Gehrke, T.; Gebauer, M. Fungal periprosthetic joint infection of the hip: A systematic review. Orthop. Rev. 2015, 7, 5748. [Google Scholar] [CrossRef]
- Jakobs, O.; Schoof, B.; Klatte, T.O.; Schmidl, S.; Fensky, F.; Guenther, D.; Frommelt, L.; Gehrke, T.; Gebauer, M. Fungal periprosthetic joint infection in total knee arthroplasty: A systematic review. Orthop. Rev. 2015, 7, 5623. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Viasus, D.; Carratala, J. Pathogenesis of invasive fungal infections. Curr. Opin. Infect. Dis. 2013, 26, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.W.; van den Bekerom, M.P.; van der Stappen, J.; Nolte, P.A.; Colen, S. 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections. Acta Orthop. 2013, 84, 517–523. [Google Scholar] [CrossRef]
- Siddiqi, A.; George, N.E.; White, P.B.; Szczech, B.W.; Thompson, J.V.; Etcheson, J.I.; Gwam, C.U.; Caughran, A.T.; Delanois, R.E.; Nace, J. Articulating spacers as a modified one-stage revision total knee arthroplasty: A preliminary analysis. Surg. Technol. Int. 2018, 32, 239–248. [Google Scholar]
- Kandel, C.E.; Jenkinson, R.; Daneman, N.; Backstein, D.; Hansen, B.E.; Muller, M.P.; Katz, K.C.; Widdifield, J.; Bogoch, E.; Ward, S.; et al. Predictors of treatment failure for hip and knee prosthetic joint infections in the setting of 1- and 2-stage exchange arthroplasty: A multicenter retrospective cohort. Open Forum Infect. Dis. 2019, 6, ofz452. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, X.; Du, Y.; Peng, Y.; Wu, W.; Zhou, Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: A retrospective study. Med. Sci. Monit. 2018, 24, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Levack, A.E.; Cyphert, E.L.; Bostrom, M.P.; Hernandez, C.J.; von Recum, H.A.; Carli, A.V. Current options and emerging biomaterials for periprosthetic joint infection. Curr. Rheumatol. Rep. 2018, 20, 33. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: A review of the literature (2005–2009). Rev. Iberoam. Micol. 2009, 26, 15–22. [Google Scholar] [CrossRef]
- Nace, J.; Siddiqi, A.; Talmo, C.T.; Chen, A.F. Diagnosis and management of fungal periprosthetic joint infections. J. Am. Acad. Orthop. Surg. 2019, 27, e804–e818. [Google Scholar] [CrossRef]
- Alves, M.J.; Grenho, L.; Lopes, C.; Borges, J.; Vaz, F.; Vaz, I.P.; Fernandes, M.H. Antibacterial effect and biocompatibility of a novel nanostructured ZnO-coated gutta-percha cone for improved endodontic treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Coad, B.R.; Kidd, S.E.; Ellis, D.H.; Griesser, H.J. Biomaterials surfaces capable of resisting fungal attachment and biofilm formation. Biotechnol. Adv. 2014, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zumbuehl, A.; Ferreira, L.; Kuhn, D.; Astashkina, A.; Long, L.; Yeo, Y.; Iaconis, T.; Ghannoum, M.; Fink, G.R.; Langer, R.; et al. Antifungal hydrogels. Proc. Natl. Acad. Sci. USA 2007, 104, 12994–12998. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casas, A.; Aguilera-Correa, J.J.; Mediero, A.; Esteban, J.; Jimenez-Morales, A. Functionalization of sol-gel coatings with organophosphorus compounds for prosthetic devices. Colloids Surf. B Biointerfaces 2019, 181, 973–980. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Sukhorukova, I.V.; Sheveyko, A.N.; Kiryukhantsev-Korneev, P.V.; Zhitnyak, I.Y.; Gloushankova, N.A.; Denisenko, E.A.; Filippovich, S.Y.; Ignatov, S.G.; Shtansky, D.V. Toward bioactive yet antibacterial surfaces. Colloids Surf. B Biointerfaces 2015, 135, 158–165. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Garcia-Casas, A.; Mediero, A.; Romera, D.; Mulero, F.; Cuevas-Lopez, I.; Jimenez-Morales, A.; Esteban, J. A New Antibiotic-Loaded Sol-Gel Can Prevent Bacterial Prosthetic Joint Infection: From in vitro Studies to an in vivo Model. Front. Microbiol. 2019, 10, 2935. [Google Scholar] [CrossRef]
- Toirac, B.; Garcia-Casas, A.; Cifuentes, S.; Aguilera-Correa, J.J.; Esteban, J.; Mediero, A.; Jiménez-Morales, A. Electrochemical characterization of coatings for local prevention of Candidainfections on titanium-based biomaterials. Prog. Org. Coat. 2020, 146, 105681. [Google Scholar] [CrossRef]
- Wang, X.; Ahmed, N.B.; Alvarez, G.S.; Tuttolomondo, M.V.; Helary, C.; Desimone, M.F.; Coradin, T. Sol-gel encapsulation of biomolecules and cells for medicinal applications. Curr. Top. Med. Chem. 2015, 15, 223–244. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Lattif, A.A.; Mukherjee, P.K.; Chandra, J.; Swindell, K.; Lockhart, S.R.; Diekema, D.J.; Pfaller, M.A.; Ghannoum, M.A. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. Int. J. Med. Microbiol. 2010, 300, 265–270. [Google Scholar] [CrossRef]
- Klotz, S.A.; Drutz, D.J.; Zajic, J.E. Factors governing adherence of Candida species to plastic surfaces. Infect. Immun. 1985, 50, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Panagoda, G.J.; Ellepola, A.N.; Samaranayake, L.P. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses 2001, 44, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Pryszcz, L.P.; Nemeth, T.; Gacser, A.; Gabaldon, T. Unexpected genomic variability in clinical and environmental strains of the pathogenic yeast Candida parapsilosis. Genome Biol. Evolut. 2013, 5, 2382–2392. [Google Scholar] [CrossRef]
- Hoyer, L.L.; Green, C.B.; Oh, S.H.; Zhao, X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—A sticky pursuit. Med. Mycol. 2008, 46, 1–15. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Katiyar, S.K.; Park, S.; Edlind, T.D.; Perlin, D.S. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2008, 52, 2305–2312. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs for Antifungal Agents. Version 10.0. 2020. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 8 July 2020).
- Kohler, J.R.; Acosta-Zaldivar, M.; Qi, W. Phosphate in virulence of candida albicans and candida glabrata. J. Fungi 2020, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Ikeh, M.A.; Kastora, S.L.; Day, A.M.; Herrero-de-Dios, C.M.; Tarrant, E.; Waldron, K.J.; Banks, A.P.; Bain, J.M.; Lydall, D.; Veal, E.A.; et al. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol. Biol. Cell 2016, 27, 2784–2801. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Clinical isolates and laboratory reference Candida species and strains have varying abilities to form biofilms. FEMS Yeast Res. 2013, 13, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; de Rycker, J.; Chaudhari, A.; Coutinho, E.; Yoshida, Y.; Van Meerbeek, B.; Mesquita, M.F.; da Silva, W.J.; Yoshihara, K.; Vandamme, K.; et al. Titanium implant functionalization with phosphate-containing polymers may favour in vivo osseointegration. J. Clin. Periodontol. 2017, 44, 950–960. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romera, D.; Toirac, B.; Aguilera-Correa, J.-J.; García-Casas, A.; Mediero, A.; Jiménez-Morales, A.; Esteban, J. A Biodegradable Antifungal-Loaded Sol–Gel Coating for the Prevention and Local Treatment of Yeast Prosthetic-Joint Infections. Materials 2020, 13, 3144. https://doi.org/10.3390/ma13143144
Romera D, Toirac B, Aguilera-Correa J-J, García-Casas A, Mediero A, Jiménez-Morales A, Esteban J. A Biodegradable Antifungal-Loaded Sol–Gel Coating for the Prevention and Local Treatment of Yeast Prosthetic-Joint Infections. Materials. 2020; 13(14):3144. https://doi.org/10.3390/ma13143144
Chicago/Turabian StyleRomera, David, Beatriz Toirac, John-Jairo Aguilera-Correa, Amaya García-Casas, Aránzazu Mediero, Antonia Jiménez-Morales, and Jaime Esteban. 2020. "A Biodegradable Antifungal-Loaded Sol–Gel Coating for the Prevention and Local Treatment of Yeast Prosthetic-Joint Infections" Materials 13, no. 14: 3144. https://doi.org/10.3390/ma13143144
APA StyleRomera, D., Toirac, B., Aguilera-Correa, J.-J., García-Casas, A., Mediero, A., Jiménez-Morales, A., & Esteban, J. (2020). A Biodegradable Antifungal-Loaded Sol–Gel Coating for the Prevention and Local Treatment of Yeast Prosthetic-Joint Infections. Materials, 13(14), 3144. https://doi.org/10.3390/ma13143144





