Evaluating a Proprietary Tannin-Blend Product as an Alternative to Monensin and Tylosin Phosphate in Feedlot Cattle Diets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Use
2.2. Animals and Treatments
2.3. Feeding and Management Description
2.4. Feedlot Performance Measures
2.5. Health Response Measures
2.6. Carcass Trait Measures
2.7. Fecal Starch
2.8. Statistical Analysis
3. Results
3.1. Feedlot Performance
3.2. Health
3.3. Carcass Characteristics
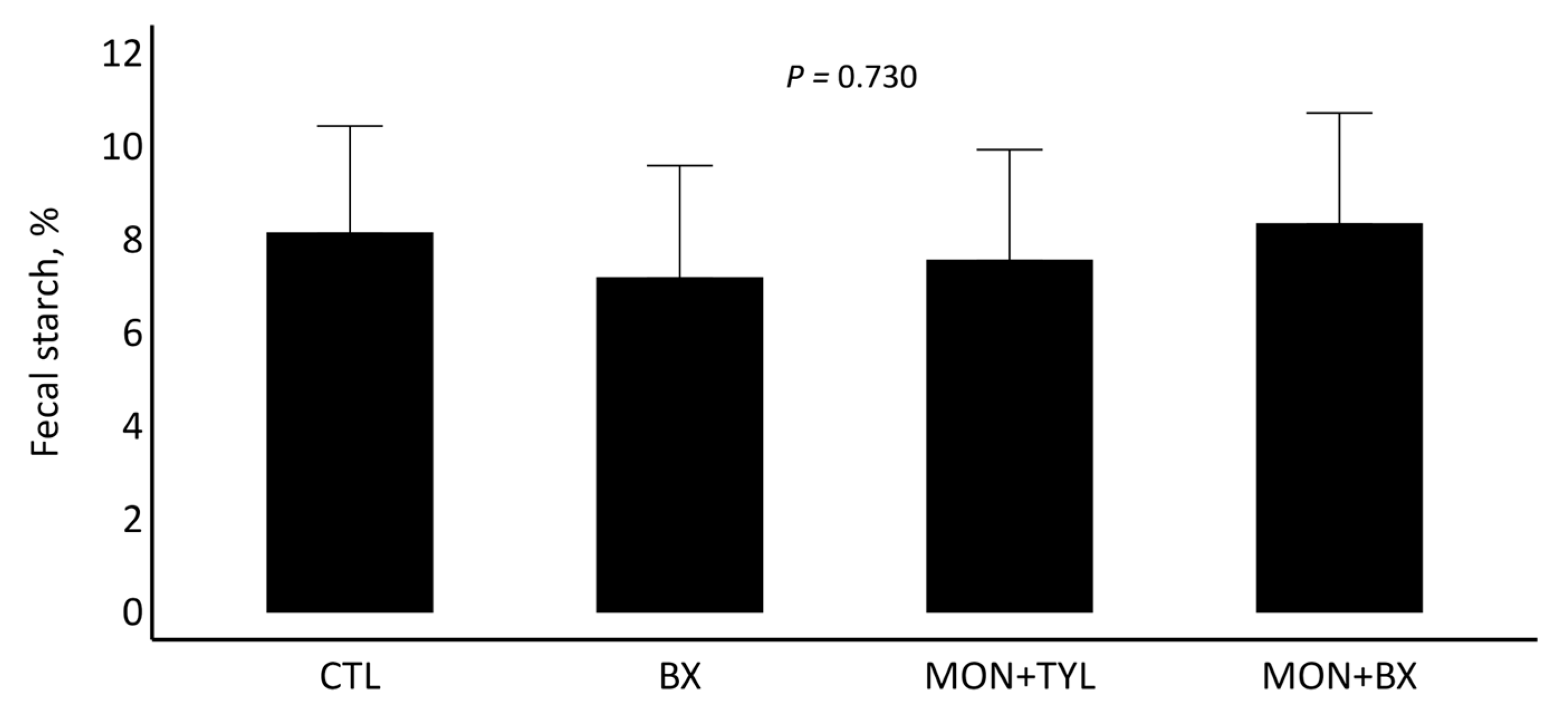
3.4. Fecal Starch
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. Acidosis in cattle: A review. J. Anim. Sci. 1998, 76, 275–286. [Google Scholar] [CrossRef]
- Amachawadi, R.G.; Nagaraja, T.G. Liver abscesses in cattle: A review of incidence in Holsteins and of bacteriology and vaccine approaches to control in feedlot cattle. J. Anim. Sci. 2016, 94, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, T.G.; Chengappa, M.M. Liver abscesses in feedlot cattle: A review. J. Anim. Sci. 1998, 76, 287–298. [Google Scholar] [CrossRef]
- Duffield, T.F.; Merrill, J.K.; Bagg, R.N. Meta-analysis of the effects of monensin in beef cattle on feed efficiency, body weight gain, and dry matter intake. J. Anim. Sci. 2012, 90, 4583–4592. [Google Scholar] [CrossRef]
- Samuelson, K.L.; Hubbert, M.E.; Galyean, M.L.; Löest, C.A. Nutritional recommendations of feedlot consulting nutritionists: The 2015 New Mexico State and Texas Tech University survey. J. Anim. Sci. 2016, 94, 2648–2663. [Google Scholar] [CrossRef]
- Silvestre, A.M.; Souza, J.M.; Millen, D.D. Adoption of adaptation protocols and feed additives to improve performance of feedlot cattle. J. Appl. Anim. Res. 2023, 51, 282–299. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Lechtenberg, K.F. Liver abscesses in feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 351–369. [Google Scholar] [CrossRef] [PubMed]
- USDA–APHIS–VS–CEAH–NAHMS. Feedlot 2011 Part IV: Health and Health Management on U.S. Feedlots with a Capacity of 1,000 or More Head. USDA, Fort Collins, CO, 2013. Available online: https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_dr_PartIV.pdf (accessed on 15 January 2025).
- Wileman, B.W.; Thomson, D.U.; Reinhardt, C.D.; Renter, D.G. Analysis of modern technologies commonly used in beef cattle production: Conventional beef production versus nonconventional production using meta-analysis. J. Anim. Sci. 2009, 87, 3418–3426. [Google Scholar] [CrossRef]
- OJEU. Regulation (EC) No 1831/2003 of the European Parliament and the Council of September 22, 2003, on Additives for Use in Animal Nutrition. Official Journal of European Union, 2003, L268-36 in OJEU of 10/18/2003. Available online: http://data.europa.eu/eli/reg/2003/1831/oj (accessed on 10 February 2025).
- Roberts, M.C. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol. Lett. 2008, 282, 147–159. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unraveling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martinez, G.D.; Miranda-Romero, L.A.; Hernandez-Garcia, P.A. Effects of dietary tannins supplementation on growth performance, rumen fermentation, and enteric methane emissions in beef cattle: A meta-analysis. Sustainability 2021, 13, 7410. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M. Effect of Lipid-Encapsulated Acacia Tannin Extract on Feed Intake, Nutrient Digestibility and Methane Emission in Sheep. Animals 2019, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Maggiolino, A.; Lorenzo, J.M.; Quiñones, J.; Latorre, M.A.; Blando, F.; Centoducati, G.; Dahl, G.E.; De Palo, P. Effects of dietary supplementation with Pinus taeda hydrolyzed lignin on in vivo performances, in vitro nutrient apparent digestibility, and gas emission in beef steers. Anim. Feed Sci. Technol. 2019, 255, 114217. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; Francavilla, M.; Neglia, G.; Esposito, L.; Caroprese, M. Extracts from Microalga Chlorella sorokiniana Exert an Anti-Proliferative Effect and Modulate Cytokines in Sheep Peripheral Blood Mononuclear Cells. Animals 2019, 9, 45. [Google Scholar] [CrossRef]
- Makmur, M.; Zain, M.; Sholikin, M.M.; Suharlina; Jayanegara, A. Modulatory effects of dietary tannins on polyunsaturated fatty acid biohydrogenation in the rumen: A meta-analysis. Heliyon 2022, 29, e09828. [Google Scholar] [CrossRef]
- Li, J.; Monje-Galvan, V. In Vitro and In Silico Studies of Antimicrobial Saponins: A Review. Processes 2023, 11, 2856. [Google Scholar] [CrossRef]
- Kholif, A.E. A Review of Effect of Saponins on Ruminal Fermentation, Health and Performance of Ruminants. Vet. Sci. 2023, 10, 450. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Hernandez, K.; Hernandez, C.; Hume, M.E.; Valencia, E.; Fulford, J.D. Effects of Plant Tannin Supplementation on Animal Responses and In Vivo Ruminal Bacterial Populations Associated with Bloat in Heifers Grazing Wheat Forage. Prof. Anim. Sci. 2012, 28, 464–472. [Google Scholar] [CrossRef]
- Sgoifo Rossi, C.A.; Grossi, S.; Compiani, R.; Baldi, G. Effect of a Blend of Essential Oils, Bioflavonoids, and Tannins on Production Performance, Health, Immune Functionality, and Antioxidant Status in Fattening Beef Cattle. Large Anim. Rev. 2023, 29, 163–170. [Google Scholar]
- Nascimento, K.S.; Bomfim, L.E.L.M.; Couto, V.R.M.; Silva, M.B.; Lopes, A.L.A.; Fernandes, M.H.M.R.; Manella, M.Q.; Ferraz Junior, M.V.C.; Fernandes, J.J.R. Growth performance and carcass characteristics of bulls fed tannins associated with or not with monensin. Transl. Anim. Sci. 2024, 8, txae136. [Google Scholar] [CrossRef]
- Rivera-Méndez, C.; Plascencia, A.; Torrentera, N.; Zinn, R.A. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J. Appl. Anim. Res. 2017, 45, 199–203. [Google Scholar] [CrossRef]
- Barajas, R.B.; Cervantes, J.; Espino, M.A.; Camacho, A.; Verdugo, M.; Flores, L.R.; Loneli, J.J.; Romo, J.A. Effect of tannin extracts supplementation on feedlot performance and plasma urea nitrogen of yearling bulls fed dry-ground corn-based diets containing corn-DDG and cane molasses. J. Anim. Sci. 2012, 90, 600. [Google Scholar]
- Manella, M.Q.; Coan, R.; Mesquita, W.; Paula, E.; Branco Arnandes, R. Proteic dry supplements enriched with a blend of tannin-based extract enhance the performance of grass-fed cattle during the rainy season in tropical regions. J. Anim. Sci. 2024, 102, 762–763. [Google Scholar] [CrossRef]
- Carvalho, P.H.V.; Latack, B.C.; Ferraz, M.V.C.; Nolasco, L.J.R.P.; Meireles, W.R.; Oliveira, H.O.M.; Zinn, R.A. Influence of low-level tannin supplementation on comparative growth performance of Holstein and Angus × Holstein cross calf-fed concentrate-based finishing diets for 328 d. J. Anim. Sci. 2024, 102, skae087. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Tabke, M.C.; Sarturi, J.O.; Galyean, M.L.; Trojan, S.J.; Brooks, J.C.; Johnson, B.J.; Martin, J.; Baggerman, J.; Thompson, A.J. Effects of tannic acid on growth performance, carcass characteristics, digestibility, nitrogen volatilization, and meat lipid oxidation of steers fed steam-flaked corn-based finishing diets. J. Anim. Sci. 2017, 95, 5124–5136. [Google Scholar] [CrossRef]
- Ferracini, J.G.; Lelis, A.L.J.; Polli, D.; Gasparim, M.B.; Feba, L.T.; Prado, I.N.; Millen, D.D. Feedlot performance of Nellore bulls fed high-concentrate diets containing the association of tannins and saponins with sodium monensin. Rev. Bras. Zootec. 2024, 53, e20230104. [Google Scholar] [CrossRef]
- Bowman-Schnug, S.M.; Fuerniss, L.K.; Cameron, J.D.; Beckett, J.L.; Ahsin, M.; van Vliet, S.; Hufstedler, G.D.; Johnson, B.J. Replacement of Monensin with a Proprietary Tannin-Blend Additive in Calf-Fed Holstein Steer Diets. Vet. Sci. 2025, 12, 166. [Google Scholar] [CrossRef]
- FASS. Guide for Care and Use of Agricultural Animals in Agricultural Research and Teaching; Federation of Animal Science Society: Savoy, IL, USA, 2010. [Google Scholar]
- USDA AMS. United States Standards for Grades of Carcass Beef. 2017. Available online: https://www.ams.usda.gov/sites/default/files/media/CarcassBeefStandard.pdf (accessed on 15 January 2025).
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distiller grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef] [PubMed]
- Fitri, A.; Yanza, Y.R.; Jayanegara, A.; Ridwan, R.; Astuti, W.D.; Sarwono, K.A.; Fidriyanto, R.; Rohmatussolihat, R.; Widyastuti, Y.; Obitsu, T. Divergence effects between dietary Acacia and Quebracho tannin extracts in nutrient utilization, performance, and methane emission of ruminants: A meta-analysis. Anim. Sci. J. 2022, 93, e13765. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Magnani, E.; Silva, T.H.; Sakamoto, L.; Manella, M.Q.; Dias, F.M.G.N.; Mercadante, M.E.; Henry, D.; Marcatto, J.O.S.; Paula, E.M.; Branco, R.H. Tannin-based product in feedlot diet as a strategy to reduce enteric methane emissions of Nellore cattle finished under tropical conditions. Transl. Anim. Sci. 2023, 7, txad048. [Google Scholar] [CrossRef]
- Perna Junior, F.; Nogueira, R.G.S.; Carvalho, R.F.; Cassiano, E.C.O.; Rodrigues, P.H.M. Use of tannin extract as a strategy to reduce methane in Nellore and Holstein cattle and its effect on intake, digestibility, microbial efficiency, and ruminal fermentation. J. Anim. Physiol. Anim. Nutr. 2022, 107, 89–102. [Google Scholar] [CrossRef]
- Perna Junior, F.; Cassiano, E.C.O.; Martins, M.F.; Romero, L.A.; Zapata, D.C.V.; Pinedo, L.A.; Marino, C.T.; Rodrigues, P.H.M. Effect of tannins-rich extract from Acacia mearnsii or monensin as feed additives on ruminal fermentation efficiency in cattle. Livest. Sci. 2017, 203, 21–29. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- Manella, M.Q.; Campanini, A.; Boin, C.; Paula, E.; Branco Arnandes, R.; Lopes, V. Use of a commercial blend of tannins as feed additive for feed lot cattle on large pen trial improves carcass weight and carcass feed conversion. J. Anim. Sci. 2024, 102 (Suppl. S3), 778–779. [Google Scholar] [CrossRef]
- Ebert, P.J.; Bailey, E.A.; Schrek, A.L.; Jennings, J.S.; Cole, N.A. Effect of condensed tannin extract supplementation on growth performance, nitrogen balance, gas emissions, and energetic losses of beef steers. J. Anim. Sci. 2017, 95, 1345–1355. [Google Scholar] [CrossRef]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial Mechanisms to Overcome Inhibitory Effects of Dietary Tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.N.; Pereira, M.A.; Oliveira, C.D.; Oliveira, C.C.; Silva, R.B.; Pereira, R.A.; DeVries, T.J.; Pereira, M.N. Effect of Low Dietary Concentrations of Acacia mearnsii Tannin Extract on Chewing, Ruminal Fermentation, Digestibility, Nitrogen Partition, and Performance of Dairy Cows. J. Dairy Sci. 2023, 106, 3203–3216. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.D. Relationships Among Soluble Phenolics, Insoluble Proanthocyanidins and Fiber in East African Browse Species. J. Range Manag. 1986, 39, 5–7. [Google Scholar] [CrossRef]
- Herrick, R.; Rogers, C.; McEvers, T.; Amachawadi, R.; Nagaraja, T.; Maxwell, C.; Reinbold, J.; Lawrence, T. Exploratory observational quantification of liver abscess incidence, specific to region and cattle type, and their associations to viscera value and bacterial flora. Appl. Anim. Sci. 2022, 38, 170–182. [Google Scholar] [CrossRef]
- Broadway, P.R.; Nagaraja, T.G.; Lawrence, T.E.; Galyean, M.L.; Hales, K.E. Liver abscesses—New perspectives on a historic fed-cattle issue. Appl. Anim. Sci. 2024, 40, 237–243. [Google Scholar] [CrossRef]
- Pinnell, L.J.; Young, J.D.; Thompson, T.W.; Wolfe, C.A.; Bryant, T.C.; Nair, M.N.; Richeson, J.T.; Morley, P.S. Establishing the link between microbial communities in bovine liver abscesses and the gastrointestinal tract. Anim. Microbiome 2023, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Davedow, T.; Narvaez-Bravo, C.; Zaheer, R.; Sanderson, H.; Rodas-Gonzalez, A.; Klima, C.; Booker, C.W.; Hannon, S.J.; Bras, A.L.; Gow, S.; et al. Investigation of a reduction in tylosin on the prevalence of liver abscesses and antimicrobial resistance in enterococci in feedlot cattle. Front. Vet. Sci. 2020, 7, 90. [Google Scholar] [CrossRef]
- Cabral, C.; Redondo, E.; Delgado, F. Reduction of liver abscesses in feedlot cattle by the addition of tannins on diet. Rev. Investig. Agropecu. 2019, 45, 211–215. [Google Scholar]
- Potter, E.L.; Wray, M.I.; Muller, R.D.; Grueter, H.P.; McAskill, J.; Young, D.C. Effect of Monensin and Tylosin on Average Daily Gain, Feed Efficiency and Liver Abscess Incidence in Feedlot Cattle. J. Anim. Sci. 1985, 61, 1058–1065. [Google Scholar] [CrossRef]
- Choi, J.; Liu, G.; Goo, D.; Wang, J.; Bowker, B.; Zhuang, H.; Kim, W.K. Effects of tannic acid supplementation on growth performance, gut health, and meat production and quality of broiler chickens raised in floor pens for 42 days. Front. Physiol. 2022, 13, 1082009. [Google Scholar] [CrossRef]
- Buyse, K.; Noten, N.V.; Delezie, E.; Goethals, L.; Janssens, G.P.; Lourenço, M. Chestnut tannins in broiler diets: Affecting intestinal development in different feeding phases. Front. Vet. Sci. 2022, 9, 996524. [Google Scholar] [CrossRef] [PubMed]
- Casanova, N.A.; Redondo, L.M.; Redondo, E.A.; Joaquim, P.E.; Dominguez, J.E.; Fernández-Miyakawa, M.E.; Chacana, P.A. Efficacy of Chestnut and Quebracho Wood Extracts to Control Salmonella in Poultry. J. Appl. Microbiol. 2021, 131, 135–145. [Google Scholar] [CrossRef] [PubMed]
| Item | Finishing Diet |
|---|---|
| Ingredients, % of DM 1 | |
| Flaked corn | 59.62 |
| High-moisture corn | 21.14 |
| Triticale silage | 4.26 |
| Supplement 2 | 3.82 |
| Palm oil | 3.48 |
| Distillers grains | 2.95 |
| Wheat straw | 2.46 |
| Alfalfa hay | 2.27 |
| Nutrient content, % of DM 3 | |
| Dry matter | 73.22 |
| Crude protein | 11.80 |
| Non-protein nitrogen | 2.42 |
| Acid detergent fiber (ADF) | 6.86 |
| Neutral detergent fiber (NDF) | 13.48 |
| peNDF 4 | 6.57 |
| Crude fat | 6.91 |
| NEm 5, Mcal/kg | 2.47 |
| NEg 6, Mcal/kg | 1.56 |
| Ca | 0.71 |
| P | 0.31 |
| Treatment 1 | ||||||
|---|---|---|---|---|---|---|
| CTL | BX | MON + TYL | MON + BX | SEM 2 | p-Value | |
| Shrunk body weight | ||||||
| Initial, kg | 254 | 254 | 254 | 254 | 9.2 | 0.499 |
| Reimplant, kg | 496 | 494 | 496 | 499 | 10.3 | 0.265 |
| Carcass-adjusted final 3, kg | 637 ab | 632 b | 639 ab | 642 a | 4.7 | 0.002 |
| ADG | ||||||
| Overall, kg | 1.67 ab | 1.65 b | 1.68 ab | 1.69 a | 0.053 | 0.002 |
| D0 to reimplant, kg | 1.82 | 1.81 | 1.83 | 1.85 | 0.096 | 0.294 |
| Reimplant to harvest, kg | 1.44 | 1.41 | 1.47 | 1.46 | 0.033 | 0.305 |
| DMI | ||||||
| Overall, kg | 9.34 a | 9.18 ab | 8.94 c | 9.03 bc | 0.179 | <0.001 |
| D0 to reimplant, kg | 8.98 a | 8.84 ab | 8.50 c | 8.71 b | 0.256 | <0.001 |
| Reimplant to harvest, kg | 9.79 a | 9.61 ab | 9.50 ab | 9.43 b | 0.189 | 0.005 |
| F:G | ||||||
| Overall | 5.60 b | 5.58 b | 5.34 a | 5.35 a | 0.096 | <0.001 |
| D0 to reimplant | 4.94 b | 4.90 b | 4.66 a | 4.71 a | 0.124 | <0.001 |
| Reimplant to harvest * | 6.85 | 6.82 | 6.48 | 6.48 | 0.173 | 0.105 |
| Treatment 1 | ||||||
|---|---|---|---|---|---|---|
| CTL | BX | MON + TYL | MON + BX | SEM 2 | p-Value | |
| Pens, n | 12 | 12 | 12 | 12 | ||
| Head per treatment | 745 | 747 | 750 | 744 | ||
| Morbidity, % | 5.31 | 5.76 | 5.77 | 5.00 | 1.046 | 0.898 |
| Respiratory morbidity, % | 4.02 | 3.87 | 4.41 | 3.50 | 0.958 | 0.847 |
| First treatment, % | 3.54 | 3.08 | 3.77 | 3.02 | 0.874 | 0.836 |
| Second treatment, % | 0.30 | 0.13 | 0.40 | 0.25 | 0.279 | 0.810 |
| Third treatment, % | 0.00 | 0.15 | 0.29 | 0.13 | 0.205 | 0.911 |
| Bloat, % | 0.48 | 0.73 | 0.29 | 0.27 | 0.205 | 0.559 |
| Mortality, % | 0.97 | 1.75 | 1.02 | 0.94 | 0.501 | 0.452 |
| Respiratory, % | 0.32 | 1.02 | 0.73 | 0.67 | 0.384 | 0.537 |
| Digestive, % | 0.48 | 0.73 | 0.29 | 0.13 | 0.325 | 0.390 |
| Treatment 1 | ||||||
|---|---|---|---|---|---|---|
| CTL | BX | MON + TYL | MON + BX | SEM 2 | p-Value | |
| HCW, kg | 408 ab | 404 b | 409 ab | 411 a | 3.0 | 0.001 |
| Yield grade | 2.80 ab | 2.73 b | 2.94 a | 2.93 a | 0.215 | 0.004 |
| Ribeye area, sq cm | 93.6 | 93.5 | 93.2 | 92.5 | 1.65 | 0.198 |
| Fat thickness, cm | 1.55 ab | 1.51 b | 1.57 ab | 1.59 a | 0.134 | 0.035 |
| KPH, % | 3.34 | 3.21 | 3.38 | 3.30 | 0.211 | 0.060 |
| Marbling score 3 | 484 ab | 479 b | 487 ab | 497 a | 11.7 | 0.046 |
| Quality grade distribution * | 0.073 | |||||
| Prime, % | 2.9 | 1.7 | 2.6 | 2.8 | 0.89 | |
| Choice, % | 79.3 | 77.3 | 81.2 | 81.7 | 2.85 | |
| Select, % | 15.3 | 16.2 | 12.5 | 12.0 | 3.54 | |
| Liver score distribution * | <0.001 | |||||
| Abscess prevalence, % | 43.1 a | 36.8 a | 18.3 c | 28.5 b | 2.49 | <0.001 |
| A+ prevalence, % | 28.7 a | 25.7 a | 12.0 c | 17.9 b | 1.92 | <0.001 |
| A prevalence, % | 14.2 a | 11.0 a | 6.2 b | 10.5 a | 1.65 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felizari, L.D.; Fuerniss, L.K.; Beckett, J.L.; Secrist, D.S.; Hufstedler, G.D.; Johnson, B.J. Evaluating a Proprietary Tannin-Blend Product as an Alternative to Monensin and Tylosin Phosphate in Feedlot Cattle Diets. Vet. Sci. 2025, 12, 446. https://doi.org/10.3390/vetsci12050446
Felizari LD, Fuerniss LK, Beckett JL, Secrist DS, Hufstedler GD, Johnson BJ. Evaluating a Proprietary Tannin-Blend Product as an Alternative to Monensin and Tylosin Phosphate in Feedlot Cattle Diets. Veterinary Sciences. 2025; 12(5):446. https://doi.org/10.3390/vetsci12050446
Chicago/Turabian StyleFelizari, Luana D., Luke K. Fuerniss, Jonathan L. Beckett, David S. Secrist, Guy D. Hufstedler, and Bradley J. Johnson. 2025. "Evaluating a Proprietary Tannin-Blend Product as an Alternative to Monensin and Tylosin Phosphate in Feedlot Cattle Diets" Veterinary Sciences 12, no. 5: 446. https://doi.org/10.3390/vetsci12050446
APA StyleFelizari, L. D., Fuerniss, L. K., Beckett, J. L., Secrist, D. S., Hufstedler, G. D., & Johnson, B. J. (2025). Evaluating a Proprietary Tannin-Blend Product as an Alternative to Monensin and Tylosin Phosphate in Feedlot Cattle Diets. Veterinary Sciences, 12(5), 446. https://doi.org/10.3390/vetsci12050446







