Evaluation of Safety and Immunogenicity of a Recombinant Receptor-Binding Domain (RBD)-Tetanus Toxoid (TT) Conjugated SARS-CoV-2 Vaccine (PastoCovac) in Recipients of Autologous Hematopoietic Stem Cell Transplantation Compared to the Healthy Controls; A Prospective, Open-Label Clinical Trial
Abstract
:1. Background
2. Methods
2.1. Study Design and Participants
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Regulatory and Ethical Approval and Written Informed Consent
2.5. Procedures
2.6. Anti-SARS-CoV-2 Antibody Evaluation
2.7. Outcome
2.8. Safety Assessments
2.9. Statistical Analysis
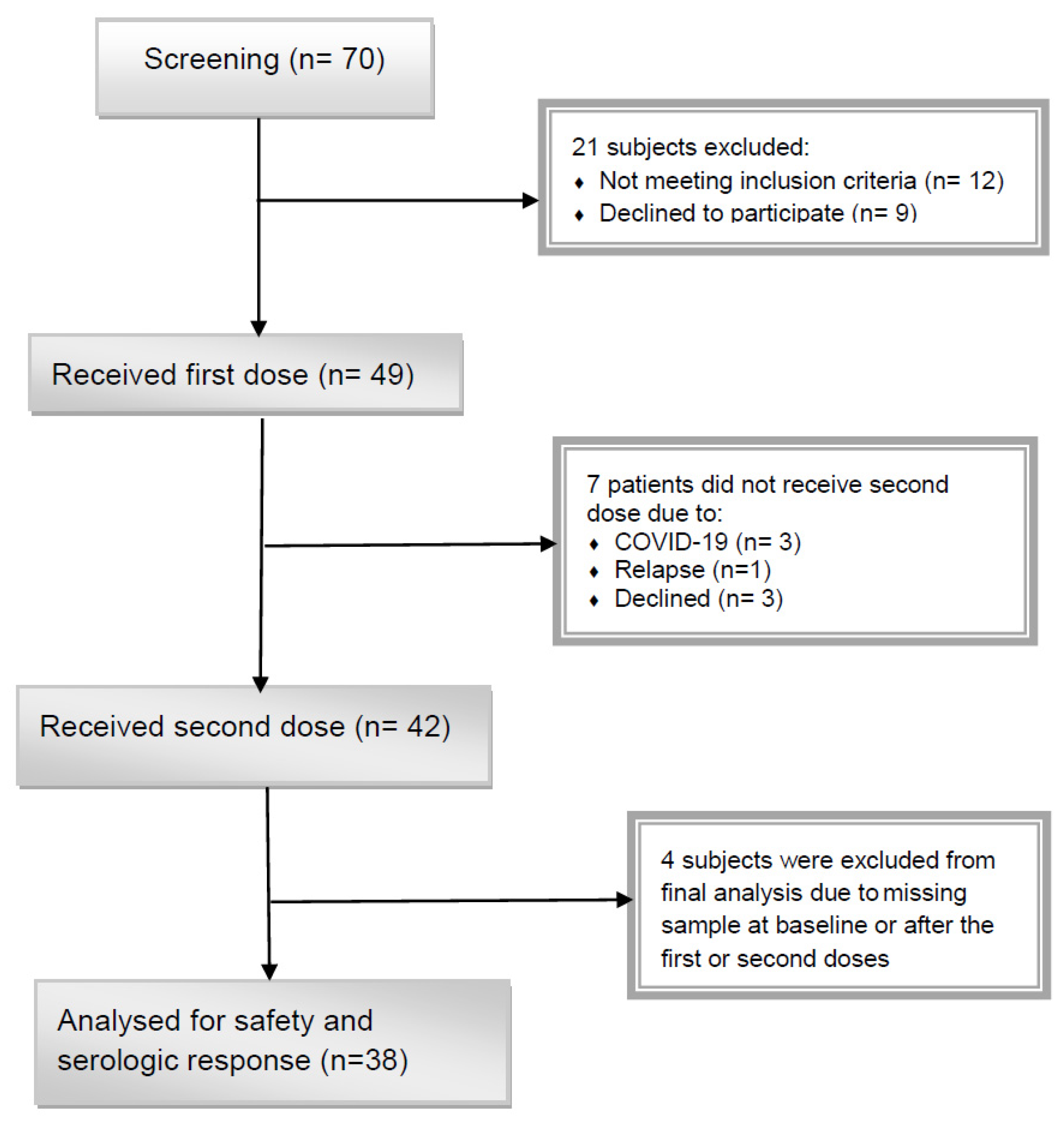
3. Results
3.1. Patient Characteristics
3.2. Serological Outcomes
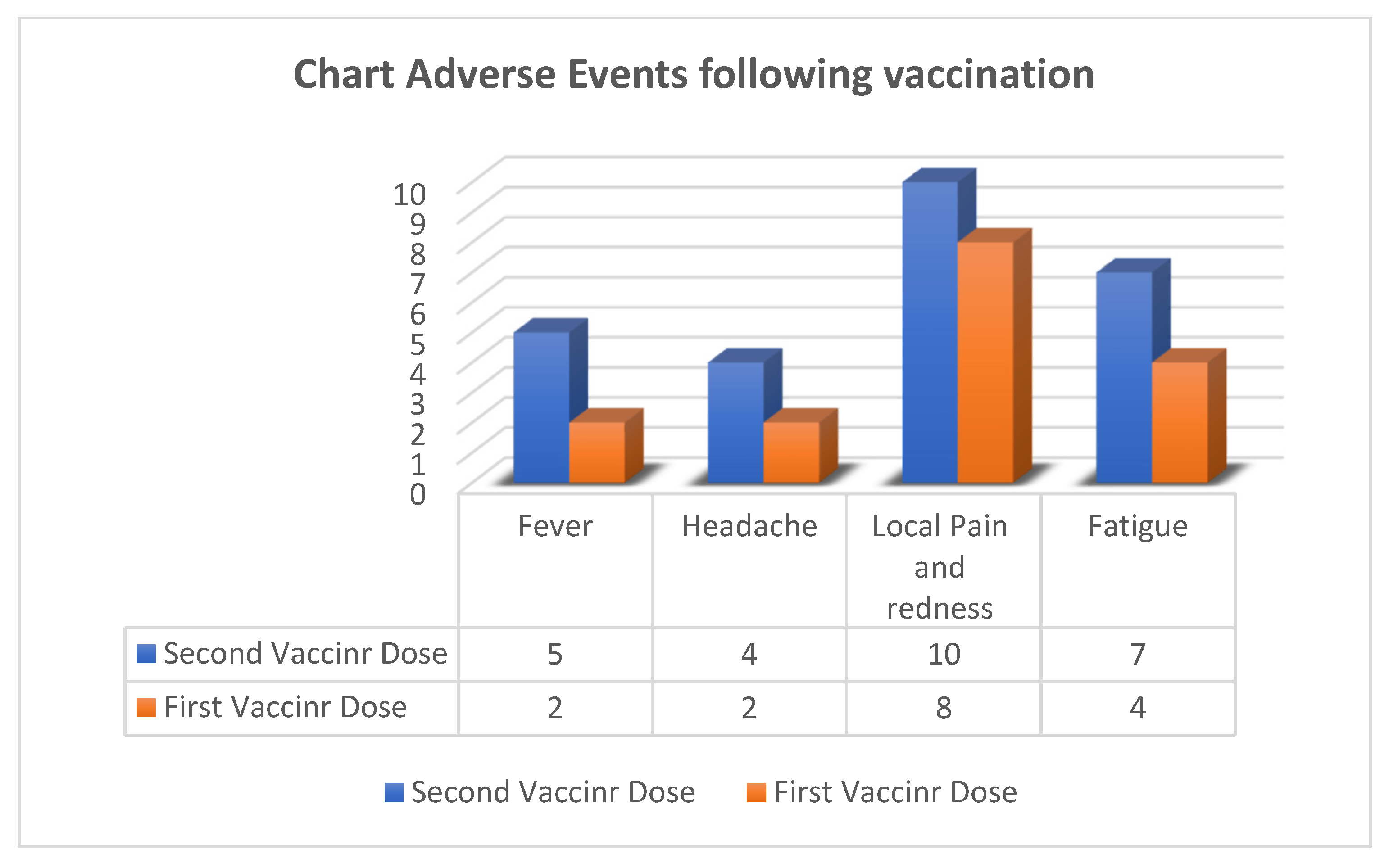
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ljungman, P.; de la Camara, R.; Mikulska, M.; Tridello, G.; Aguado, B.; Al Zahrani, M.; Apperley, J.; Berceanu, A.; Bofarull, R.M.; Calbacho, M.; et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia 2021, 35, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhatt, N.S.; Martin, A.S.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.-A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef] [PubMed]
- EBMT: COVID-19 Vaccines. Version 8. Available online: https://www.ebmt.org/covid-19-and-bmt (accessed on 1 March 2022).
- Ljungman, P.; Mikulska, M.; De La Camara, R.; Basak, G.W.; Chabannon, C.; Corbacioglu, S.; Duarte, R.; Dolstra, H.; Lankester, A.C.; Mohty, M.; et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020, 55, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Shen, L.; He, L.; Tang, K. Immune response to vaccination against SARS-CoV-2 in hematopoietic stem cell transplantation and CAR T-cell therapy recipients. J. Hematol. Oncol. 2022, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Du, K.; Luo, M.; Shen, K.; Zhou, Y.; Guo, K.; Liu, Y.; Yin, C.; Li, Y.; Li, G.; et al. Serologic response and safety of COVID-19 vaccination in HSCT or CAR T-cell recipients: A systematic review and meta-analysis. Exp. Hematol. Oncol. 2022, 11, 46. [Google Scholar] [CrossRef]
- Huang, A.; Cicin-Sain, C.; Pasin, C.; Epp, S.; Audigé, A.; Müller, N.J.; Nilsson, J.; Bankova, A.; Wolfensberger, N.; Vilinovszki, O.; et al. Antibody Response to SARS-CoV-2 Vaccination in Patients following Allogeneic Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2022, 28, 214.e1–214.e11. [Google Scholar] [CrossRef]
- Haggenburg, S.; Lissenberg-Witte, B.I.; van Binnendijk, R.S.; Hartog, G.D.; Bhoekhan, M.S.; Haverkate, N.J.E.; De Rooij, D.M.; van Meerloo, J.; Cloos, J.; Kootstra, N.A.; et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022, 6, 1537–1546. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E. Whole Inactivated Virus and Protein-Based COVID-19 Vaccines. Annu. Rev. Med. 2022, 73, 55–64. [Google Scholar] [CrossRef]
- Hernández-Bernal, F.; Ricardo-Cobas, M.C.; Martín-Bauta, Y.; Navarro-Rodríguez, Z.; Piñera-Martínez, M.; Quintana-Guerra, J.; Urrutia-Pérez, K.; Urrutia-Pérez, K.; Chávez-Chong, C.O.; Azor-Hernández, J.L.; et al. Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study). EClinicalMedicine 2022, 46, 101383. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and efficacy of NVX- CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Salimian, J.; Ahmadi, A.; Amani, J.; Olad, G.; Halabian, R.; Saffaei, A.; Arabfard, M.; Nasiri, M.; Nazarian, S.; Abolghasemi, H.; et al. Safety and immunogenicity of a recombinant receptor-binding domain-based protein subunit vaccine (Noora vaccine™) against COVID-19 in adults: A randomized, double-blind, placebo-controlled, Phase 1 trial. J. Med. Virol. 2022. [Google Scholar] [CrossRef]
- Valdes-Balbin, Y.; Santana-Mederos, D.; Quintero, L.; Fernández, S.; Rodriguez, L.; Ramirez, B.S.; Perez-Nicado, R.; Acosta, C.; Méndez, Y.; Ricardo, M.G.; et al. SARS-CoV-2 RBD-Tetanus Toxoid Conjugate Vaccine Induces a Strong Neutralizing Immunity in Preclinical Studies. ACS Chem. Biol. 2021, 16, 1223–1233. [Google Scholar] [CrossRef]
- Eugenia-Toledo-Romaní, M.; Verdecia-Sánchez, L.; Rodríguez-González, M.; Rodríguez-Noda, L.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sánchez-Ramírez, B.; Pérez-Nicado, R.; González-Mugica, R.; Hernández-García, T.; et al. Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: Open label phase I and phase IIa clinical trials. Vaccine 2022, 40, 4220–4230. [Google Scholar] [CrossRef]
- Kwiecińska-Piróg, J.; Przekwas, J.; Kraszewska, Z.; Sękowska, A.; Brodzka, S.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Zacharski, M.; Mańkowska-Cyl, A.; et al. The Differences in the Level of Anti-SARS-CoV-2 Antibodies after mRNA Vaccine between Convalescent and Non-Previously Infected People Disappear after the Second Dose—Study in Healthcare Workers Group in Poland. Vaccines 2021, 9, 1402. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Service. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Available online: https://www.meddra.org/ (accessed on 5 November 2021).
- Shah, G.L.; DeWolf, S.; Lee, Y.J.; Tamari, R.; Dahi, P.B.; Lavery, J.A.; Ruiz, J.D.; Devlin, S.M.; Cho, C.; Peled, J.U.; et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J. Clin. Investig. 2020, 130, 6656–6667. [Google Scholar] [CrossRef]
- Majcherek, M.; Matkowska-Kocjan, A.; Szymczak, D.; Karasek, M.; Szeremet, A.; Kiraga, A.; Milanowska, A.; Kuznik, E.; Kujawa, K.; Wrobel, T.; et al. Two Doses of BNT162b2 mRNA Vaccine in Patients after Hematopoietic Stem Cell Transplantation: Humoral Response and Serological Conversion Predictors. Cancers 2022, 14, 325. [Google Scholar] [CrossRef]
- Attolico, I.; Tarantini, F.; Carluccio, P.; Schifone, C.P.; Delia, M.; Gagliardi, V.P.; Perrone, T.; Gaudio, F.; Longo, C.; Giordano, A.; et al. Serological response following BNT162b2 anti-SARS-CoV-2 mRNA vaccination in haematopoietic stem cell transplantation patients. Br. J. Haematol. 2021, 196, 928–931. [Google Scholar] [CrossRef]
- Salvini, M.; Maggi, F.; Damonte, C.; Mortara, L.; Bruno, A.; Mora, B.; Brociner, M.; Mattarucchi, R.; Ingrassia, A.; Sirocchi, D.; et al. Immunogenicity of anti-SARS-CoV-2 Comirnaty vaccine in patients with lymphomas and myeloma who underwent autologous stem cell transplantation. Bone Marrow Transplant. 2021, 57, 137–139. [Google Scholar] [CrossRef]
- Harris, A.E.; Styczynski, J.; Bodge, M.; Mohty, M.; Savani, B.N.; Ljungman, P. Pretransplant vaccinations in allogeneic stem cell transplantation donors and recipients: An often-missed opportunity for immunoprotection? Bone Marrow Transplant. 2015, 50, 899–903. [Google Scholar] [CrossRef]
- Pao, M.; Papadopoulos, E.B.; Chou, J.; Glenn, H.; Castro-Malaspina, H.; Jakubowski, A.A.; Kernan, N.; Perales, M.A.; Prokop, S.; Scaradavou, A.; et al. Response to Pneumococcal (PNCRM7) and Haemophilus Influenzae Conjugate Vaccines (HIB) in Pediatric and Adult Recipients of an Allogeneic Hematopoietic Cell Transplantation (alloHCT). Biol. Blood Marrow Transplant. 2008, 14, 1022–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Dalla Pria, A.; Lightstone, L.; Xu, X.N.; et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021, 397, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.; Tut, G.; Bruton, R.; Faustini, S.; Stephens, C.; Saunders, P.; Bentley, C.; Hilyard, K.; Brown, K.; Amirthalingam, G.; et al. mRNA vaccination in people over 80 years of age induces strong humoral immune responses against SARS-CoV-2 with cross neutralization of P.1 Brazilian variant. eLife 2021, 10, e69375. [Google Scholar] [CrossRef] [PubMed]
- Jullien, M.; Coste-Burel, M.; Clemenceau, B.; Letailleur, V.; Guillaume, T.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Imbert, B.; Ollier, J.; et al. Anti-SARS-CoV-2 vaccines in recipient and/or donor before allotransplant. eJHaem 2022, 3, 484–487. [Google Scholar] [CrossRef]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef]
- Tamari, R.; Politikos, I.; Knorr, D.A.; Vardhana, S.A.; Young, J.C.; Marcello, L.T.; Doddi, S.; Devlin, S.M.; Ramanathan, L.V.; Pessin, M.S.; et al. Predictors of Humoral Response to SARS-CoV-2 Vaccination after Hematopoietic Cell Transplantation and CAR T-cell Therapy. Blood Cancer Discov. 2021, 2, 577–585. [Google Scholar] [CrossRef]
- Piñana, J.L.; López-Corral, L.; Martino, R.; Montoro, J.; Vazquez, L.; Pérez, A.; Martin-Martin, G.; Facal-Malvar, A.; Ferrer, E.; Pascual, M.; et al. SARS-CoV -2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am. J. Hematol. 2021, 97, 30–42. [Google Scholar] [CrossRef]
- Canti, L.; Humblet-Baron, S.; Desombere, I.; Neumann, J.; Pannus, P.; Heyndrickx, L.; Henry, A.; Servais, S.; Willems, E.; Ehx, G.; et al. Predictors of neutralizing antibody response to BNT162b2 vaccination in allogeneic hematopoietic stem cell transplant recipients. J. Hematol. Oncol. 2021, 14, 174. [Google Scholar] [CrossRef]

| Arm | Characteristic | Number (%) | the Mean (SD) of ISR | ||||
|---|---|---|---|---|---|---|---|
| Before First Dose | After First Dose | After Second Dose | p Value | ||||
| Control Healthy Group | Sex | Female | 22 (44) | 1.92 ± 1.49 | 2.67 ± 1.77 | 3.02 ± 1.69 | 0.89 |
| Male | 28 (56) | 1.90 ± 1.21 | 2.81 ± 1.64 | 3.00 ± 1.51 | |||
| Age (* Mean in years) | ≤40 | 35 (70) | 1.83 ± 1.26 | 2.83 ± 1.64 | 3.08 ± 1.43 | 0.68 | |
| >40 | 15 (30) | 2.09 ± 1.50 | 2.56 ± 1.82 | 2.85 ± 1.92 | |||
| Total | 50 (100) | 1.91 ± 1.33 | 2.75 ± 1.68 | 3.01 ± 1.58 | --- | ||
| Auto-HCT Group | Sex | Female | 16 (42.1) | 1.40 ± 0.81 | 2.56 ± 1.65 | 3.94 ± 2.07 | 0.82 |
| Male | 22 (57.9) | 1.37 ± 0.80 | 2.42 ± 1.71 | 3.75 ± 1.89 | |||
| Age (* Mean in years) | ≤40 | 10 (24) | 1.48 ± 0.97 | 2.39 ± 1.61 | 3.90 ± 2.10 | 0.78 | |
| >40 | 28 (76) | 1.29 ± 0.57 | 2.47 ± 1.67 | 3.61 ± 1.70 | |||
| Background disease | Lymphoma | 18 (47.4) | 1.29 ± 0.81 | 2.49 ± 1.72 | 3.90 ± 2.11 | 0.87 | |
| MM | 20 (52.6) | 1.47 ± 0.78 | 2.47 ± 1.67 | 3.61 ± 1.70 | |||
| Lymphocyte Count (cells/µL) | <1000 | 16 (42.1) | 1.26 ± 0.48 | 2.55 ± 1.90 | 3.14 ± 1.68 | 0.29 | |
| ≥1000 | 22 (57.9) | 1.48 ± 0.96 | 2.43 ± 1.52 | 4.21 ± 1.95 | |||
| Pre-HSCT COVID-19 vaccination | Yes | 34 (89.5) | 1.47 ± 0.80 | 2.59 ± 1.72 | 3.93 ± 1.90 | 0.04 | |
| No | 4 (10.5) | 0.72 ± 0.15 | 1.49 ± 0.66 | 2.29 ± 1.12 | |||
| Pre-HSCT PCR-positive COVID-19 | Yes | 21 (55.3) | 1.50 ± 0.89 | 2.83 ± 1.68 | 4.21 ± 1.80 | 0.046 | |
| No | 17 (44.7) | 1.25 ± 0.65 | 2.05 ± 1.60 | 3.20 ± 1.91 | |||
| Median (range) time between HSCT and Vaccination in days | <130 | 19 (50) | 1.44 ± 0.87 | 2.29 ± 1.49 | 3.58 ± 1.87 | 0.56 | |
| ≥130 | 19 (50) | 1.34 ± 0.73 | 2.67 ± 1.67 | 3.93 ± 1.96 | |||
| Total | 38 (100) | 1.39 ± 0.79 | 2.48 ± 1.67 | 3.75 ± 1.89 | --- | ||
| Total | 88 | 1.68 ± 1.15 | 2.63 ± 1.67 | 3.33 ± 1.75 | --- | ||
| Effect | Univariate | Multivariate | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Patients Age (≥50.5 vs. <50.5) | 0.80 (0.22–2.95) | 0.74 | ||
| Patients Sex (Male vs. Female) | 1.83 (0.47–7.07) | 0.37 | ||
| Background disease (Lymphoma vs. MM) | 0.67 (0.18–2.49) | 0.55 | ||
| Pre-HSCT PCR-positive COVID-19 (Yes vs. No) | 3.58 (0.87–14.65) | 0.07 | 6.24 (1.17–33.15) | 0.03 |
| Pre-HSCT COVID-19 vaccination (Yes vs. No) | 0.14 | |||
| Lymphocyte counts at vaccination (≥1000 vs. <1000) | 5.20 (1.15–23.54) | 0.03 | 8.57 (1.51–48.75) | 0.02 |
| Time between HSCT and Vaccination Median in Days (≥130 vs. <130) | 1.95 (0.52–7.31) | 0.32 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkhordar, M.; Ahmadvand, M.; Sharifi Aliabadi, L.; Noorani, S.S.; Bagheri Amiri, F.; Janbabai, G.; Sorouri, R.; Asadi Milani, M.; Vaezi, M. Evaluation of Safety and Immunogenicity of a Recombinant Receptor-Binding Domain (RBD)-Tetanus Toxoid (TT) Conjugated SARS-CoV-2 Vaccine (PastoCovac) in Recipients of Autologous Hematopoietic Stem Cell Transplantation Compared to the Healthy Controls; A Prospective, Open-Label Clinical Trial. Vaccines 2023, 11, 117. https://doi.org/10.3390/vaccines11010117
Barkhordar M, Ahmadvand M, Sharifi Aliabadi L, Noorani SS, Bagheri Amiri F, Janbabai G, Sorouri R, Asadi Milani M, Vaezi M. Evaluation of Safety and Immunogenicity of a Recombinant Receptor-Binding Domain (RBD)-Tetanus Toxoid (TT) Conjugated SARS-CoV-2 Vaccine (PastoCovac) in Recipients of Autologous Hematopoietic Stem Cell Transplantation Compared to the Healthy Controls; A Prospective, Open-Label Clinical Trial. Vaccines. 2023; 11(1):117. https://doi.org/10.3390/vaccines11010117
Chicago/Turabian StyleBarkhordar, Maryam, Mohammad Ahmadvand, Leyla Sharifi Aliabadi, Seied Saeid Noorani, Fahimeh Bagheri Amiri, Ghasem Janbabai, Rahim Sorouri, Mona Asadi Milani, and Mohammad Vaezi. 2023. "Evaluation of Safety and Immunogenicity of a Recombinant Receptor-Binding Domain (RBD)-Tetanus Toxoid (TT) Conjugated SARS-CoV-2 Vaccine (PastoCovac) in Recipients of Autologous Hematopoietic Stem Cell Transplantation Compared to the Healthy Controls; A Prospective, Open-Label Clinical Trial" Vaccines 11, no. 1: 117. https://doi.org/10.3390/vaccines11010117





