Immune Response to CoronaVac and Its Safety in Patients with Type 2 Diabetes Compared with Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Definitions
2.3. Procedure
2.4. Outcomes
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcome
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.2.3. Reactogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 March 2022).
- World Health Organization. Evaluation of COVID-19 Vaccine Effectiveness. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1 (accessed on 1 March 2022).
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef]
- Joshi, N.; Caputo, G.M.; Weitekamp, M.R.; Karchmer, A. Infections in Patients with Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J. Infection and Diabetes Mellitus. Diabetes Care 1980, 3, 187–197. [Google Scholar] [CrossRef]
- Mowat, A.G.; Baum, J. Chemotaxis of Polymorphonuclear Leukocytes from Patients with Diabetes Mellitus. N. Engl. J. Med. 1971, 284, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bagdade, J.D.; Root, R.K.; Bulger, R.J. Impaired Leukocyte Function in Patients with Poorly Controlled Diabetes. Diabetes 1974, 23, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bagdade, J.D.; Stewart, M.; Walters, E. Impaired Granulocyte Adherence: A Reversible Defect in Host Defense in Patients with Poorly Controlled Diabetes. Diabetes 1978, 27, 677–681. [Google Scholar] [CrossRef]
- van Oss, C.J.; Border, J.R. Influence of Intermittent Hyperglycemic Glucose Levels on the Phagocytosis of Microorganisms by Human Granulocytes in Vitro. Immunol. Commun. 1978, 7, 669–676. [Google Scholar] [CrossRef]
- Davidson, N.; Sowden, J.; Fletcher, J. Defective phagocytosis in insulin-controlled diabetics: Evidence for a reaction between glucose and opsonizing proteins. J. Clin. Pathol. 1984, 37, 783–786. [Google Scholar] [CrossRef]
- Alexiewicz, J.M.; Kumar, D.; Smogorzewski, M.; Klin, M.; Massry, S.G. Polymorphonuclear Leukocytes in Non-Insulin-dependent Diabetes Mellitus: Abnormalities in Metabolism and Function. Ann. Intern. Med. 1995, 123, 919–924. [Google Scholar] [CrossRef]
- Leibovici, L.; Yehezkelli, Y.; Porter, A.; Regev, A.; Krauze, I.; Harell, D. Influence of Diabetes Mellitus and Glycaemic Control on the Characteristics and Outcome of Common Infections. Diabet. Med. 1996, 13, 457–463. [Google Scholar] [CrossRef]
- Kwoun, M.O.; Ling, P.R.; Lydon, E.; Imrich, A.; Qu, Z.; Palombo, J.; Bistrian, B.R. Immunologic Effects of Acute Hyperglycemia in Nondiabetic Rats. J. Parenter. Enter. Nutr. 1997, 21, 91–95. [Google Scholar] [CrossRef] [PubMed]
- McManus, L.M.; Bloodworth, R.C.; Prihoda, T.J.; Blodgett, J.L.; Pinckard, R.N. Agonist-dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia. J. Leukoc. Biol. 2001, 70, 395–404. [Google Scholar] [CrossRef] [PubMed]
- MacRury, S.M.; Gemmell, C.G.; Paterson, K.R.; MacCuish, A.C. Changes in phagocytic function with glycaemic control in diabetic patients. J. Clin. Pathol. 1989, 42, 1143–1147. [Google Scholar] [CrossRef]
- Rassias, A.J.; Marrin, C.A.S.; Arruda, J.; Whalen, P.K.; Beach, M.; Yeager, M.P. Insulin Infusion Improves Neutrophil Function in Diabetic Cardiac Surgery Patients. Obstet. Anesth. Dig. 1999, 88, 1011–1016. [Google Scholar] [CrossRef]
- Rassias, A.J.; Givan, A.L.; Marrin, C.A.S.; Whalen, K.; Pahl, J.; Yeager, M.P. Insulin Increases Neutrophil Count and Phagocytic Capacity After Cardiac Surgery. Obstet. Anesthesia Dig. 2002, 94, 1113–1119. [Google Scholar] [CrossRef]
- Von Känel, R.; Mills, P.J.; Dimsdale, J.E. Short-term hyperglycemia induces lymphopenia and lymphocyte subset redistribution. Life Sci. 2001, 69, 255–262. [Google Scholar] [CrossRef]
- Bouter, K.P.; Meyling, F.H.; Hoekstra, J.B.; Masurel, N.; Erkelens, D.W.; Diepersloot, R.J. Influence of blood glucose levels on peripheral lymphocytes in patients with diabetes mellitus. Diabetes Res. 1992, 19, 77–80. [Google Scholar]
- Pérez-Galarza, J.; Prócel, C.; Cañadas, C.; Aguirre, D.; Pibaque, R.; Bedón, R.; Sempértegui, F.; Drexhage, H.; Baldeón, L. Immune Response to SARS-CoV-2 Infection in Obesity and T2D: Literature Review. Vaccines 2021, 9, 102. [Google Scholar] [CrossRef]
- World Health Organization. Background Document on the Inactivated Vaccine Sinovac-Coronavac against COVID-19. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1 (accessed on 1 March 2022).
- Notifier. WHO validates Sinovac COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. Available online: https://notifier.in/item/0qm7qm8ujt3xt78kltzmzbiiw342ykk3/2709341.html (accessed on 1 March 2022).
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 serological tests: Towards harmonization of anti-spike assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef]
- Rus, K.R.; Korva, M.; Knap, N.; Županc, T.A.; Poljak, M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J. Clin. Virol. 2021, 139, 104820. [Google Scholar] [CrossRef]
- Rangsrisaeneepitak, V.; Porntharukchareon, T.; Dechates, B.; Sirisreetreerux, S.; Tawinprai, K. Antibody levels in people with diabetes after one dose of the ChAdOx1 nCoV-19 (AZD1222) vaccine. Diabetol. Int. 2022, 13, 637–643. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Garneau, H.; Camous, X.; Dupuis, G.; Pawelec, G.; Baehl, S.; Tessier, D.; Frost, E.H.; Frasca, D.; Larbi, A.; et al. Predictors of the antibody response to influenza vaccination in older adults with type 2 diabetes. BMJ Open Diabetes Res. Care 2015, 3, e000140. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, A.B.; Forouhi, M.; Karimi, F.; Moghadam, A.S.; Naeini, L.G.; Kokabian, P.; Naderi, D. Immunogenicity of COVID-19 vaccines in patients with diabetes mellitus: A systematic review. Front. Immunol. 2022, 13, 940357. [Google Scholar] [CrossRef]
- Berg, J.M.V.D.; Remmelzwaal, S.; Blom, M.T.; van Hoek, B.A.C.E.; Swart, K.M.A.; Overbeek, J.A.; Burchell, G.L.; Herings, R.M.C.; Elders, P.J.M. Effectiveness of COVID-19 Vaccines in Adults with Diabetes Mellitus: A Systematic Review. Vaccines 2022, 11, 24. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Danasekara, S.; Gomes, L.; Fernando, S.; Guruge, D.; Ranasinghe, T.; Gunasekera, B.; Kamaladasa, A.; Kuruppu, H.; et al. Comparison of the immunogenicity of five COVID-19 vaccines in Sri Lanka. Immunology 2022, 167, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-J.; Chan, K.-H.; Hung, I.F.-N. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines 2021, 9, 989. [Google Scholar] [CrossRef]
- Xiang, F.; Long, B.; He, J.; Cheng, F.; Zhang, S.; Liu, Q.; Chen, Z.; Li, H.; Chen, M.; Peng, M.; et al. Impaired antibody responses were observed in patients with type 2 diabetes mellitus after receiving the inactivated COVID-19 vaccines. Virol. J. 2023, 20, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Lv, J.; Huang, T.; Zhang, R.; Zhang, D.; Luo, L.; Wei, S.; Liu, X.; Zhang, S.; et al. Evaluation of Immunogenicity and Safety of Vero Cell-Derived Inactivated COVID-19 Vaccine in Older Patients with Hypertension and Diabetes Mellitus. Vaccines 2022, 10, 1020. [Google Scholar] [CrossRef]
- Alqassieh, R.; Suleiman, A.; Abu-Halaweh, S.; Santarisi, A.; Shatnawi, O.; Shdaifat, L.; Tarifi, A.; Al-Tamimi, M.; Al-Shudifat, A.-E.; Alsmadi, H.; et al. Pfizer-BioNTech and Sinopharm: A Comparative Study on Post-Vaccination Antibody Titers. Vaccines 2021, 9, 1223. [Google Scholar] [CrossRef]
- Marfella, R.; Sardu, C.; D’Onofrio, N.; Prattichizzo, F.; Scisciola, L.; Messina, V.; La Grotta, R.; Balestrieri, M.L.; Maggi, P.; Napoli, C.; et al. Glycaemic control is associated with SARS-CoV-2 breakthrough infections in vaccinated patients with type 2 diabetes. Nat. Commun. 2022, 13, 2318. [Google Scholar] [CrossRef]
- Ou, X.; Jiang, J.; Lin, B.; Liu, Q.; Lin, W.; Chen, G.; Wen, J. Antibody responses to COVID-19 vaccination in people with obesity: A systematic review and meta-analysis. Influ. Other Respir. Viruses 2022, 17, e13078. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Liu, Q.; Mei, H.; Wang, Y.; Cui, G.; Zhao, S. Serological reactivity of inactivated SARS-CoV-2 vaccine based on an S-RBD neutralizing antibody assay. Int. J. Infect. Dis. 2022, 117, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Naruse, H.; Ito, H.; Izawa, H.; Sarai, M.; Ishii, J.; Sakaguchi, E.; Murakami, R.; Ando, T.; Fujigaki, H.; Saito, K. Immunogenicity of BNT162b2 mRNA COVID-19 Vaccine in Patients with Cardiovascular Disease. J. Clin. Med. 2021, 10, 5498. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-M.; Li, Q.; Yi, J.-X.; Cai, X.-J.; Xie, L.; Fang, W.; Qiu, J.-F.; Xu, C.-W.; He, C.-L.; Xu, X.-R.; et al. Investigation of Lymphocyte Subsets in Peripheral Blood of Patients with Dyslipidemia. Int. J. Gen. Med. 2021, 14, 5573–5579. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chung, H.; Lee, J.-E.; Kim, J.; Hwang, J.; Chung, Y. Immunologic Aspects of Dyslipidemia: A Critical Regulator of Adaptive Immunity and Immune Disorders. J. Lipid Atheroscler. 2021, 10, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Nicolay, U.; Del Giudice, G.; Rappuoli, R. Influence of Statins on Influenza Vaccine Response in Elderly Individuals. J. Infect. Dis. 2015, 213, 1224–1228. [Google Scholar] [CrossRef]

| Baseline Characteristics | Type 2 Diabetic Patients | Healthcare Workers | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 52.48 ± 10.71 | 52.11 ± 10.10 | |
| Male, n (%) | 13 (48.15) | 26 (48.15) | |
| BW, median (IQR) | 76 (60, 86) | 65.25 (55–73.5) | 0.044 |
| BMI, median (IQR) | 28.01 (24.93, 31.45) | 23.61 (21.51, 26.24) | <0.001 |
| <25, n (%) | 3 (11.11) | 36 (66.67) | |
| ≥25, n (%) | 20 (74.07) | 18 (33.33) | |
| Haemoglobin A1C, % (IQR) | 7.04 (6.20, 8.10) | NA | |
| ≤6.5, n (%) | 8 (29.63) | NA | |
| 6.6–7, n (%) | 8 (29.63) | NA | |
| 7.1–7.9, n (%) | 4 (14.81) | NA | |
| ≥8, n (%) | 7 (25.93) | NA | |
| Number of diabetes medications, % (IQR) | 3, (2–4) | NA | |
| 1, n (%) | 4 (15.38) | NA | |
| 2, n (%) | 8 (30.77) | NA | |
| 3, n (%) | 6 (23.08) | NA | |
| 4, n (%) | 7 (26.92) | NA | |
| 5, n (%) | 1 (3.85) | NA | |
| Insulin use, n (%) | 4 (14.81) | NA | |
| GLP-1 RA use, n (%) | 4 (14.81) | NA | |
| Comorbidities | 27 (100.00) | 9 (16.67) | <0.001 |
| Hypertension, n (%) | 18 (66.67) | 8 (14.81) | <0.001 |
| Dyslipidaemia, n (%) | 25 (92.59) | 5 (9.26) | <0.001 |
| Coronary artery disease, n (%) | 3 (11.11) | 1 (1.85) | 0.105 |
| Chronic kidney disease, n (%) | 1 (3.70) | 0 (0.00) | 0.333 |
| Cirrhosis, n (%) | 3 (11.11) | 0 (0.00) | 0.034 |
| Steroid use, n (%) | 1 (3.70) | 0 (0.00) | - |
| End-stage kidney disease, n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
| HIV + status n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
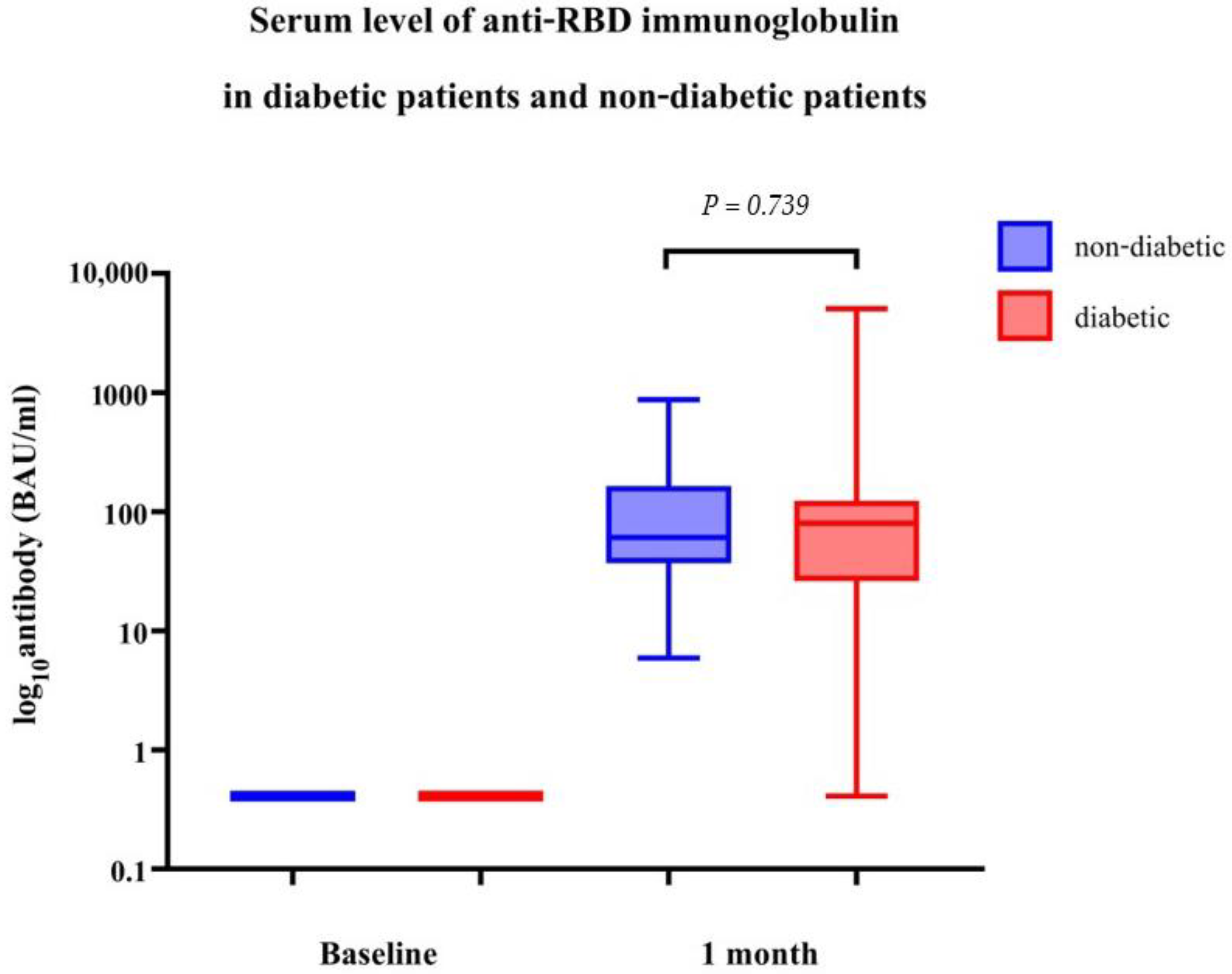
| Geometric Mean of Anti-RBD Immunoglobulin (BAU/mL) (95% CI) | Geometric Ratio | p-Value | |
|---|---|---|---|
| DM | 57.68 (29.08, 114.44) | 0.85 (0.32, 2.26) | 0.739 |
| Non-DM | 72.49 (55.77, 94.22) | Ref. |
| Type 2 Diabetic Patients | Geometric Mean of Anti-RBD Immunoglobulin (BAU/mL) (95% CI) | Geometric Ratio | p-Value |
|---|---|---|---|
| Level of HbA1c (%) | |||
| HbA1C ≤ 6.5 | 48.24 (14.77, 157.49) | Ref. | |
| HbA1C 6.6–7 | 30.95 (6.53, 146.75) | 0.64 (0.11, 3.63) | 0.602 |
| HbA1C 7.1–7.9 | 56.57 (9.38, 341.12) | 1.17 (0.26, 5.35) | 0.830 |
| HbA1C ≥ 8 | 145.82 (20.89, 1017.671) | 3.02 (0.43, 21.32) | 0.253 |
| Number of DM drugs | |||
| 1 | 83.79 (10.99, 638.89) | Ref. | |
| 2 | 31.81 (5.25, 192.77) | 0.38 (0.35, 3.06) | 0.346 |
| 3 | 44.67 (9.26, 215.47) | 0.53 (0.09, 3.29) | 0.480 |
| 4 | 100.39 (17.41, 578.75) | 1.20 (0.16, 8.83) | 0.853 |
| 5 | 39.31 | 0.47 (0.13, 1.69) | 0.232 |
| GLP1-RA | |||
| Use | 27.39(1.58, 474.19) | 0.44 (0.07, 2.77) | 0.367 |
| Non-use | 62.24 (28.66, 135.18) | Ref. | |
| Insulin | |||
| Use | 63.17 (16.85, 236.82) | 1.11 (0.36, 3.40) | 0.846 |
| Non-use | 56.79 (25.44, 126.75) | Ref. | |
| BMI (kg/m2) | |||
| <25 | 40.05 (4.99–321.63) | Ref. | |
| ≥25 | 65.55 (31.58, 136.06) | 1.64 (0.26–10.29) | 0.585 |
| Age (years) | |||
| 18–30 | 99.96 | Ref. | |
| 31–60 | 52.15 (24.76, 109.82) | 0.52 (0.24, 1.11) | 0.090 |
| >60 | 73.72 (5.90, 921.82) | 0.74 (0.10, 5.26) | 0.752 |
| Sex | |||
| Male | 71.01 (24.63, 204.69) | Ref. | |
| Female | 47.57 (17.27, 131.05) | 0.67 (1.17, 2.69) | 0.558 |
| Comorbidities | |||
| HT | |||
| HT | 42.51 (17.75, 101.84) | 0.40 (0.10, 1.59) | 0.183 |
| Non-HT | 106.24 (31.08, 363.16) | Ref. | |
| DLP | |||
| DLP | 50.04 (24.59, 101.83) | 0.15 (0.07, 0.30) | <0.001 |
| Non-DLP | 341.64 (119.88, 973.58) | Ref. | |
| CAD | |||
| CAD | 11.30 (0.00, 27763.21) | 0.16 (0.01, 4.05) | 0.253 |
| Non-CAD | 70.73 (38.14, 131.20) | Ref. | |
| Cirrhosis | |||
| Cirrhosis | 120.37 (9.48, 1529.01) | 2.29 (0.63, 8.28) | 0.197 |
| Non-Cirrhosis | 52.62 (24.67, 112.25) | Ref. | |
| CKD | |||
| CKD | 100.60 | 1.78 (0.86, 3.68) | 0.114 |
| Non-CKD | 56.47 (27.71, 115.06) | Ref. | |
| Steroid | |||
| Steroid use | 0.41 | 0.01 (0.00, 0.01) | <0.001 |
| Non-steroid use | 69.77 (38.84, 125.34) | Ref. |
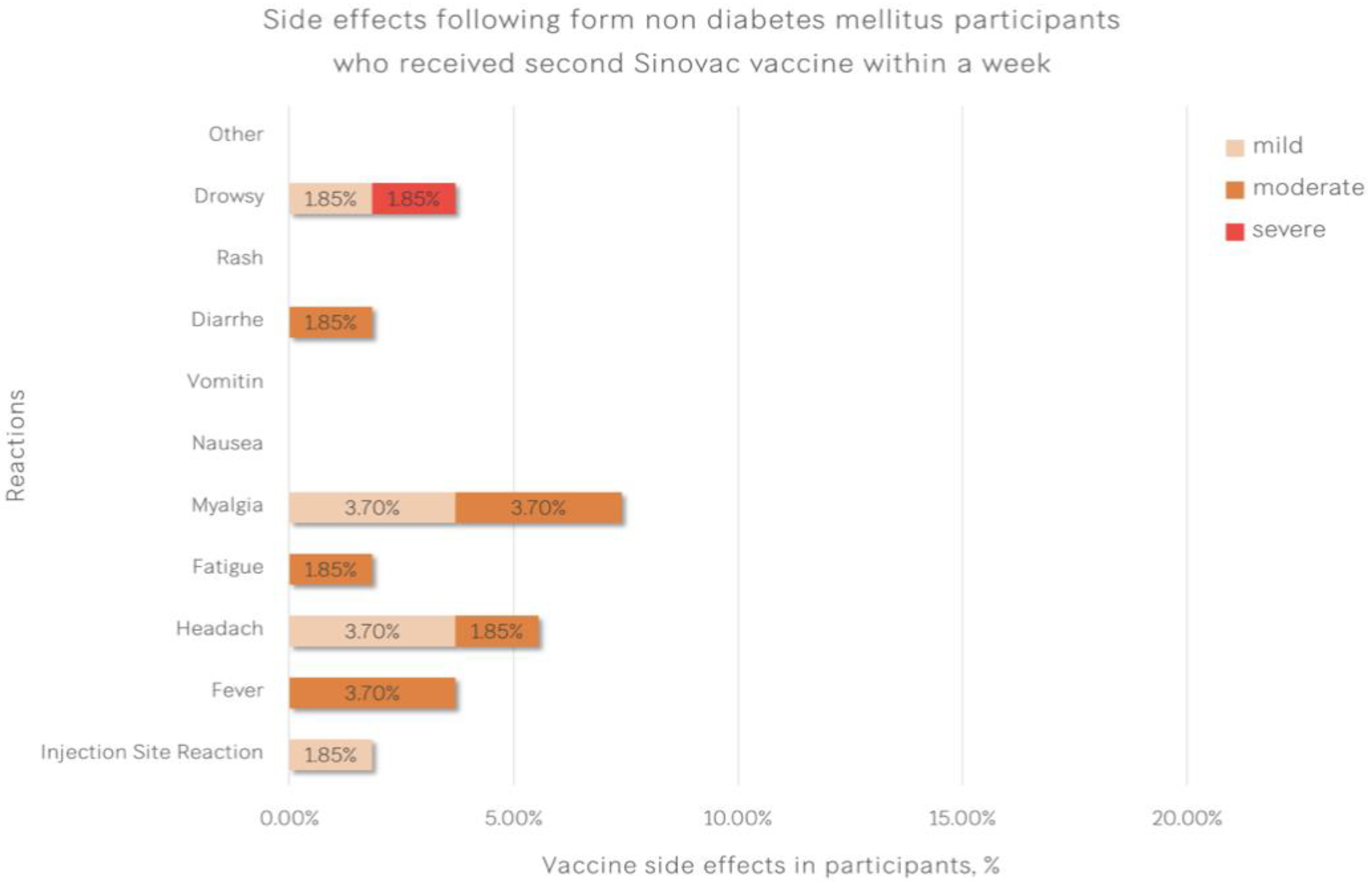
| Reactions | Type 2 Diabetic Patients (%) | Healthcare Workers (%) | p-Value |
|---|---|---|---|
| Injection Site Reaction | 4 (14.81) | 1 (1.85) | 0.040 |
| Fever | 2 (7.41) | 2 (3.70) | 0.597 |
| Headache | 3 (11.11) | 3 (5.56) | 0.395 |
| Fatigue | 3 (11.11) | 1 (1.85) | 0.105 |
| Myalgia | 4 (14.81) | 4 (7.41) | 0.431 |
| Nausea | 1 (3.70) | 0 (0.00) | 0.333 |
| Vomiting | 1 (3.70) | 0 (0.00) | 0.333 |
| Diarrhoea | 1 (3.70) | 1 (1.85) | 1.000 |
| Rash | 0 (0.00) | 0 (0.00) | - |
| Drowsiness | 1 (3.70) | 2 (3.70) | 1.000 |
| Other | 0 (0.00) | 0 (0.00) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dechates, B.; Porntharukchareon, T.; Sirisreetreerux, S.; Therawit, P.; Worawitchawong, S.; Sornsamdang, G.; Soonklang, K.; Tawinprai, K. Immune Response to CoronaVac and Its Safety in Patients with Type 2 Diabetes Compared with Healthcare Workers. Vaccines 2023, 11, 684. https://doi.org/10.3390/vaccines11030684
Dechates B, Porntharukchareon T, Sirisreetreerux S, Therawit P, Worawitchawong S, Sornsamdang G, Soonklang K, Tawinprai K. Immune Response to CoronaVac and Its Safety in Patients with Type 2 Diabetes Compared with Healthcare Workers. Vaccines. 2023; 11(3):684. https://doi.org/10.3390/vaccines11030684
Chicago/Turabian StyleDechates, Bothamai, Thachanun Porntharukchareon, Supamas Sirisreetreerux, Phonthip Therawit, Supanat Worawitchawong, Gaidganok Sornsamdang, Kamonwan Soonklang, and Kriangkrai Tawinprai. 2023. "Immune Response to CoronaVac and Its Safety in Patients with Type 2 Diabetes Compared with Healthcare Workers" Vaccines 11, no. 3: 684. https://doi.org/10.3390/vaccines11030684
APA StyleDechates, B., Porntharukchareon, T., Sirisreetreerux, S., Therawit, P., Worawitchawong, S., Sornsamdang, G., Soonklang, K., & Tawinprai, K. (2023). Immune Response to CoronaVac and Its Safety in Patients with Type 2 Diabetes Compared with Healthcare Workers. Vaccines, 11(3), 684. https://doi.org/10.3390/vaccines11030684





