Longitudinal Assessment of Lipoprotein(a) Levels in Perinatally HIV-Infected Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Lipid Profiles
2.3. Neuroimaging Details
2.4. HIV Characteristics
2.5. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
3.1.1. Retrospective Longitudinal Substudy
3.1.2. Cohort Substudy
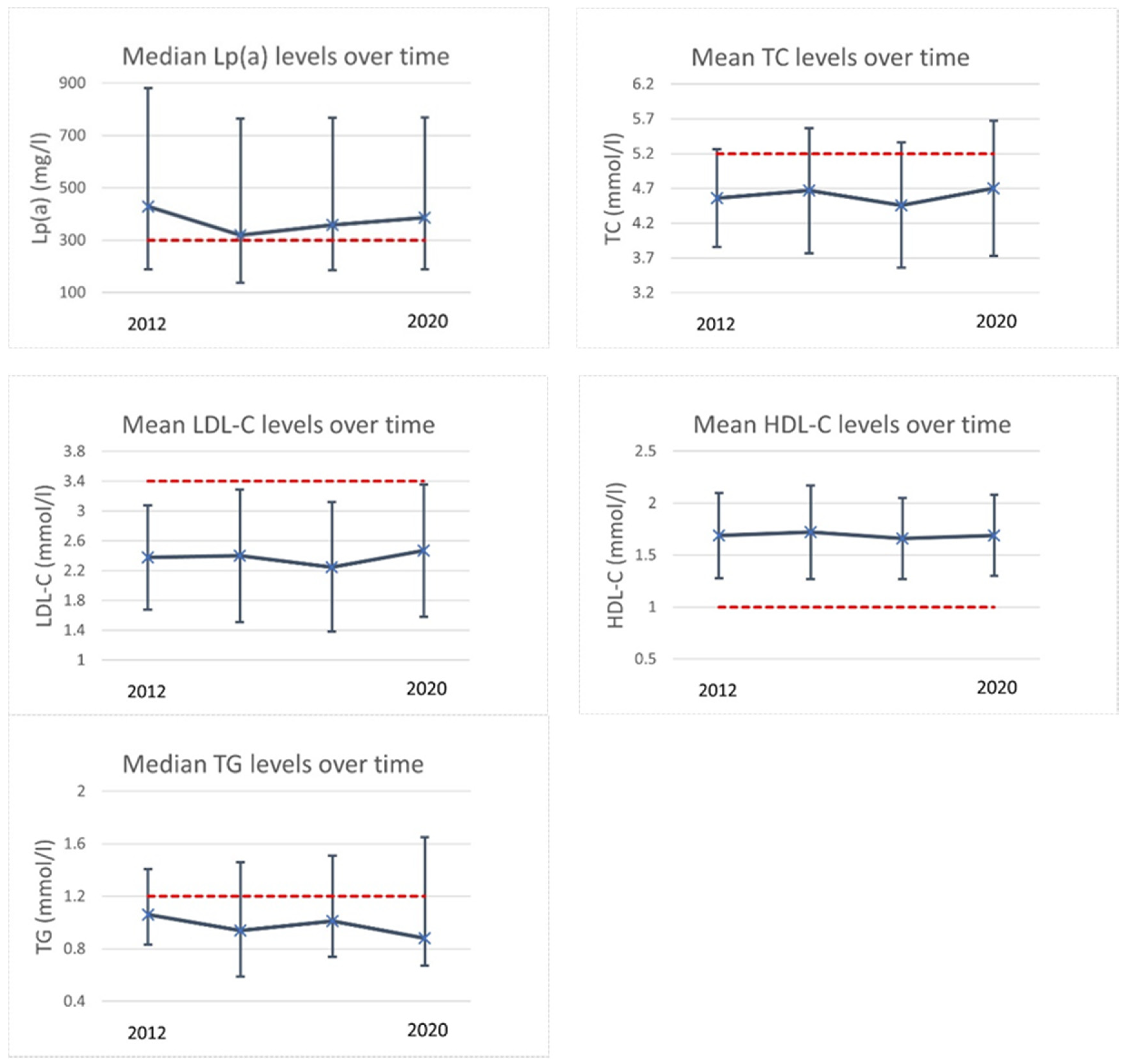
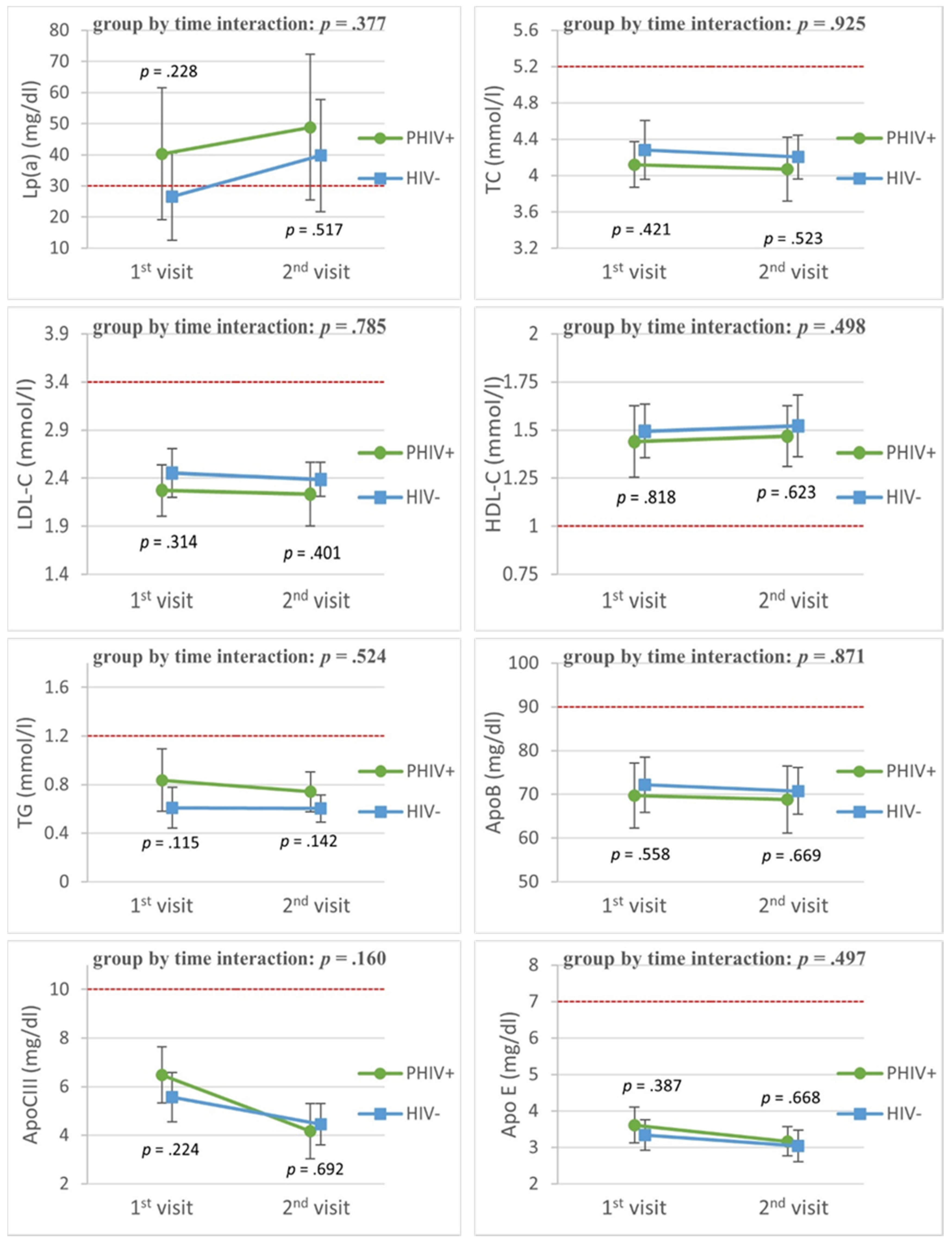
3.2. Lipid Profiles over Time
3.2.1. Retrospective Longitudinal Substudy
3.2.2. Cohort Substudy
3.3. Factors Associated with Lp(a)
3.3.1. Retrospective Longitudinal Substudy
3.3.2. Cohort Substudy
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Stelzle, D.; Lee, K.K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Barlow-Mosha, L.; Eckard, A.R.; McComsey, G.A.; Musoke, P.M. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J. Int. AIDS Soc. 2013, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.S.; Grobbee, D.E.; Burgner, D.; Cheung, M.M.; Kurniati, N.; Sastroasmoro, S.; Uiterwaal, C.S. Cardiovascular manifestations of HIV infection in children. Eur. J. Prev. Cardiol. 2015, 22, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Tawakol, A.; Burdo, T.H.; Abbara, S.; Wei, J.; Vijayakumar, J.; Corsini, E.; Abdelbaky, A.; Zanni, M.V.; Hoffmann, U.; et al. Arterial inflammation in patients with HIV. JAMA 2012, 308, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friis-Møller, N.; Sabin, C.A.; Weber, R.; d’Arminio Monforte, A.; El-Sadr, W.M.; Reiss, P.; Thiébaut, R.; Morfeldt, L.; De Wit, S.; Pradier, C.; et al. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 2003, 349, 1993–2003. [Google Scholar] [PubMed] [Green Version]
- Périard, D.; Telenti, A.; Sudre, P.; Cheseaux, J.J.; Halfon, P.; Reymond, M.J.; Marcovina, S.M.; Glauser, M.P.; Nicod, P.; Darioli, R.; et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation 1999, 100, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Prasad, A.; Choi, Y.S.; Xing, C.; Clopton, P.; Witztum, J.L.; Tsimikas, S. LPA Gene, Ethnicity, and Cardiovascular Events. Circulation 2017, 135, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Mauss, S.; Berger, F.; Schmutz, G.; Henke, J.; Richter, W.O. Lipoprotein(a) in patients initiating antiretroviral therapy. HIV Med. 2008, 9, 415–420. [Google Scholar] [CrossRef]
- Koppel, K.; Bratt, G.; Eriksson, M.; Sandström, E. Serum lipid levels associated with increased risk for cardiovascular disease is associated with highly active antiretroviral therapy (HAART) in HIV-1 infection. Int. J. STD AIDS 2000, 11, 451–455. [Google Scholar] [CrossRef]
- Van den Hof, M.; Klein Haneveld, M.J.; Blokhuis, C.; Scherpbier, H.J.; Jansen, H.; Kootstra, N.A.; Dallinga-Thie, G.M.; Van Deventer, S.; Tsimikas, S.; Pajkrt, D.; et al. Elevated Lipoprotein(a) in Perinatally HIV-Infected Children Compared With Healthy Ethnicity-Matched Controls. Open Forum Infect. Dis. 2019, 6, ofz301. [Google Scholar]
- Charakida, M.; Donald, A.E.; Green, H.; Storry, C.; Clapson, M.; Caslake, M.; Dunn, D.T.; Halcox, J.P.; Gibb, D.M.; Klein, N.J.; et al. Early structural and functional changes of the vasculature in HIV-infected children: Impact of disease and antiretroviral therapy. Circulation 2005, 112, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Lainka, E.; Oezbek, S.; Falck, M.; Ndagijimana, J.; Niehues, T. Marked dyslipidemia in human immunodeficiency virus-infected children on protease inhibitor-containing antiretroviral therapy. Pediatrics 2002, 110, e56. [Google Scholar] [CrossRef] [Green Version]
- McNeal, C.J. Lipoprotein(a): Its relevance to the pediatric population. J. Clin. Lipidol. 2015, 9, S57–S66. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.R.; Dahlen, G.H.; Jarpa, R.A.; Webber, L.S.; Berenson, G.S. Racial (black-white) differences in serum lipoprotein (a) distribution and its relation to parental myocardial infarction in children. Bogalusa Heart Study. Circulation 1991, 84, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.P.; Wang, M.; Pirruccello, J.P.; Ellinor, P.T.; Ng, K.; Kathiresan, S.; Khera, A.V. Lp(a) (Lipoprotein[a]) Concentrations and Incident Atherosclerotic Cardiovascular Disease: New Insights From a Large National Biobank. Arterioscler. Thromb. Vasc. Biol. 2001, 41, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; van Bilsen, W.P.; Smit, C.; Fraaij, P.L.; Warris, A.; Kuijpers, T.W.; Geelen, S.P.; Wolfs, T.F.; Scherpbier, H.J.; van Rossum, A.M.; et al. Country of birth does not influence long-term clinical, virologic, and immunological outcome of HIV-infected children living in the Netherlands: A cohort study comparing children born in the Netherlands with children born in Sub-Saharan Africa. J. Acquir. Immune Defic. Syndr. (1999) 2015, 68, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Saleheen, D.; Haycock, P.C.; Zhao, W.; Rasheed, A.; Taleb, A.; Imran, A.; Abbas, S.; Majeed, F.; Akhtar, S.; Qamar, N.; et al. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: A mendelian randomisation analysis. The lancet. Diabetes Endocrinol. 2015, 5, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Van den Hof, M.; Ter Haar, A.M.; Scherpbier, H.J.; van der Lee, J.H.; Reiss, P.; Wit, F.; Oostrom, K.J.; Pajkrt, D. Neurocognitive Development in Perinatally Human Immunodeficiency Virus-infected Adolescents on Long-term Treatment, Compared to Healthy Matched Controls: A Longitudinal Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 70, 1364–1371. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, S213–S256. [Google Scholar] [CrossRef] [Green Version]
- Mahley, R.W.; Innerarity, T.L.; Rall, S.C., Jr.; Weisgraber, K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984, 25, 1277–1294. [Google Scholar] [CrossRef]
- Huff, M.W.; Hegele, R.A. Apolipoprotein C-III: Going back to the future for a lipid drug target. Circ. Res. 2013, 112, 1405–1408. [Google Scholar] [CrossRef] [Green Version]
- Moriarty, P.M.; Varvel, S.A.; Gordts, P.L.; McConnell, J.P.; Tsimikas, S. Lipoprotein(a) Mass Levels Increase Significantly According to APOE Genotype: An Analysis of 431 239 Patients. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Van den Hof, M.; Jellema, P.; Ter Haar, A.M.; Scherpbier, H.J.; Schrantee, A.; Kaiser, A.; Caan, M.; Majoie, C.; Reiss, P.; Wit, F.; et al. Normal structural brain development in adolescents treated for perinatally acquired HIV: A longitudinal imaging study. AIDS 2021, 35, 1221–1228. [Google Scholar] [CrossRef]
- Stichting HIV Monitoring (SHM). Available online: https://www.hiv-monitoring.nl/english/ (accessed on 20 May 2021).
- R Core Team. R: A Language and Environment for Statistical; R Foundation for Statistical Computing, Vienna, Austria. 2013. Available online: http://www.r-project.org/ (accessed on 27 May 2021).
- Ram, N.; Gerstorf, D. Time-structured and net intraindividual variability: Tools for examining the development of dynamic characteristics and processes. Psychol. Aging 2009, 24, 778–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.; Cao, J.; Steffen, B.T.; Post, W.S.; Stein, J.H.; Tattersall, M.C.; Kaufman, J.D.; McConnell, J.P.; Hoefner, D.M.; Warnick, R.; et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 996–1001. [Google Scholar] [CrossRef] [Green Version]
- Paré, G.; Çaku, A.; McQueen, M.; Anand, S.S.; Enas, E.; Clarke, R.; Boffa, M.B.; Koschinsky, M.; Wang, X.; Yusuf, S.; et al. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. Circulation 2019, 139, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Obisesan, T.O.; Aliyu, M.H.; Adediran, A.S.; Bond, V.; Maxwell, C.J.; Rotimi, C.N. Correlates of serum lipoprotein (A) in children and adolescents in the United States. The third National Health Nutrition and Examination Survey (NHANES-III). Lipids Health Dis. 2004, 3, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, L.; Hutten, B.; Wiegman, A.; Kastelein, J.; Hof, M. Lipoprotein(A) Levels Over Time: A Long-Term Follow-Up Study Of A Large Cohort Of Children. Atherosclerosis 2019, 287, e59. [Google Scholar] [CrossRef]
- Tsimikas, S. The re-emergence of lipoprotein(a) in a broader clinical arena. Prog. Cardiovasc. Dis. 2016, 59, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; Mccomsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 71, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Babyak, M.A. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom. Med. 2004, 66, 411–421. [Google Scholar] [PubMed] [Green Version]
- Fatica, E.M.; Meeusen, J.W.; Vasile, V.C.; Jaffe, A.S.; Donato, L.J. Measuring the contribution of Lp(a) cholesterol towards LDL-C interpretation. Clin. Biochem. 2020, 86, 45–51. [Google Scholar] [CrossRef]
- Yeang, C.; Witztum, J.L.; Tsimikas, S. Novel method for quantification of lipoprotein(a)-cholesterol: Implications for improving accuracy of LDL-C measurements. J. Lipid Res. 2021, 62, 100053. [Google Scholar] [CrossRef] [PubMed]
- Brandstätter, A.; Lingenhel, A.; Zwiauer, K.; Strobl, W.; Kronenberg, F. Decrease of Lp(a) during weight reduction in obese children is modified by the apo(a) kringle-IV copy number variation. Int. J. Obes. 2009, 33, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Valdés Hernández, M.C.; Muñoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 2015, 4, 001140. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Caan, M.W.; Mutsaerts, H.J.; Scherpbier, H.J.; Kuijpers, T.W.; Reiss, P.; Majoie, C.B.; Pajkrt, D. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology 2016, 86, 19–27. [Google Scholar] [CrossRef]
- Hoare, J.; Fouche, J.P.; Phillips, N.; Joska, J.A.; Myer, L.; Zar, H.J.; Stein, D.J. Structural brain changes in perinatally HIV-infected young adolescents in South Africa. AIDS 2018, 32, 2707–2718. [Google Scholar] [CrossRef] [PubMed]
- Uban, K.A.; Herting, M.M.; Williams, P.L.; Ajmera, T.; Gautam, P.; Huo, Y.; Malee, K.M.; Yogev, R.; Csernansky, J.G.; Wang, L.; et al. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS 2015, 29, 1035–1044. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar]
- Tsimikas, S.; Clopton, P.; Brilakis, E.S.; Marcovina, S.M.; Khera, A.; Miller, E.R.; de Lemos, J.A.; Witztum, J.L. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: Results from the Dallas Heart Study. Circulation 2009, 119, 1711–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, E.M.; Ellis, K.L.; Barrett, P.; Watts, G.F.; Hung, J.; Beilby, J.P.; Thompson, P.L.; Stobie, P.; McQuillan, B.M. Lipoprotein(a) and apolipoprotein(a) isoform size: Associations with angiographic extent and severity of coronary artery disease, and carotid artery plaque. Atherosclerosis 2018, 275, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lapinleimu, J.; Raitakari, O.T.; Lapinleimu, H.; Pahkala, K.; Rönnemaa, T.; Simell, O.G.; Viikari, J.S. High lipoprotein(a) concentrations are associated with impaired endothelial function in children. J. Pediatrics 2015, 166, 947–952.e522. [Google Scholar] [CrossRef]
- Gencer, B.; Kronenberg, F.; Stroes, E.S.; Mach, F. Lipoprotein(a): The revenant. Eur. Heart J. 2017, 38, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
| First Assessment (n = 36) | Last Assessment (n = 36) | |
|---|---|---|
| Age (y) | 8.0 (5.7–10.8) | 11.1 (3.6) |
| Male sex | 24 (67%) | |
| Ethnic background | ||
| Black | 33 (92%) | |
| Other | 3 (8%) | |
| Adopted | 30 (83%) | |
| Height (m) | 1.12 (0.95–1.30) | 1.47 (1.33–1.62) |
| Weight (kg) | 19.8 (14.4–30.2) | 42.0 (29.2–52.5) |
| Body Mass Index | 16.2 (15.2–17.8) | 18.7 (16.2–21.5) |
| APOE genotypes | ||
| ε2/ε2 | 1 (5%) | |
| ε2/ε3 | 4 (17%) | |
| ε2/ε4 | 2 (8%) | |
| ε3/ε3 | 10 (42%) | |
| ε3/ε4 | 4 (17%) | |
| ε4/ε4 | 3 (13%) | |
| Age at start cART (y) | 2.8 (0.3–5.8) | |
| CDC category | ||
| NA | 19 (83%) | |
| B | 1 (4.3%) | |
| C | 3 (13%) | |
| HIV viral load (<40 copies/mL) | ||
| Undetectable | 32 (89%) | 36 (100%) |
| Detectable | 4 (11%) | 0 (0%) |
| * CD4+ T cell nadir (cells/µL) | 0.82 (SD: 0.31) | |
| CD4+ T cell nadir Z score | 0.03 (SD: 1.1) | |
| CD4+ T cell nadir % | 35% (31–39%) | |
| * HIV zenith (log copies/mL) | 3.6 (2.5–5.4) | |
| cART regiment, no(%) | ||
| Backbone + NtRTI | 1 (3%) | 0 (0%) |
| Backbone + NNRTI | 18 (50%) | 1 (3%) |
| Backbone + PI | 17 (47%) | 10 (28%) |
| Backbone + INSTI | 0 (0%) | 25 (69%) |
| * Exposure to cART | ||
| PI ever used | 23 (64%) | |
| Duration (y) | 3.41 (2.35–4.59) | |
| INSTI ever used | 24 (67%) | |
| Duration (y) | 1.78 (1.34–2.42) | |
| NNRTI ever used | 20 (56%) | |
| Duration (y) | 2.68 (1.32–3.95) |
| PHIV | CONTROLS | ||
|---|---|---|---|
| p | |||
| Follow-up rate | 62% | 62% | |
| Follow-up time | 4.65 (0.33) | 4.55 (0.33) | 0.343 X |
| Age (y) | |||
| At first enrollment | 13.4 (10.9–15.6) | 12.1 (11.1–15.2) | 0.655 Z |
| At second enrollment | 17.5 (15.5–20.7) | 16.4 (15.8–19.5) | 0.526 Z |
| Male sex | 12 (57%) | 9 (39%) | 0.365 Y |
| Ethnic background | 0.606 Y | ||
| Black | 17 (81%) | 18 (78%) | |
| White | 0 (0%) | 2 (9%) | |
| Other | 4 (19%) | 3 (13%) | |
| Height (m) | 1.66 (0.13) | 1.69 (0.09) | 0.297 X |
| Weight (kg) | 56.3 (49.0–69.5) | 63.0 (56.0–67.4) | 0.109 Z |
| Body Mass Index | 20.4 (19.2–22.3) | 22.2 (19.9–26.3) | 0.137 Z |
| Overweight or obese * | |||
| At first enrollment | 1 (5%) | 1 (4%) | 0.999 Y |
| At second enrollment | 2 (10%) | 7 (30%) | 0.137 Y |
| Blood Pressure (mmHg) | |||
| Systolic | 114 (109–125) | 119 (114–124) | 0.484 Z |
| Diastolic | 65 (56–73) | 64 (59–74) | 0.843 Z |
| Lifestyle | |||
| Ever Smoked | 7 (33%) | 5 (22%) | 0.504 Y |
| APOE genotypes | 0.466 Y | ||
| ε2/ε2 | 0 (0%) | 0 (0%) | |
| ε2/ε3 | 3 (17%) | 2 (10%) | |
| ε2/ε4 | 0 (0%) | 3 (14%) | |
| ε3/ε3 | 9 (50%) | 10 (48%) | |
| ε3/ε4 | 6 (33%) | 5 (24%) | |
| ε4/ε4 | 0 (0%) | 1 (5%) | |
| Age at HIV diagnosis (y) | 1.72 (0.83–4.16) | ||
| CDC | |||
| NA | 8 (38%) | ||
| B | 8 (38%) | ||
| C | 5 (24%) | ||
| Age at treatment initiation (y) | 2.52 (1.20–5.97) | ||
| cART duration (y) | 14.86 (9.51–19.57) | ||
| cART use | |||
| At 1st enrollment | 20 (95%) | ||
| At 2nd enrollment | 20 (95%) | ||
| Current regimen | |||
| Backbone + INSTI | 12 (60%) | ||
| Backbone + PI | 4 (20%) | ||
| Backbone + NNRTI | 4 (20%) | ||
| CD4+ nadir | 460 (300–570) | ||
| CD4+ Z score | −0.82 (0.61) | ||
| HIV zenith (log copies/mL) | 5.5 (4.9–5.8) | ||
| Undetectable HIV viral load | |||
| At 1st enrollment | 20 (95%) | ||
| At 2nd enrollment | 19 (90%) | ||
| During entire follow-up | 15 (71%) |
| Lp(a) of PHIV+ Visiting Outpatient Clinic | ||||||
|---|---|---|---|---|---|---|
| Univariable Analyses | Analyses (Adjusted for BMI) | |||||
| Coefficient | 95%CI | p | Coefficient | 95%CI | p | |
| Age | 0.99 | 0.98–1.01 | 0.402 | |||
| Male sex | 0.53 | 0.26–1.01 | 0.079 | |||
| BMI (kg/m2) | 0.98 | 0.96–1.02 | 0.029 | - | - | - |
| PI use | 0.97 | 0.89–1.05 | 0.401 | |||
| INSTI use | 0.96 | 0.90–1.02 | 0.171 | |||
| NNRTI use | 1.10 | 1.03–1.19 | 0.007 | 1.07 | 0.99–1.16 | 0.111 |
| Lipids (mmol/L) | ||||||
| TC | 1.12 | 1.06–1.18 | <0.001 | 1.14 | 1.08–1.20 | <0.001 |
| LDL-C | 1.11 | 1.05–1.18 | <0.001 | 1.14 | 1.07–1.21 | <0.001 |
| HDL-C | 1.03 | 0.92–1.16 | 0.570 | |||
| TG | 0.99 | 0.97–1.05 | 0.698 | |||
| APOE genotypes | ||||||
| ε2/ε2 | 0.18 | 0.03–0.98 | 0.049 | |||
| ε2/ε3 | 1.17 | 0.45–3.03 | 0.732 | |||
| ε2/ε4 | 2.12 | 0.61–7.39 | 0.229 | |||
| ε3/ε3 * | - | - | - | |||
| ε3/ε4 | 0.79 | 0.30–2.05 | 0.623 | |||
| ε4/ε4 | 1.28 | 0.41–3.71 | 0.636 | |||
| Lp(a) of PHIV Adolescents AND Controls | ||||||
|---|---|---|---|---|---|---|
| Univariable Analyses with Mixed Models | Multivariable Analyses with Mixed Models | |||||
| Coefficient | 95%CI | p | Coefficient | 95%CI | p | |
| HIV diagnosis | 2.41 | 0.85–6.75 | 0.106 | |||
| Age | 1.03 | 0.96–1.10 | 0.397 | |||
| Male Sex | 1.00 | 0.42–2.38 | 0.999 | |||
| Systolic BP (mmHg) | 1.00 | 0.99–1.02 | 0.744 | |||
| BMI (kg/m2) | 1.03 | 0.95–1.11 | 0.512 | |||
| Lipids (mmol/L) | ||||||
| TC | 1.6 | 1.00–2.59 | 0.048 | 0.88 | (0.31–2.74) | 0.832 |
| HDL-C | 1.02 | 0.42–1.10 | 0.958 | |||
| LDL-C | 2.29 | 1.28–3.90 | 0.004 | 2.35 | (0.53–7.78) | 0.231 |
| TG | 0.65 | 0.38–1.08 | 0.101 | |||
| APOE genotypes | ||||||
| ε2/ε2 | - | - | - | |||
| ε2/ε3 | 0.54 | 0.14–2.16 | 0.423 | |||
| ε2/ε4 | 0.04 | 0.01–0.17 | 0.001 | 0.04 | (0.007–0.24) | 0.004 |
| ε3/ε3 * | - | - | - | |||
| ε3/ε4 | 0.54 | 0.20–1.52 | 0.285 | |||
| ε4/ε4 | 0.68 | 0.06–8.25 | 0.779 | |||
| MRI parameters | ||||||
| WMH volume (mm3) | 1.00 | 0.90–1.00 | 0.123 | |||
| FA | 0.01 | −0.02 to 0.03 | 0.527 | |||
| MD | −0.02 | −0.05 to 0.01 | 0.128 | |||
| AD | −0.03 | −0.06 to 0.01 | 0.168 | |||
| RD | −0.03 | −0.06 to 0.01 | 0.141 | |||
| Lp(a) of PHIV+ adolescents | ||||||
| Univariable analyses with mixed models | ||||||
| coefficient | 95%CI | p | ||||
| HIV VL zenith (log) c/mL | 1.66 | 0.25–11.2 | 0.601 | |||
| CD4+ nadir Z-score | 1.22 | 0.55–2.72 | 0.624 | |||
| Duration of cART (years) | 1.01 | 0.92–1.11 | 0.836 | |||
| CDC category | ||||||
| B | 1.40 | 0.51–3.86 | 0.535 | |||
| C | 1.94 | 0.61–6.17 | 0.290 | |||
| Undetectable VL at both measurements | 0.79 | 0.29–2.16 | 0.652 | |||
| BMI (kg/m2) | 1.07 | 1.03–1.12 | 0.002 | |||
| PI use | 0.90 | 0.53–1.46 | 0.675 | |||
| INSTI use | 1.11 | 0.89–1.38 | 0.381 | |||
| NNRTI use | 0.90 | 0.72–1.16 | 0.434 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Genderen, J.G.; Van den Hof, M.; de Boer, C.G.; Jansen, H.P.G.; van Deventer, S.J.H.; Tsimikas, S.; Witztum, J.L.; Kastelein, J.J.P.; Pajkrt, D. Longitudinal Assessment of Lipoprotein(a) Levels in Perinatally HIV-Infected Children and Adolescents. Viruses 2021, 13, 2067. https://doi.org/10.3390/v13102067
van Genderen JG, Van den Hof M, de Boer CG, Jansen HPG, van Deventer SJH, Tsimikas S, Witztum JL, Kastelein JJP, Pajkrt D. Longitudinal Assessment of Lipoprotein(a) Levels in Perinatally HIV-Infected Children and Adolescents. Viruses. 2021; 13(10):2067. https://doi.org/10.3390/v13102067
Chicago/Turabian Stylevan Genderen, Jason G., Malon Van den Hof, Claudia G. de Boer, Hans P. G. Jansen, Sander J. H. van Deventer, Sotirios Tsimikas, Joseph L. Witztum, John J. P. Kastelein, and Dasja Pajkrt. 2021. "Longitudinal Assessment of Lipoprotein(a) Levels in Perinatally HIV-Infected Children and Adolescents" Viruses 13, no. 10: 2067. https://doi.org/10.3390/v13102067






