Neuropsychological and Psychosocial Functioning of Children with Perinatal HIV-Infection in The Netherlands
Abstract
1. Introduction
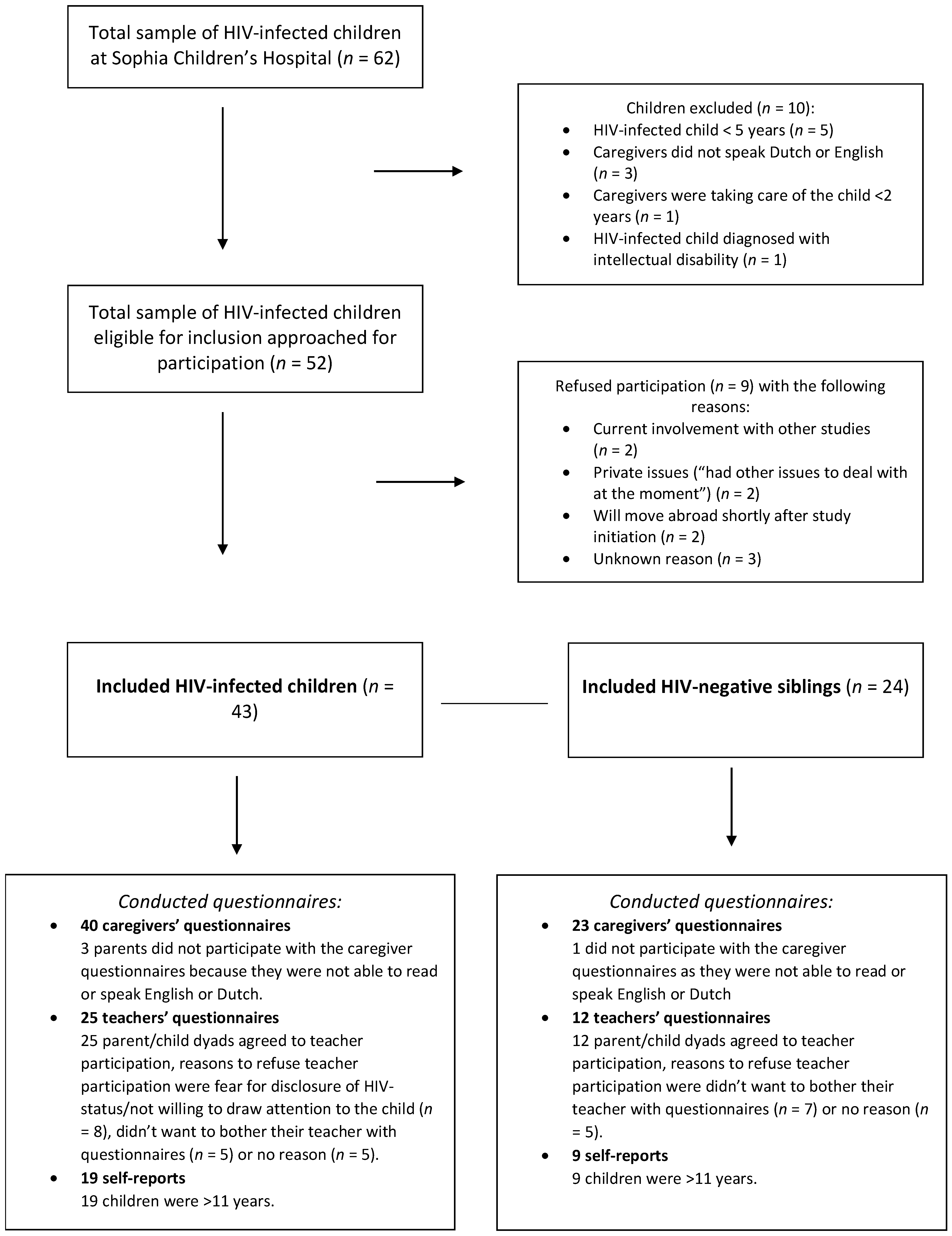
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Procedure
2.4. Neuropsychological and Psychosocial Assessment
2.4.1. Neuropsychological Assessment
Executive Functioning
Sensory Processing
2.4.2. Psychosocial Assessment
Behavioral and Emotional Functioning
Health-Related Quality of Life
2.5. Indication for Further Extensive Neuropsychological Assessment (NPA)
2.6. Socio-Demographic Characteristics
2.7. Medical Characteristics
2.8. Statistical Analyses
- Presence (or absence) of an indication for further NPA;
- Executive functioning according to the BRIEF Global Executive Composite score of caregivers’, teachers’, and self-report;
- Behavioral and emotional functioning according to the SDQ total difficulties score of the caregivers’, teachers’, and self-report.
3. Results
3.1. Participant Characteristics
3.2. Executive Functioning
3.2.1. Caregiver Reports
3.2.2. Teacher Reports
3.2.3. Self-Reports
3.3. Sensory Processing
3.3.1. Caregiver Reports
3.3.2. Self-Reports
3.4. Behavioral and Emotional Functioning
3.4.1. Caregiver Reports
3.4.2. Teacher Reports
3.4.3. Self-Reports
3.5. Health-Related Quality of Life
3.5.1. Caregiver Reports
3.5.2. Self-Reports
3.6. Reasons for Further Neuropsychological Assessment (NPA)
- Caregiver-reported SSP auditory filtering subscale (31%);
- Caregiver-reported SDQ total difficulties score (30%);
- Caregiver-reported SDQ hyperactivity/inattention subscale (25%);
- Teacher-reported SDQ emotional problems subscale (25%);
- Caregiver-reported CHQ role/social limitations due to emotional and behavioral problems subscale (26%).
3.7. Associations of Socio-Demographic/Medical Characteristics with Neuropsychological and Psychosocial Functioning
3.8. Results of Siblings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Definition of Scores in the Clinical Range
| Questionnaire | Clinical Range | References |
| Strength and Difficulties Questionnaire (SDQ) | ||
| - SDQ caregiver report and teacher report for children 5–7 years | - Scores above the 90th percentile (p90) | Mieloo et al., 2012 |
| - SDQ caregiver report, teacher report and self-report for children 8–19 years | - Scores above the 90th percentile (p90) | Goedhart, Treffers, & Van Widenfelt, 2003 |
| Behavior Rating Inventory of Executive Function (BRIEF) | ||
| - BRIEF caregiver report, teacher report and self-report for children 5–18 years | >1.5 Standard Deviation (SD) above the mean score, adjusted for age and sex | Huizinga & Smidts, 2012 |
| Sensory Profile (SP) | ||
| - SP caregiver report (Short Sensory Profile) for children 5–12 years | >2SD difference from the mean score (with three different mean scores for children 4–5 years, 6–10 years, and 11–12 years) | Dunn & Rietman, 2013 |
| - Adolescent/Adult Sensory Profile (AASP) for self-completion for children 11–18 years | >2SD difference from the mean score | Brown et al., 2007 |
| Child Health Questionnaire | ||
| - Caregiver report (CHQ-PF50) for children 5–19 years | >1SD below the mean score | Raat, Bonsel et al., 2002 |
| - Self-report (CHQ-CF87) for children 11–19 years | >1SD below the mean score | Raat, Landgraf et al., 2002 |
Appendix B. Results BRIEF Self-Reports
| HIV-Infected ChildrenMean (SD) | Siblings Mean (SD) | Dutch Normative Mean (SD) [22] | p-Value 1 | |
| BRIEF self-report | n = 19 | n = 9 | n = 1687 | |
| Total (Global Executive Composite) Score | 50.1 (10.7) | 48.3 (13.1) | 50.0 (10.0) | 0.983 a 0.618 b 0.714 c |
| Behavioral Regulation Index | 48.4 (12.0) | 49.0 (14.0) | 50.0 (10.0) | 0.495 a 0.835 b 0.911 c |
| Metacognition index | 51.6 (9.3) | 48.8 (12.3) | 50.0 (10.0) | 0.480 a 0.715 b 0.500 c |
| Inhibit | 46.7 (10.8) | 51.6 (12.0) | 50.0 (10.0) | 0.158 a 0.641 b 0.298 c |
| Shift | 50.7 (10.0) | 49.9 (13.1) | 50.0 (10.0) | 0.748 a 0.974 b 0.851 c |
| Emotional Control | 48.7 (10.4) | 47.6 (9.4) | 50.0 (10.0) | 0.585 a 0.465 b 0.775 c |
| Working Memory | 52.1 (9.6) | 49.6 (9.6) | 50.0 (10.0) | 0.347 a 0.895 b 0.525 c |
| Plan/Organize | 51.2 (9.9) | 49.3 (8.6) | 50.0 (10.0) | 0.615 a 0.841 b 0.639 c |
| Organization of materials | 49.1 (7.3) | 46.2 (10.0) | 50.0 (10.0) | 0.680 a 0.258 b 0.402 c |
| Monitor | 51.0 (10.3) | 52.0 (10.4) | 50.0 (10.0) | 0.665 a 0.550 b 0.813 c |
| Task Completion | 52.4 (8.6) | 48.7 (11.6) | 50.0 (10.0) | 0.304 a 0.691 b 0.351 c |
| a = patient vs. Dutch normative data. b = sibling vs. Dutch normative data. c = patient vs. sibling. 1 = Independent-Samples t Test. Dutch normative data [22]. A higher score indicates more problems in executive functioning. | ||||
Appendix C. Results Sensory Profile Caregivers’ Report and Self-Report
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Data Mean (SD) [23] | p-Value | |
| SSP caregivers’ report | n = 32 | n = 19 | n = 1257 | |
| Tactile sensitivity | 31.0 (3.9) | 30.6 (4.6) | 33.0 (2.7) | −0.000 *,a,1 |
| 0.009 *,b,2 | ||||
| 0.769 c,3 | ||||
| Taste/Smell Sensitivity | 17.5 (3.5) | 18.5 (2.2) | 17.8 (2.9) | 0.526 a,1 |
| 0.185 b,2 | ||||
| 0.206 c,3 | ||||
| Movement Sensitivity | 13.4 (2.2) | 14.5 (1.2) | 13.7 (1.8) | 0.418 a,1 |
| 0.017 *,b,2 | ||||
| 0.045 *,c,3 | ||||
| Underresponsive/Seeks Sensation | 28.3 (4,0) | 26.4 (7.3) | 29.0 (4.2) | 0.360 a,1 |
| 0.009 *,b,1 | ||||
| 0.308 c,1 | ||||
| Auditory filtering | 20.9 (5.6) | 20.7 (5.6) | 23.9 (3.9) | 0.000 *,a,1 |
| 0.000 *,b,1 | ||||
| 0.902 c,1 | ||||
| Low energy/weak | 27.7 (4.3) | 29.3 (1.3) | 28.6 (2.4) | 0.046 a,1 |
| 0.025 b,2 | ||||
| 0.166 c,3 | ||||
| Visual/Auditory Sensitivity | 21.3 (3,6) | 19.9 (5.3) | 21.4 (2.7) | 0.759 a,1 |
| 0.628 b,2 | ||||
| −0.492 c,3 | ||||
| * = p < 0.05. a = patient vs. Dutch normative data. b = sibling vs. Dutch normative data. c = Patient vs. Sibling. 1 = Independent-Samples t Test. 2 = Wilcoxon Signed-Ranks Test. 3 = Mann–Whitney Test. Dutch normative data [23]. A lower score indicates that more problems were reported by caregivers/parents. | ||||
| HIV-Infected Children Mean(SD) | Siblings Mean (SD) | Normative Data Mean (SD) [27] | p-Value 1 | |
| AASP self-report | n = 19 | n = 9 | n = 33 | |
| Low Registration | 33.1 (9.0) | 31.1 (9.7) | 30.5 (6.4) | 0.233 a |
| 0.811 b | ||||
| 0.607 c | ||||
| Sensation seeking | 42.1 (8.7) | 41.8 (9.0) | 51.2 (6.8) | 0.000 *,a |
| 0.001 *,b | ||||
| 0.927 c | ||||
| Sensory Sensitivity | 32.9 (8.4) | 35.4 (12.6) | 34.3 (7.0) | 0.529 a |
| 0.715 b | ||||
| 0.592 c | ||||
| Sensation Avoidance | 31.4 (9.0) | 33.7 (10.9) | 30.2 (8.4) | 0.641 a |
| 0.309 b | ||||
| 0.559 c | ||||
| * = p < 0.05. a = patient vs. normative data. b = sibling vs. normative data. c = Patient vs. Sibling. 1 = Independent-Samples t Test. Belgian normative data [27]. A higher score indicates more problems were reported (self-report). | ||||
Appendix D. Results SDQ Teachers’ Report and Self-Report
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Data Mean (SD) [21] | p-Value | |
| SDQ teachers’ report | n = 25 | n = 12 | n = 208 | |
| Total score | 8.8 (6.0) | 6.3 (7.7) | 7.6 (6.6) | 0.387 a,1 0.116 b,2 0.104 c,3 |
| Emotional problems | 2.6 (2.4) | 1.3 (2.1) | 1.7 (2.0) | 0.209 a,2 0.109 b,2 0.073 c,3 |
| Conduct problems | 0.8 (1.1) | 0.5 (1.0) | 1.0 (1.8) | 0.268 a,2 0.109 b,2 0.412 c,3 |
| Hyperactivity/inattention | 4.0 (3.1) | 3.3 (3.4) | 2.8 (3.0) | 0.061 a,1 0.937 b,2 0.386 c,3 |
| Peer problems | 1.5 (1.5) | 1.3 (2.3) | 2.0 (2.2) | 0.111 a,2 0.067 b,2 0.325 c,3 |
| Prosocial | 7.3 (2.2) | 7.2 (2.6) | 8.1 (2.2) | 0.068 a,2 0.158 b,1 0.948 c,3 |
| a = patient vs. Dutch normative data. b = sibling vs. Dutch normative data. c = patient vs. sibling. 1 = Independent-Samples t Test. 2 = Wilcoxon Signed-Ranks Test. 3 = Mann–Whitney Test. Dutch normative data [21]. A higher score indicates more problems in in behavioral and emotional functioning, except for the prosocial scale where a lower score indicates more problems. | ||||
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Data Mean (SD) [21] | p-Value | |
| SDQ self-report | n = 19 | n = 9 | n = 1353 | |
| Total score | 9.3 (5.9) | 10.6 (6.5) | 9.9 (4.9) | 0.607 a,1 |
| 0.690 b,1 | ||||
| 0.621 c,1 | ||||
| Emotional problems | 2.6 (2.3) | 2.3 (1.7) | 2.2 (2.0) | 0.351 a,1 |
| 0.842 b,1 | ||||
| 0.733 c,1 | ||||
| Conduct problems | 1.7 (1.7) | 2.1 (2.0) | 2.0 (1.5) | 0.421 a,2 |
| 0.863 b,2 | ||||
| 0.464 c,3 | ||||
| Hyperactivity/inattention | 3.3 (2.3) | 4.3 (2.9) | 3.8 (2.3) | 0.362 a,1 |
| 0.489 b,1 | ||||
| 0.317 c,1 | ||||
| Peer problems | 1.7 (1.5) | 1.8 (2.3) | 1.9 (1.7) | 0.686 a,2 |
| 0.511 b,2 | ||||
| 0.724 c,3 | ||||
| Prosocial | 8.0 (1.7) | 7.9 (1.5) | 7.2 (2.3) | 0.082 a,2 |
| 0.370 b,1 | ||||
| 0.802 c,3 | ||||
| a = patient vs. Dutch normative data. b = sibling vs. Dutch normative data. c = Patient vs. sibling. 1 = Independent-Samples t Test. 2 = Wilcoxon Signed-Ranks Test. 3 = Mann–Whitney Test. Dutch normative data [21]. | ||||
Appendix E. Results CHQ Caregiver-Report and Self-Report
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Data Mean (SD) [25] | p-Value | |
| CHQ caregivers’ report | n = 40 | n = 23 | n = 353 | |
| General health | 66.8 (17.4) | 76.7 (19.8) | 82.9 (13.4) | 0.000 * a,1 0.426 b,2 0.002 * c,3 |
| Physical functioning | 96.3 (13.3) | 97.0 (8.0) | 99.1 (4.3) | 0.004 * a,1 0.228 b,1 0.693 c,1 |
| Role/social limitations- emotional behavior | 88.6 (22.1) | 87.9 (18.8) | 97.9 (7.2) | 0.000 * a,1 0.021 * b,1 0.896 c,1 |
| Role/social limitations- physical | 97.1 (9.2) | 97.7 (7.8) | 95.8 (15.6) | 0.610 a,1 0.307 b,1 0.781 c,1 |
| * = p < 0.05. a = patient vs. Dutch normative data. b = sibling vs. Dutch normative data. c = patient vs. sibling. 1 = Independent-Samples t Test. 2 = Wilcoxon Signed-Ranks Test. 3 = Mann–Whitney Test. Dutch normative data [25]. A lower score indicates more problems. | ||||
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Data Mean (SD) [26] | p-Value | |
| CHQ self-report | n = 19 | n = 9 | n = 444 | |
| General health | 68.9 (17.0) | 83.5 (14.6) | 74.6 (15.9) | 0.127 a,1 |
| 0.096 b,1 | ||||
| 0.035 *,c,1 | ||||
| Physical functioning | 93.8 (8.5) | 90.5 (23.0) | 96.8 (5.4) | 0.219 a,2 |
| 0.763 b,2 | ||||
| 0.649 c,3 | ||||
| Role/social limitations-emotional | 84.2 (25.2) | 87.7 (23.2) | 92.3 (16.8) | 0.836 a,2 |
| 0.582 *,b,2 | ||||
| 0. 774 c,3 | ||||
| Role/social limitations-behavior | 94.2 (15.0) | 84.0 (27.3) | 91.4 (13.7) | 0.076 a,2 |
| 0.854 b,2 | ||||
| 0.258 c,3 | ||||
| Role/social limitations-physical | 96.5 (10.5) | 92.6 (12.4) | 96.5 (11.6) | 0.076 a,2 |
| 0.854 b,2 | ||||
| 0.288 c,3 | ||||
| * = p < 0.05. a = patient vs. Dutch normative data. b = sibling vs. Dutch normative data. c = patient vs. sibling. 1 = Independent-Samples t Test. 2 = Wilcoxon Signed-Ranks Test. 3 = Mann–Whitney Test. Dutch normative data [26]. A lower score indicates more problems. | ||||
References
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Sherr, L.; Croome, N.; Castaneda, K.P.; Bradshaw, K.; Romero, R.H. Developmental challenges in HIV infected children—An updated systematic review. Child. Youth Serv. Rev. 2014, 45, 74–89. [Google Scholar] [CrossRef]
- Kerr, S.J.; Puthanakit, T.; Malee, K.M.; Thongpibul, K.; Ly, P.S.; Sophonphan, J.; Suwanlerk, T.; Kosalaraksa, P.; Ounchanum, P.; Aurpibul, L.; et al. Increased Risk of Executive Function and Emotional Behavioral Problems Among Virologically Well-Controlled Perinatally HIV-Infected Adolescents in Thailand and Cambodia. JAIDS J. Acquir. Immune Defic. Syndr. 2019, 82, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Ming, X.; Williams, P.L.; Robertson, K.R.; Oleske, J.M.; Seage, G.R., III; The International Maternal Pediatric Adolescent AIDS Clinical Trials. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS 2009, 23, 1893. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, S.; de Sonneville, L.; Wolfs, T.F.; Licht, R.; Geelen, S.P. Neurocognitive function profile in HIV-infected school-age children. Eur. J. Paediatr. Neurol. 2008, 12, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Pajkrt, D.; Blokhuis, C.; Caan, M.W.; Kootstra, N.A. Neurodevelopmental delay in pediatric HIV/AIDS: Current perspectives. Neurobehav. HIV Med. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Sherr, L.; Mueller, J.; Varrall, R. A systematic review of cognitive development and child human immunodeficiency virus infection. Psychol. Health Med. 2009, 14, 387–404. [Google Scholar] [CrossRef]
- Ro, E.; Clark, L.A. Psychosocial functioning in the context of diagnosis: Assessment and theoretical issues. Psychol. Assess. 2009, 21, 313–324. [Google Scholar] [CrossRef]
- Mellins, C.A.; Malee, K.M. Understanding the mental health of youth living with perinatal HIV infection: Lessons learned and current challenges. J. Int. AIDS Soc. 2013, 16, 18593. [Google Scholar] [CrossRef]
- Melvin, D.; Krechevsky, D.; Divac, A.; Tacconelli, E.; Miah, J.; Giannakopoulou, C.; Waugh, S.; Hekster, B.; Byard, K. Parental reports of emotional and behavioural difficulties on the SDQ for school-age children with vertically acquired HIV infection living in London. Psychol. Health Med. 2007, 12, 40–47. [Google Scholar] [CrossRef]
- Laughton, B.; Cornell, M.; Boivin, M.; Van Rie, A. Neurodevelopment in perinatally HIV-infected children: A concern for adolescence. J. Int. AIDS Soc. 2013, 16, 18603. [Google Scholar] [CrossRef]
- Cohen, S.; Ter Stege, J.A.; Weijsenfeld, A.M.; Van Der Plas, A.; Kuijpers, T.W.; Reiss, P.; Scherpbier, H.J.; Haverman, L.; Pajkrt, D. Health-related quality of life in perinatally HIV-infected children in the Netherlands. AIDS Care 2015, 27, 1–10. [Google Scholar] [CrossRef]
- van Opstal, S.E.M.; Wagener, M.N.; Miedema, H.S.; Utens, E.M.W.J.; Aarsen, F.K.; van der Knaap, L.C.; van Gorp, E.C.M.; van Rossum, A.M.C.; Roelofs, P.D.D.M. School functioning of children with perinatal HIV-infection in high-income countries: A systematic review. PLoS ONE 2021, 16, e0252746. [Google Scholar] [CrossRef]
- Rehm, R.S.; Franck, L.S. Long-Term Goals and Normalization Strategies of Children and Families Affected by HIV/AIDS. Adv. Nurs. Sci. 2000, 23, 69–82. [Google Scholar] [CrossRef]
- Nozyce, M.L.; Lee, S.S.; Wiznia, A.; Nachman, S.; Mofenson, L.M.; Smith, M.E.; Yogev, R.; McIntosh, K.; Stanley, K.; Pelton, S. A Behavioral and Cognitive Profile of Clinically Stable HIV-Infected Children. Pediatrics 2006, 117, 763–770. [Google Scholar] [CrossRef]
- Jeremy, R.J.; Kim, S.; Nozyce, M.; Nachman, S.; McIntosh, K.; Pelton, S.I.; Yogev, R.; Wiznia, A.; Johnson, G.M.; Krogstad, P.; et al. Neuropsychological Functioning and Viral Load in Stable Antiretroviral Therapy-Experienced HIV-Infected Children. Pediatrics 2005, 115, 380–387. [Google Scholar] [CrossRef]
- Kullgren, K.A.; Morris, M.K.; Bachanas, P.J.; Jones, J.S. Prediction of Cognitive, Adaptive, and Behavioral Functioning in Preschool and School-Age Children with HIV. Child. Health Care 2004, 33, 241–256. [Google Scholar] [CrossRef]
- Medin, G.; García-Navarro, C.; Gomez, M.N.; Amador, J.T.R.; Mellado, M.J.; Jimenez, S.; Muñoz-Fernández, M.Á.; Conejo, P.R.; Saavedra, J.; Hortelano, M.G.; et al. Disease disclosure, treatment adherence, and behavioural profile in a cohort of vertically acquired HIV-infected adolescents. NeuroCoRISpeS study. AIDS Care 2016, 28, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bomba, M.; Nacinovich, R.; Oggiano, S.; Cassani, M.; Baushi, L.; Bertulli, C.; Longhi, D.; Coppini, S.; Parrinello, G.; Plebani, A.; et al. Poor health-related quality of life and abnormal psychosocial adjustment in Italian children with perinatal HIV infection receiving highly active antiretroviral treatment. AIDS Care 2010, 22, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Mieloo, C.; Raat, H.; Van Oort, F.; Bevaart, F.; Vogel, I.; Donker, M.; Jansen, W. Validity and Reliability of the Strengths and Difficulties Questionnaire in 5–6 Year Olds: Differences by Gender or by Parental Education? PLoS ONE 2012, 7, e36805. [Google Scholar] [CrossRef]
- Goedhart, A.; Treffers, P.D.; Van Widenfelt, B.J.M. Vragen naar psychische problemen bij kinderen en adolescenten. De Strengths and Difficulties Questionnaire (SDQ). Maandbl. Geest. Volksgezond. 2003, 58, 1018–1035. [Google Scholar]
- Huizinga, M.; Smidts, D. BRIEF Vragenlijst Executieve Functies Voor 5-tot 18-Jarigen: Handleiding (User’s Manual), 3rd ed.; Hogrefe Uitgevers: Amsterdam, The Nederland, 2012. [Google Scholar]
- Dunn, W.; Rietman, A. Sensory Profile-NL: Handleiding (Manual), 1st ed.; Pearson Assessment and Information BV: Amsterdam, The Nederland, 2013. [Google Scholar]
- Brown, C.E.; Dunn, W.; Rietman, A. Adolescent/Adult Sensory Profile-NL Handleiding (User’s Manual); Dutch Translation; NCS Pearson, Inc.: San Antonio, TX, USA, 2007. [Google Scholar]
- Raat, H.; Bonsel, G.J.; Essink-Bot, M.-L.; Landgraf, J.M.; Gemke, R.J. Reliability and validity of comprehensive health status measures in children: The Child Health Questionnaire in relation to the Health Utilities Index. J. Clin. Epidemiol. 2002, 55, 67–76. [Google Scholar] [CrossRef]
- Raat, H.; Landgraf, J.; Bonsel, G.; Gemke, R.J.B.J.; Essink-Bot, M.L. Reliability and validity of the child health question-naire-child form (CHQ-CF87) in a Dutch adolescent population. Qual. Life Res. 2002, 11, 575–581. [Google Scholar] [CrossRef] [PubMed]
- De la Marche, W.; Steyaert, J.; Noens, I. Atypical sensory processing in adolescents with an autism spectrum disorder and their non-affected siblings. Res. Autism Spectr. Disord. 2012, 6, 639–645. [Google Scholar] [CrossRef]
- Dunn, W. The Sensations of Everyday Life: Empirical, Theoretical, and Pragmatic Considerations. Am. J. Occup. Ther. 2001, 55, 608–620. [Google Scholar] [CrossRef]
- Brown, C.; Dunn, W. Adolescent/Adult Sensory Profile; Pearson San Antonio: San Antonio, TX, USA, 2002. [Google Scholar]
- McIntosh, D.N.; Miller, L.J.; Shyu, V.; Dunn, W. Development and validation of the short sensory profile. In The Sensory Profile: Examiner’s Manual; Dunn, W., Ed.; Psychological Corporation: San Antonio, TX, USA, 1999; pp. 59–73. [Google Scholar]
- Goodman, R. Psychometric Properties of the Strengths and Difficulties Questionnaire. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 1337–1345. [Google Scholar] [CrossRef]
- Vogels, A.; Siebelink, B.; Theunissen, M.; de Wolff, M.; Reijneveld, S. Vergelijking van de KIVPA en de SDQ als Signaleringsinstrument Voor Problemen bij Adolescenten in de Jeugdgezondheidszorg; TNO: Leiden, The Nederland, 2001. [Google Scholar]
- Van Widenfelt, B.M.; Goedhart, A.W.; Treffers, P.D.; Goodman, R. Dutch version of the Strengths and Difficulties Question-naire (SDQ). Eur. Child Adolesc. Psychiatry 2003, 12, 281–289. [Google Scholar] [CrossRef]
- Landgraf, J.; Abetz, L.; Ware, J. The CHQ User’s Manual; The Health Institute, New England Medical Center: Boston, MA, USA, 1996. [Google Scholar]
- HealthActCHQ. Child Health Questionnaire Scoring and Interpretation Manual; HealthActCHQ: Cambridge, MA, USA, 2008. [Google Scholar]
- Centraal Bureau voor de Statistiek (CBS). Netherlands Central Bureau of Statistics. Dutch Standard Classification of Occupations 1992: Edition 2010; Statistics Netherlands: Voorburg/Heerlen, The Nederland, 2010. [Google Scholar]
- Centers for Disease Control (CDC). Revision of the CDC surveillance case definition for acquired immunodeficiency syn-drome. J. Am. Med. Assoc. 1987, 258, 1143–1154. [Google Scholar] [CrossRef]
- Phillips, N.; Amos, T.; Kuo, C.; Hoare, J.; Ipser, J.; Thomas, K.G.; Stein, D.J. HIV-Associated Cognitive Impairment in Peri-natally Infected Children: A Meta-analysis. Pediatrics 2016, 138, e20160893. [Google Scholar] [CrossRef]
- Rowe, K.; Buivydaite, R.; Heinsohn, T.; Rahimzadeh, M.; Wagner, R.G.; Scerif, G.; Stein, A. Executive function in HIV-affected children and adolescents: A systematic review and meta-analyses. AIDS Care 2021, 33, 833–857. [Google Scholar] [CrossRef]
- Cohen, S.; Caan, M.W.; Mutsaerts, H.-J.; Scherpbier, H.J.; Kuijpers, T.W.; Reiss, P.; Pajkrt, D. Cerebral injury in perinatal-ly HIV-infected children compared to matched healthy controls. Neurology 2016, 86, 19–27. [Google Scholar] [CrossRef]
- Hoare, J.; Myer, L.; Heany, S.; Fouche, J.-P.; Phillips, N.J.; Zar, H.; Stein, D. Cognition, Structural Brain Changes, and Systemic Inflammation in Adolescents Living with HIV on Antiretroviral Therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 84, 114–121. [Google Scholar] [CrossRef]
- Du Plessis, S.; Vink, M.; Joska, J.A.; Koutsilieri, E.; Stein, D.J.; Emsley, R. HIV infection and the fronto–striatal system: A systematic review and meta-analysis of fMRI studies. AIDS 2014, 28, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Malee, K.M.; Chernoff, M.C.; Sirois, P.A.; Williams, P.L.; Garvie, P.A.; Kammerer, B.L.; Harris, L.L.; Nozyce, M.L.; Yildirim, C.; Nichols, S.L.; et al. Impact of Perinatally Acquired HIV Disease Upon Longitudinal Changes in Memory and Executive Functioning. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Lentoor, A.G. Clinico-Immunological Status and Neurocognitive Function of Perinatally Acquired HIV-Positive Children on cART: A Cross-Sectional Correlational Study in South Africa. Front. Neurol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Sirois, P.A.; Chernoff, M.C.; Malee, K.M.; Garvie, P.A.; Harris, L.L.; Williams, P.L.; Yildirim, C. Associations of memory and executive functioning with academic and adaptive functioning among youth with perinatal HIV exposure and/or infec-tion. J. Pediatr. Infect. Dis. Soc. 2016, 5 (Suppl. 1), S24–S32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomaidis, L.; Bertou, G.; Critselis, E.; Spoulou, V.; Kafetzis, D.A.; Theodoridou, M. Cognitive and psychosocial develop-ment of HIV pediatric patients receiving highly active anti-retroviral therapy: A case-control study. BMC Pediatr. 2010, 10, 99. [Google Scholar] [CrossRef]
- Diamantopoulou, S.; Rydell, A.-M.; Thorell, L.B.; Bohlin, G. Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Dev. Neuropsychol. 2007, 32, 521–542. [Google Scholar] [CrossRef]
- Bunford, N.; Brandt, N.E.; Golden, C.; Dykstra, J.B.; Suhr, J.A.; Owens, J.S. Attention-Deficit/Hyperactivity Disorder Symptoms Mediate the Association between Deficits in Executive Functioning and Social Impairment in Children. J. Abnorm. Child Psychol. 2014, 43, 133–147. [Google Scholar] [CrossRef]
- Butler, L. Social Cognition and HIV: Exploring the Profile of Cognitive Impairments in HIV-Associated Neurocognitive Disorders (HAND). Ph.D. Thesis, University of East London, London, UK, 2016. [Google Scholar]
- Ownsworth, T.L.; McFarland, K.; Young, R.M. The investigation of factors underlying deficits in self-awareness and self-regulation. Brain Inj. 2002, 16, 291–309. [Google Scholar] [CrossRef]
- McQuade, J.D.; Mendoza, S.A.; Larsen, K.L.; Breaux, R.P. The Nature of Social Positive Illusory Bias: Reflection of Social Impairment, Self-Protective Motivation, or Poor Executive Functioning? J. Abnorm. Child Psychol. 2017, 45, 289–300. [Google Scholar] [CrossRef]
- Owens, J.S.; Goldfine, M.E.; Evangelista, N.M.; Hoza, B.; Kaiser, N.M. A Critical Review of Self-perceptions and the Positive Illusory Bias in Children with ADHD. Clin. Child Fam. Psychol. Rev. 2007, 10, 335–351. [Google Scholar] [CrossRef]
- Chi, P.; Li, X. Impact of Parental HIV/AIDS on Children’s Psychological Well-Being: A Systematic Review of Global Literature. AIDS Behav. 2013, 17, 2554–2574. [Google Scholar] [CrossRef]
- Jacobs, E.; Miller, L.C.; Tirella, L.G. Developmental and Behavioral Performance of Internationally Adopted Preschoolers: A Pilot Study. Child Psychiatry Hum. Dev. 2009, 41, 15–29. [Google Scholar] [CrossRef]
- Bradley, R.H.; Corwyn, R. Socioeconomic Status and Child Development. Annu. Rev. Psychol. 2002, 53, 371–399. [Google Scholar] [CrossRef] [PubMed]
- Reiss, F. Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Soc. Sci. Med. 2013, 90, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.E.; Ford, T.; Williams, R.; Russell, G. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): A systematic review. Child Psychiatry Hum. Dev. 2016, 47, 440–458. [Google Scholar] [CrossRef]
- Rabiner, D.L.; Godwin, J.; Dodge, K.A. Predicting academic achievement and attainment: The contribution of early academic skills, attention difficulties, and social competence. School Psychol. Rev. 2016, 45, 250–267. [Google Scholar] [CrossRef]
- Sprangers, M.A.; Schwartz, C.E. Integrating response shift into health-related quality of life research: A theoretical model. Soc. Sci. Med. 1999, 48, 1507–1515. [Google Scholar] [CrossRef]
- van Zellem, L.; Buysse, C.; Madderom, M.; Legerstee, J.S.; Aarsen, F.; Tibboel, D.; Utens, E.M. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015, 41, 1057–1066. [Google Scholar] [CrossRef]
- Pinquart, M. Do the Parent–Child Relationship and Parenting Behaviors Differ Between Families with a Child with and without Chronic Illness? A Meta-Analysis. J. Pediatr. Psychol. 2013, 38, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, C.M.; Clawson, A.H.; Mullins, L.L.; Brinkman, T.M.; Pui, C.; Hudson, M.M.; Krull, K.R. The relationship of child executive functions to parenting capacities in childhood acute lymphoblastic leukemia survivors. Pediatr. Blood Cancer 2019, 66, e27761. [Google Scholar] [CrossRef] [PubMed]
- Aarsen, F.K.; Paquier, P.; Reddingius, R.E.; Streng, I.C.; Arts, W.F.M.; Evera-Preesman, M.; Catsman-Berrevoets, C.E. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer 2005, 106, 396–402. [Google Scholar] [CrossRef] [PubMed]
| Variable | HIV-Infected Children (n = 43) | Siblings (n = 24) | p-Value |
|---|---|---|---|
| Sex child | 0.45 1 | ||
| Male | 46.5% | 50.0% | |
| Mean age child in years at inclusion | 9.9 | 9.8 | 0.98 2 |
| Ethnicity | 0.18 1 | ||
| African | 46.5% | 66.7% | |
| Mixed | 37.2% | 16.7% | |
| Other | 16.3% | 16.7% | |
| Home environment | 0.89 1 | ||
| Living with at least one biological parent | 51.2% | 50.0% | |
| Adopted | 37.2% | 41.7% | |
| Other | 11.6% | 8.3% | |
| Born in the Netherlands | 46.5% | 45.8% | 0.96 1 |
| SES | 0.65 1 | ||
| Low | 44.2% | 37.5% | |
| Middle | 25.6% | 37.5% | |
| High | 30.2% | 25.0% | |
| CDC-nadir | |||
| N | 18.6% | ||
| A | 25.6% | ||
| B | 18.6% | ||
| C | 37.2% |
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Mean (SD) [22] | p-Value 1 | |
|---|---|---|---|---|
| BRIEF caregivers’ report | n = 40 | n = 23 | n = 3333 | |
| Global Executive Composite Score | 46.0 (10.4) | 51.3 (9.3) | 50.0 (10.0) | 0.012 * a 0.534 b 0.054 c |
| Behavioral Regulation Index | 45.1 (11.3) | 53.4 (9.2) | 50.0 (10.0) | 0.002 * a 0.105 b 0.005 * c |
| Metacognition index | 46.9 (9.8) | 49.8 (9.3) | 50.0 (10.0) | 0.054 a 0.935 b 0.255 c |
| Inhibit | 46.4 (10.9) | 51.5 (10.3) | 50.0 (10.0) | 0.024 * a 0.480 b 0.077 c |
| Shift | 46.9 (11.5) | 54.1 (10.3) | 50.0 (10.0) | 0.054 a 0.049 * b 0.017 * c |
| Emotional control | 45.1 (9.9) | 52.8 (8.1) | 50.0 (10.0) | 0.002 * a 0.184 b 0.002 * c |
| Initiate | 47.2 (9.0) | 48.4 (9.0) | 50.0 (10.0) | 0.076 a 0.441 b 0.579 c |
| Working Memory | 51.0 (9.6) | 51.6 (8.3) | 50.0 (10.0) | 0.521 a 0.441 b 0.794 c |
| Plan/Organize | 47.4 (9.1) | 49.8 (10.1) | 50.0 (10.0) | 0.099 a 0.935 b 0.373 c |
| Organization of materials | 46.3 (9.4) | 48.4 (9.7) | 50.0 (10.0) | 0.021 * a 0.453 b 0.388 c |
| Monitor | 44.4 (10.1) | 49.8 (9.2) | 50.0 (10.0) | 0.000 * a 0.935 b 0.047 * c |
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Mean (SD) [22] | p-Value | |
|---|---|---|---|---|
| BRIEF teachers’ report | n = 25 | n = 12 | n = 941 | |
| Global Executive Composite Score | 54.0 (6.5) | 49.1 (8.0) | 50.0 (10.0) | 0.049 * a,1 0.751 b,1 0.054 c,1 |
| Behavioral Regulation Index | 52.9 (7.1) | 50.6 (9.3) | 50.0 (10.0) | 0.097 a,2 0.842 b,1 0.435 c,3 |
| Metacognition Index | 54.8 (8.2) | 47.8 (7.4) | 50.0 (10.0) | 0.018 * a,1 0.454 b,1 0.017 * c,1 |
| Inhibit | 50.9 (7.5) | 52.7 (8.9) | 50.0 (10.0) | 0.662 a,1 0.358 b,1 0.526 c,1 |
| Shift | 54.8 (8.6) | 49.7 (7.9) | 50.0 (10.0) | 0.018 * a,1 0.909 b,1 0.074 c,3 |
| Emotional control | 52.0 (8.8) | 50.6 (8.7) | 50.0 (10.0) | 0.210 a,2 0.842 b,1 0.672 c,3 |
| Initiate | 54.9 (10.4) | 47.8 (8.1) | 50.0 (10.0) | 0.016 * a,1 0.454 b,1 0.046* c,1 |
| Working Memory | 54.7 (8.2) | 49.0 (8.0) | 50.0 (10.0) | 0.021 * a,1 0.730 b,1 0.055 c,1 |
| Plan/Organize | 55.5 (8.7) | 48.4 (7.9) | 50.0 (10.0) | 0.007 * a,1 0.586 b,1 0.024 * c,1 |
| Organization of materials | 51.5 (7.3) | 49.5 (7.1) | 50.0 (10.0) | 0.418 a,2 0.863 b,1 0.397 c,3 |
| Monitor | 50.8 (9.7) | 47.4 (6.5) | 50.0 (10.0) | 0.678 a,1 0.373 b,1 0.275 c,1 |
| HIV-Infected Children Mean (SD) | Siblings Mean (SD) | Dutch Normative Data [21] Mean (SD) | p-Value | |
|---|---|---|---|---|
| SDQ caregivers’ report | n = 40 | n = 23 | n = 300 | |
| Total difficulties score | 10.3 (5.3) | 11.3 (6.3) | 6.7 (5.3) | 0.000 * a,1 0.000 * b,1 0.485 c,1 |
| Emotional problems | 2.5 (2.0) | 3.1 (2.4) | 1.8 (1.9) | 0.025 * a,1 0.002 * b,1 0.287 c,1 |
| Conduct problems | 1.4 (1.7) | 2.0 (1.6) | 1.0 (1.4) | 0.121 a,1 0.005 * b,2 0.044 * c,3 |
| Hyperactivity/inattention | 4.5 (2.6) | 5.0 (3.0) | 2.7 (2.7) | 0.000 * a,1 0.000 * b,1 0.451 c,1 |
| Peer problems | 2.0 (1.6) | 1.3 (1.7) | 1.1 (1.6) | 0.001 * a,1 0.243 b,2 0.047 * c,3 |
| Prosocial | 8.3 (2.5) | 8.4 (1.8) | 8.5 (1.5) | 0.469 a,1 0.913 b,2 0.788 c,3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Opstal, S.E.M.; Dogterom, E.J.; Wagener, M.N.; Aarsen, F.K.; Miedema, H.S.; Roelofs, P.D.D.M.; van der Knaap, L.C.; Fraaij, P.L.A.; Stol, K.; Rietman, A.B.; et al. Neuropsychological and Psychosocial Functioning of Children with Perinatal HIV-Infection in The Netherlands. Viruses 2021, 13, 1947. https://doi.org/10.3390/v13101947
van Opstal SEM, Dogterom EJ, Wagener MN, Aarsen FK, Miedema HS, Roelofs PDDM, van der Knaap LC, Fraaij PLA, Stol K, Rietman AB, et al. Neuropsychological and Psychosocial Functioning of Children with Perinatal HIV-Infection in The Netherlands. Viruses. 2021; 13(10):1947. https://doi.org/10.3390/v13101947
Chicago/Turabian Stylevan Opstal, Stefanie E. M., Emma J. Dogterom, Marlies N. Wagener, Femke K. Aarsen, Harald S. Miedema, Pepijn D. D. M. Roelofs, Linda C. van der Knaap, Pieter L. A. Fraaij, Kim Stol, André B. Rietman, and et al. 2021. "Neuropsychological and Psychosocial Functioning of Children with Perinatal HIV-Infection in The Netherlands" Viruses 13, no. 10: 1947. https://doi.org/10.3390/v13101947
APA Stylevan Opstal, S. E. M., Dogterom, E. J., Wagener, M. N., Aarsen, F. K., Miedema, H. S., Roelofs, P. D. D. M., van der Knaap, L. C., Fraaij, P. L. A., Stol, K., Rietman, A. B., van Gorp, E. C. M., van Rossum, A. M. C., & Utens, E. M. W. J. (2021). Neuropsychological and Psychosocial Functioning of Children with Perinatal HIV-Infection in The Netherlands. Viruses, 13(10), 1947. https://doi.org/10.3390/v13101947






