Role of Environmental Photocatalysts and Organic Matter on the Degradation and Toxicity of Metformin Hydrochloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Particles
2.3. Batch Reactor Studies
2.4. Toxicological Assay
3. Results and Discussion
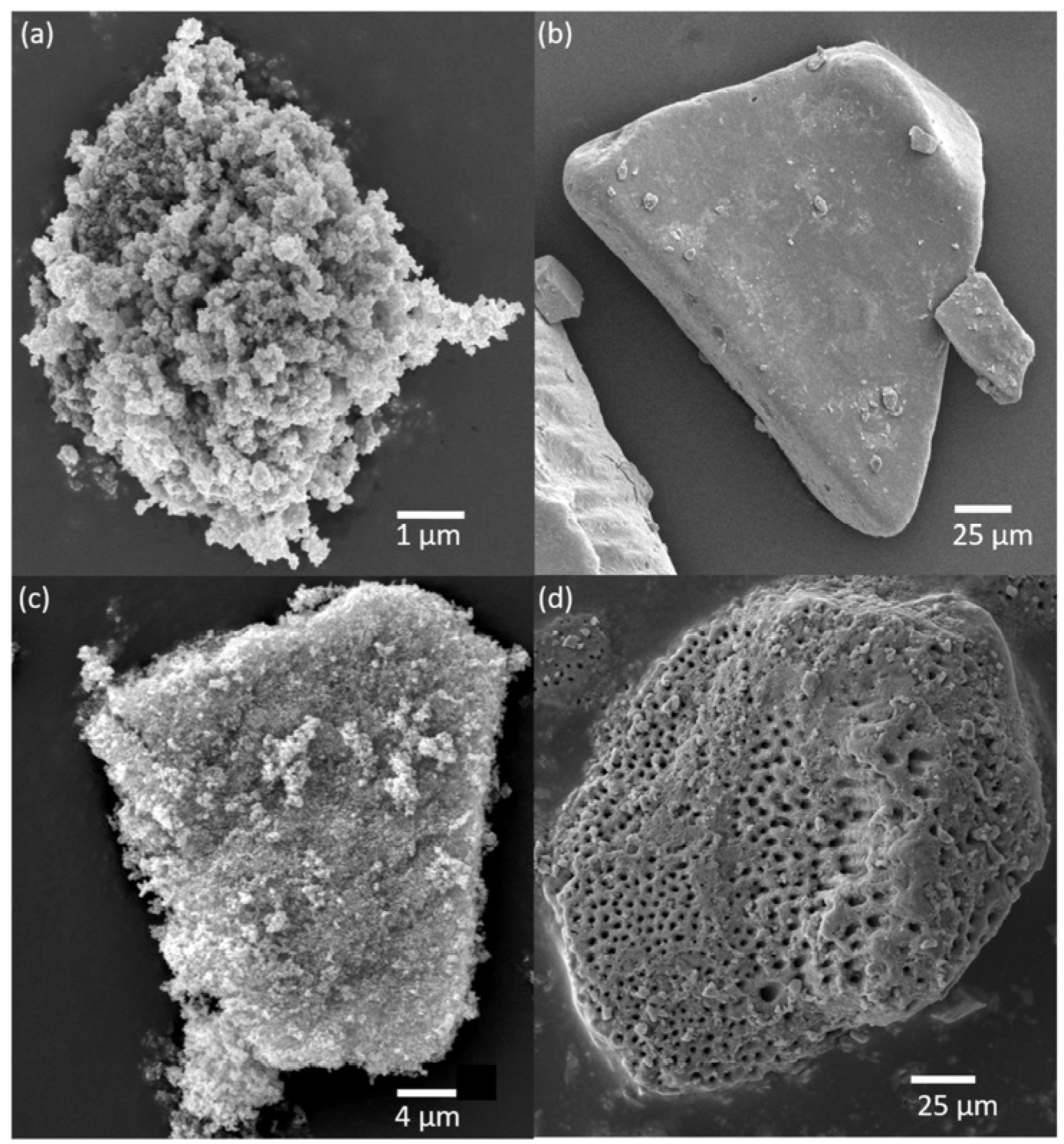
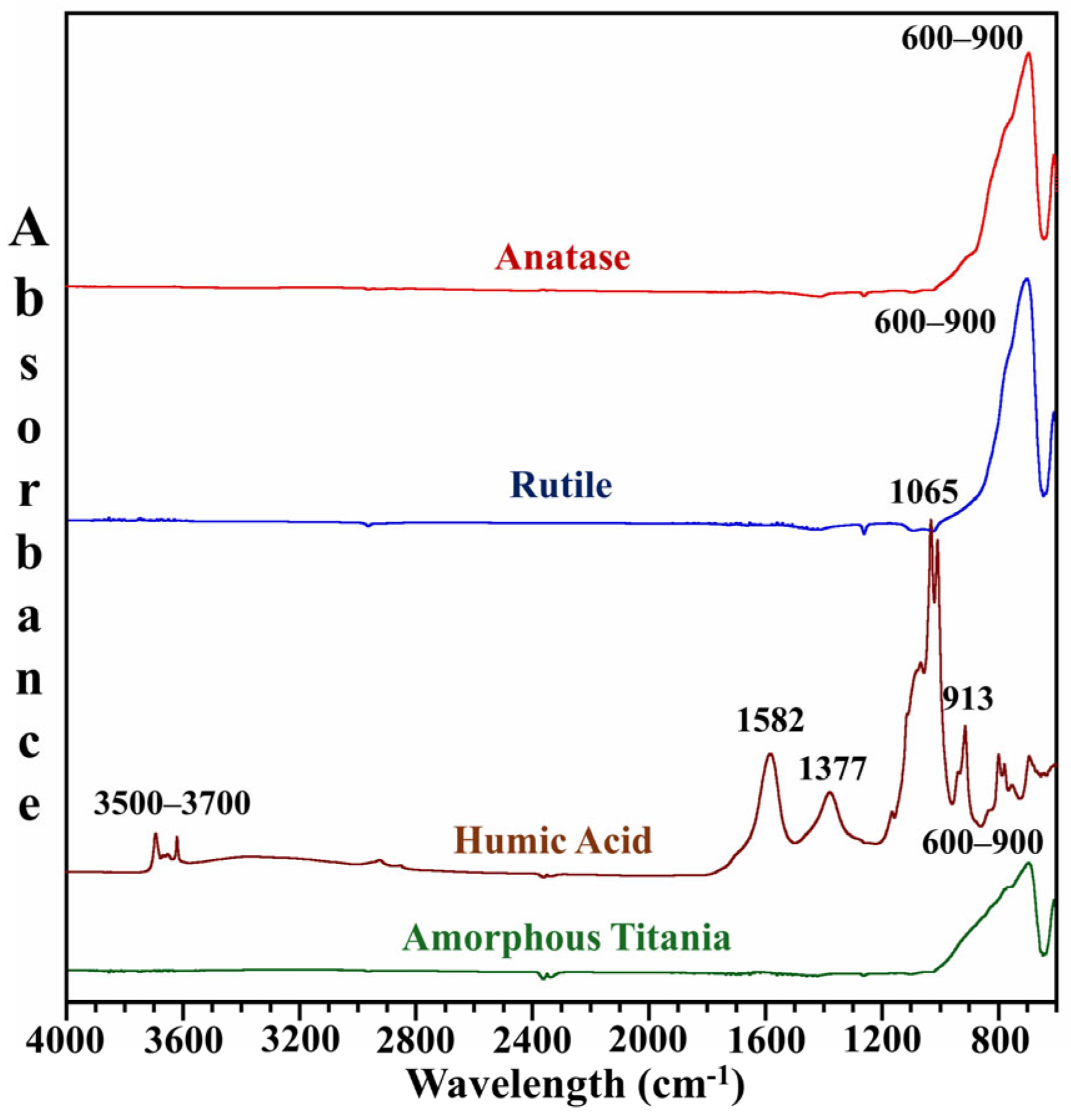
3.1. Characterization of Mineral Particles
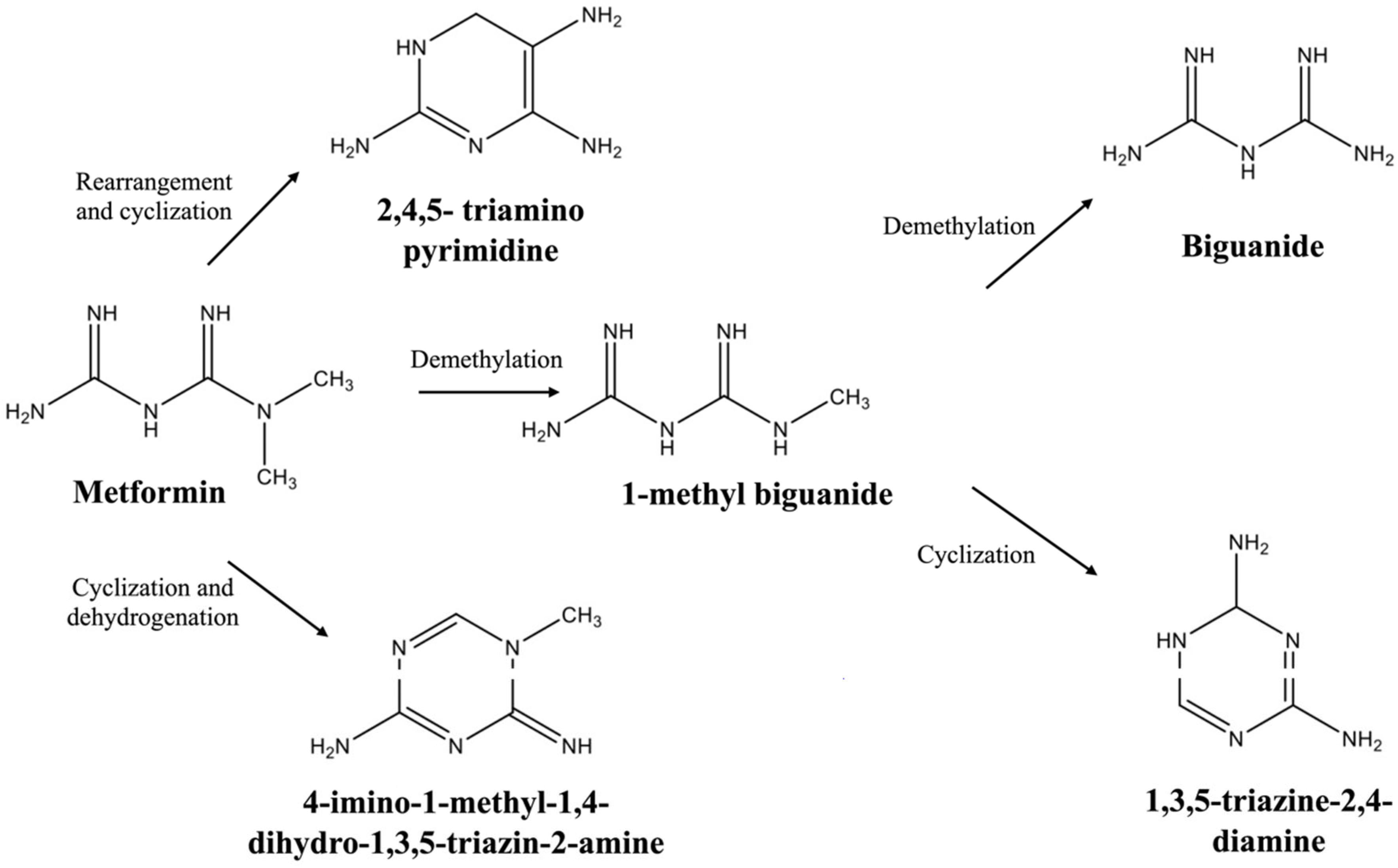
3.2. Degradation Studies
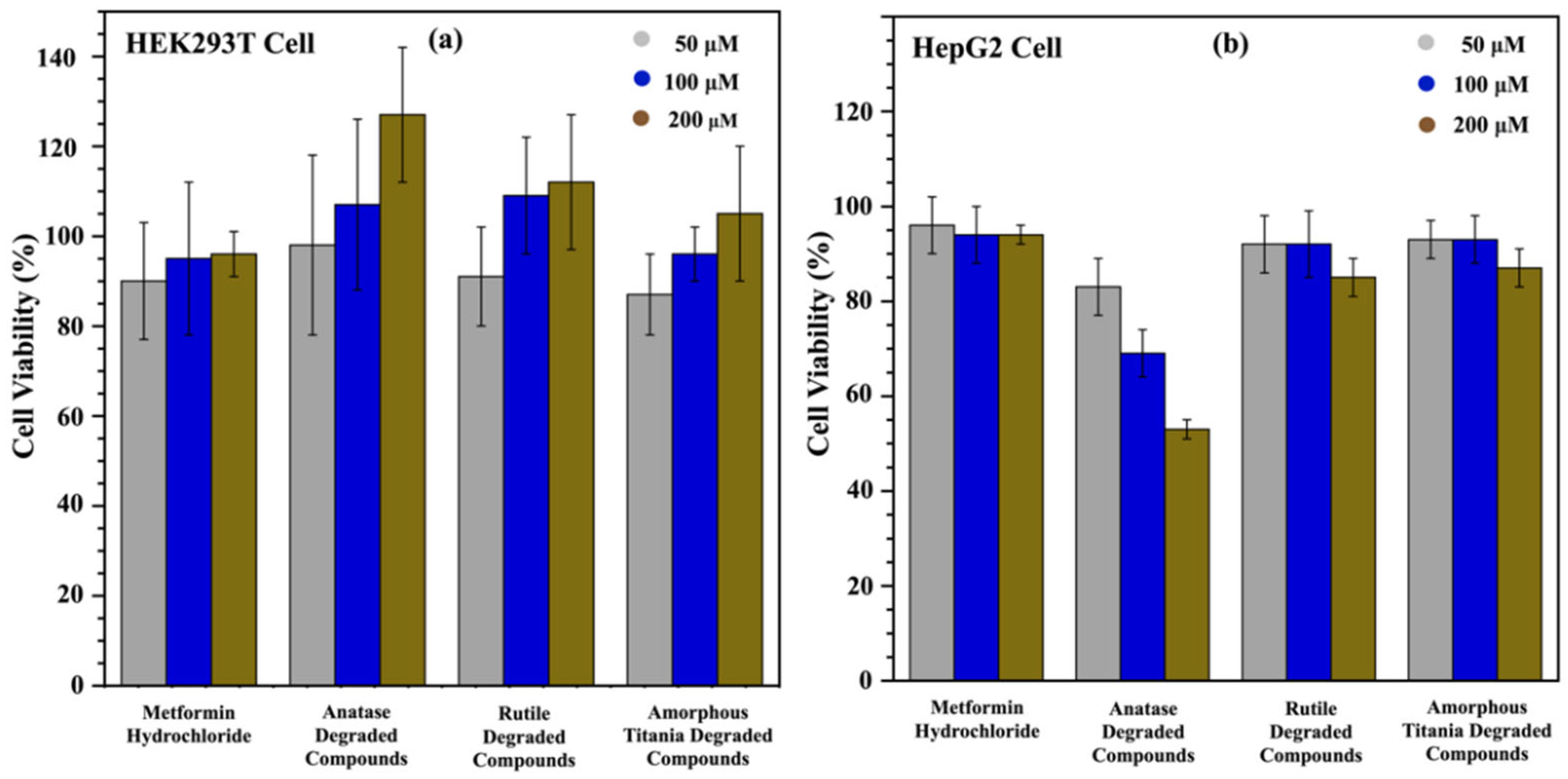
3.3. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Arias Espana, V.A.; Liu, Y.; Jit, J. Emerging Contaminants in the Environment: Risk-Based Analysis for Better Management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.; Shmerling, M. Is Metformin a Wonder Drug? Harvard Health Publishing: Cambridge, MA, USA, 2024. [Google Scholar]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. J. Res. Med. Sci. 2014, 19, 658–664. [Google Scholar] [PubMed]
- Yang, C.; Liu, K.; Yang, S.; Zhu, W.; Tong, L.; Shi, J.; Wang, Y. Prediction of Metformin Adsorption on Subsurface Sediments Based on Quantitative Experiment and Artificial Neural Network Modeling. Sci. Total Environ. 2023, 899, 165666. [Google Scholar] [CrossRef]
- Meštrović, T. Metformin Chemistry. News-Medical.Net. Available online: https://www.news-medical.net/health/Metformin-Chemistry.aspx (accessed on 10 May 2024).
- Judeh, A.A.; Sarief, A.; Umar, Y.; Ashwaq, O.; Haque, S.M.; Judeh, A.A.; Sarief, A.; Umar, Y.; Ashwaq, O.; Haque, S.M. Development, computational studies and validation of spectrophotometric method of metformin hydrochloride in pharmaceutical formulations. J. Chil. Chem. Soc. 2020, 65, 4895–4899. [Google Scholar] [CrossRef]
- Sinn, M.; Riede, L.; Fleming, J.R.; Funck, D.; Lutz, H.; Bachmann, A.; Mayans, O.; Hartig, J.S. Metformin Hydrolase Is a Recently Evolved Nickel-Dependent Heteromeric Ureohydrolase. Nat. Commun. 2024, 15, 8045. [Google Scholar] [CrossRef]
- Niemuth, N.J.; Jordan, R.; Crago, J.; Blanksma, C.; Johnson, R.; Klaper, R.D. Metformin Exposure at Environmentally Relevant Concentrations Causes Potential Endocrine Disruption in Adult Male Fish. Environ. Toxicol. Chem. 2015, 34, 291–296. [Google Scholar] [CrossRef]
- Fu, G.L.; Meng, Q.Y.; Chen, Y.; Xin, J.Z.; Liu, J.H.; Dang, W.; Lu, H.L. Metformin Exposure Altered Intestinal Microbiota Composition and Metabolites in Amphibian Larvae. Ecotoxicol. Environ. Saf. 2023, 267, 115617. [Google Scholar] [CrossRef]
- Eggen, T.; Lillo, C. Antidiabetic II Drug Metformin in Plants: Uptake and Translocation to Edible Parts of Cereals, Oily Seeds, Beans, Tomato, Squash, Carrots, and Potatoes. J. Agric. Food Chem. 2012, 60, 6929–6935. [Google Scholar] [CrossRef]
- Luo, M.; Zheng, L.; Wang, S.; Zhu, J.; Tang, Z.; Gao, P. Characterization of Four Major Degradation Products in Metformin by 2D LC-QTOF/MS/MS. J. Pharm. Biomed. Anal. 2021, 192, 113662. [Google Scholar] [CrossRef]
- Carbuloni, C.F.; Savoia, J.E.; Santos, J.S.P.; Pereira, C.A.A.; Marques, R.G.; Ribeiro, V.A.S.; Ferrari, A.M. Degradation of Metformin in Water by TiO2–ZrO2 Photocatalysis. J. Environ. Manage. 2020, 262, 110347. [Google Scholar] [CrossRef] [PubMed]
- Tisler, S.; Zwiener, C. Formation and Occurrence of Transformation Products of Metformin in Wastewater and Surface Water. Sci. Total Environ. 2018, 628–629, 1121–1129. [Google Scholar] [CrossRef]
- Quintão, F.J.O.; Freitas, J.R.L.; de Fátima Machado, C.; Aquino, S.F.; de Queiroz Silva, S.; de Cássia Franco Afonso, R.J. Characterization of Metformin By-Products under Photolysis, Photocatalysis, Ozonation and Chlorination by High-Performance Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2016, 30, 2360–2368. [Google Scholar] [CrossRef]
- Cristina Stenger, F.; Catia Block, L.; Correa, R.; Alves de Freitas, R.; Mari Belle Bresolin, T. HPLC Stability Indicating Assay Method for Metformin Hydrochloride in Bulk Drug and Tablets and Cytotoxicity of Degradation Products. Curr. Pharm. Anal. 2012, 8, 368–374. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Rubasinghege, G. Mechanistic Study on Iron Solubility in Atmospheric Mineral Dust Aerosol: Roles of Titanium, Dissolved Oxygen, and Solar Flux in Solutions Containing Different Acid Anions. ACS Earth Sp. Chem. 2020, 4, 101–111. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Hurab, O.; Rubasinghege, G. Atmospheric Processing and Iron Mobilization of Ilmenite: Iron-Containing Ternary Oxide in Mineral Dust Aerosol. J. Phys. Chem. A 2018, 122, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Rubasinghege, G.; Gurung, R.; Rijal, H.; Maldonado-Torres, S.; Chan, A.; Acharya, S.; Rogelj, S.; Piyasena, M. Abiotic Degradation and Environmental Toxicity of Ibuprofen: Roles of Mineral Particles and Solar Radiation. Water Res. 2018, 131, 22–32. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium Dioxide Nanoparticles (TiO2 NPs) Promote Growth and Ameliorate Salinity Stress Effects on Essential Oil Profile and Biochemical Attributes of Dracocephalum Moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Adeyinka, G.C.; Moodley, B. Effect of Aqueous Concentration of Humic Acid on the Sorption of Polychlorinated Biphenyls onto Soil Particle Grain Sizes. J. Soils Sediments 2019, 19, 1543–1553. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-Spectroscopic Characterization of Humic Acids and Humin Fractions Obtained by Advanced NaOH, Na4P2O7, and Na2CO3 Extraction Procedures. J. Plant Nutr. Soil. Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Rodrigues, A.; Brito, A.; Janknecht, P.; Proena, M.F.; Nogueira, R. Quantification of Humic Acids in Surface Water: Effects of Divalent Cations, PH, and Filtration. J. Environ. Monit. 2009, 11, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Aguer, J.P.; Richard, C.; Andreux, F. Effect of Light on Humic Substances: Production of Reactive Species. Analusis 1999, 27, 387–389. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Jeong, Y.J.; Cho, I.S.; Lee, C.-G.; Park, S.-J.; Alvarez, P.J.J. The Inhibitory Mechanism of Humic Acids on Photocatalytic Generation of Reactive Oxygen Species by TiO2 Depends on the Crystalline Phase. Chem. Eng. J. 2023, 476, 146785. [Google Scholar] [CrossRef]
- Ellepola, N.; Rubasinghege, G. Heterogeneous Photocatalysis of Amoxicillin under Natural Conditions and High-Intensity Light: Fate, Transformation, and Mineralogical Impacts. Environments 2022, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge Carrier Trapping, Recombination and Transfer during TiO2 Photocatalysis: An Overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Sakar, M.; Mithun Prakash, R.; Trong-On, D. Insights into the Tio2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, O. Interplay between Reactive Oxygen and Nitrogen Species in Living Organisms. Chem. Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Z.; Feng, Z.; Zhao, H.; Zhao, G. Fast Generation of Hydroxyl Radicals by Rerouting the Electron Transfer Pathway via Constructed Chemical Channels during the Photo-Electro-Reduction of Oxygen. Environ. Sci. Technol. 2022, 56, 1331–1340. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Sienkiewicz, A.; Wanag, A.; Rokicka-Konieczna, P.; Morawski, A.W. The Role of Adsorption in the Photocatalytic Decomposition of Dyes on APTES-Modified TiO2 Nanomaterials. Catalysts 2021, 11, 172. [Google Scholar] [CrossRef]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of Active Oxidative Species in TiO2 Photocatalysis Using the Fluorescence Technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Wöll, C. Photocatalytic Activity of Bulk TiO2 Anatase and Rutile Single Crystals Using Infrared Absorption Spectroscopy. Phys. Rev. Lett. 2011, 106, 138302. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Herrmann, J.M. Comparison of the Photoelectronic and Photocatalytic Activities of Various Anatase and Rutile Forms of Titania in Pure Liquid Organic Phases and in Aqueous Solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Kakuma, Y.; Nosaka, A.Y.; Nosaka, Y. Difference in TiO2 Photocatalytic Mechanism between Rutile and Anatase Studied by the Detection of Active Oxygen and Surface Species in Water. Phys. Chem. Chem. Phys. 2015, 17, 18691–18698. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S. Photocatalytic Activity of Amorphous−Anatase Mixture of Titanium(IV) Oxide Particles Suspended in Aqueous Solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef]
- Vital-Grappin, A.D.; Ariza-Tarazona, M.C.; Luna-Hernández, V.M.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Siligardi, C.; Cedillo-González, E.I. The Role of the Reactive Species Involved in the Photocatalytic Degradation of HDPE Microplastics Using C,N-TiO2 Powders. Polymers 2021, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Caglar, B.; Guner, E.K.; Ersoy, S.; Caglar, S.; Özdemir, A.O.; Özdokur, K.V.; Doğan, B.; İçer, F.; Çırak, Ç. Bi2S3 Nanorods Decorated on Bentonite Nanocomposite for Enhanced Visible-Light-Driven Photocatalytic Performance towards Degradation of Organic Dyes. J. Alloys Compd. 2021, 885, 160964. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; Li, P.; Tan, Y.; Ren, Y. Ultraviolet Photolysis of Metformin: Mechanisms of Environmental Factors, Identification of Intermediates, and Density Functional Theory Calculations. Environ. Sci. Pollut. Res. 2020, 27, 17043–17053. [Google Scholar] [CrossRef] [PubMed]
- Nascarella, M.A.; Calabrese, E.J. A Method to Evaluate Hormesis in Nanoparticle Dose-Responses. Dose-Response 2012, 10, 344–354. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial Biogenesis and Metabolic Hyperactivation Limits the Application of MTT Assay in the Estimation of Radiation Induced Growth Inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef]

| Surface | Rate Constant (h−1) | R2 | Half-Life (hours) |
|---|---|---|---|
| Anatase | 0.0356 | 0.965 | 19.5 |
| Rutile | 0.0015 | 0.930 | 462 |
| Amorphous Titania | 0.0032 | 0.981 | 216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Regalado, J.; Kanaththage, M.I.; Patidar, P.L.; Rubasinghege, G. Role of Environmental Photocatalysts and Organic Matter on the Degradation and Toxicity of Metformin Hydrochloride. Toxics 2025, 13, 407. https://doi.org/10.3390/toxics13050407
Khan R, Regalado J, Kanaththage MI, Patidar PL, Rubasinghege G. Role of Environmental Photocatalysts and Organic Matter on the Degradation and Toxicity of Metformin Hydrochloride. Toxics. 2025; 13(5):407. https://doi.org/10.3390/toxics13050407
Chicago/Turabian StyleKhan, Rifat, Jaqueline Regalado, Malsha Indeewari Kanaththage, Praveen L. Patidar, and Gayan Rubasinghege. 2025. "Role of Environmental Photocatalysts and Organic Matter on the Degradation and Toxicity of Metformin Hydrochloride" Toxics 13, no. 5: 407. https://doi.org/10.3390/toxics13050407
APA StyleKhan, R., Regalado, J., Kanaththage, M. I., Patidar, P. L., & Rubasinghege, G. (2025). Role of Environmental Photocatalysts and Organic Matter on the Degradation and Toxicity of Metformin Hydrochloride. Toxics, 13(5), 407. https://doi.org/10.3390/toxics13050407







