Overlooked Photochemical Risk of Antimicrobial Fragrances: Formation of Potent Allergens and Their Mechanistic Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photochemical and Scavenging Experiments
2.3. Laser Flash Photolysis and Electron Paramagnetic Resonance
2.4. Analytical Methods
2.5. Toxicity Evaluation
3. Results and Discussion
3.1. Kinetic of CA Photochemical Transformation
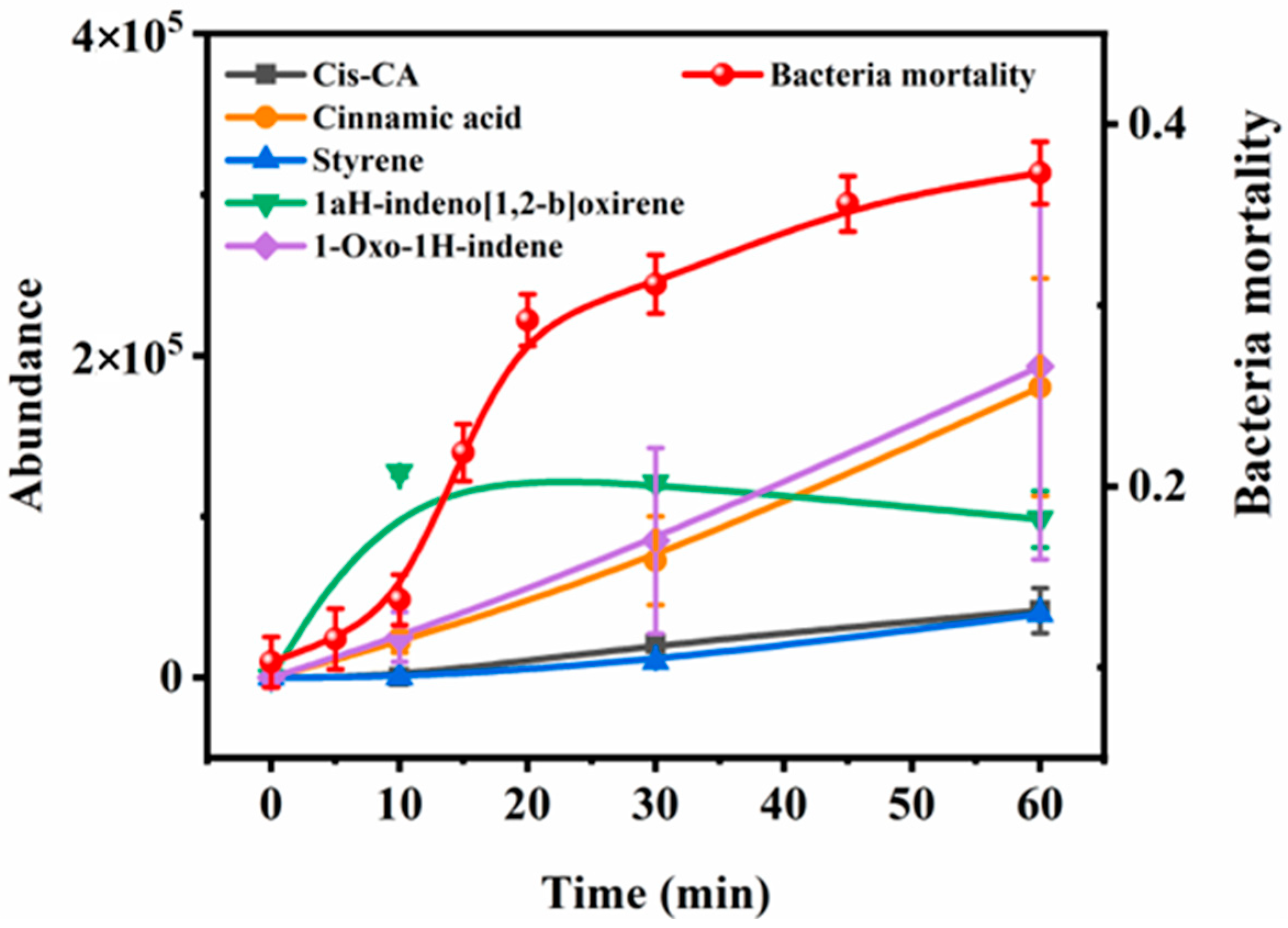
3.2. Identification of Transformation Products of CA and Toxicity Evaluation
3.3. Photodegradation Mechanisms
3.3.1. The Identification of Transient Intermediates
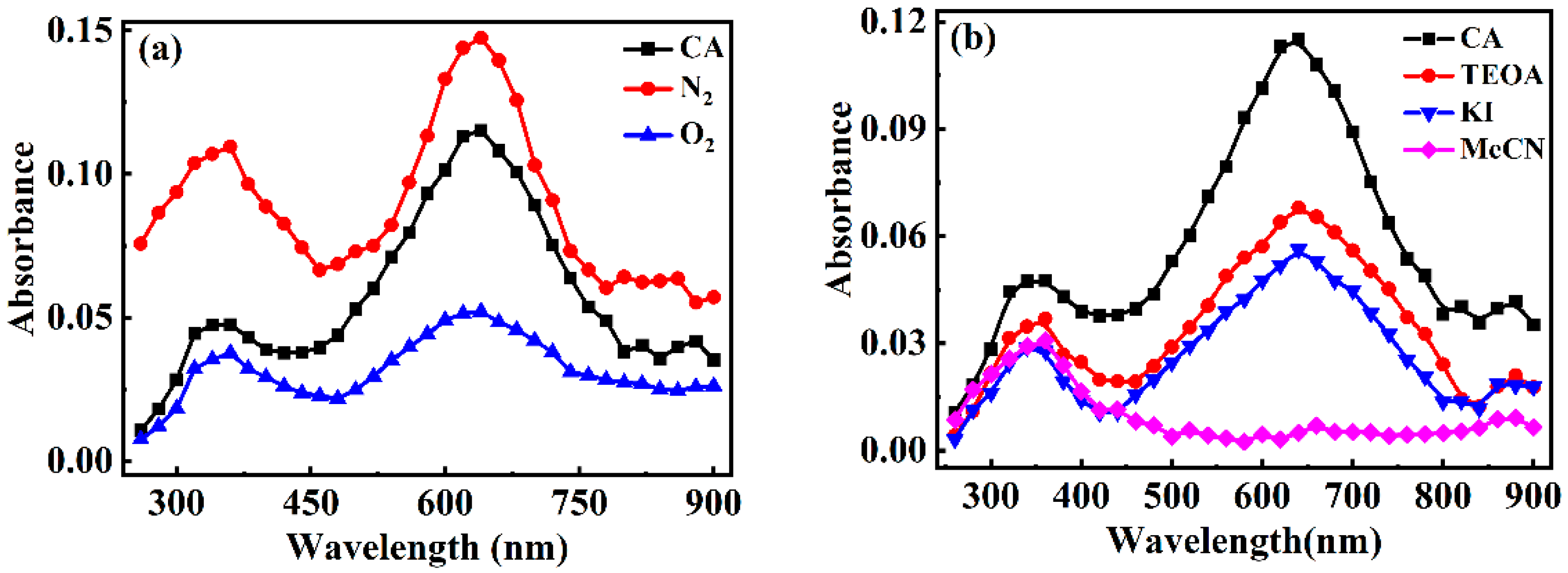
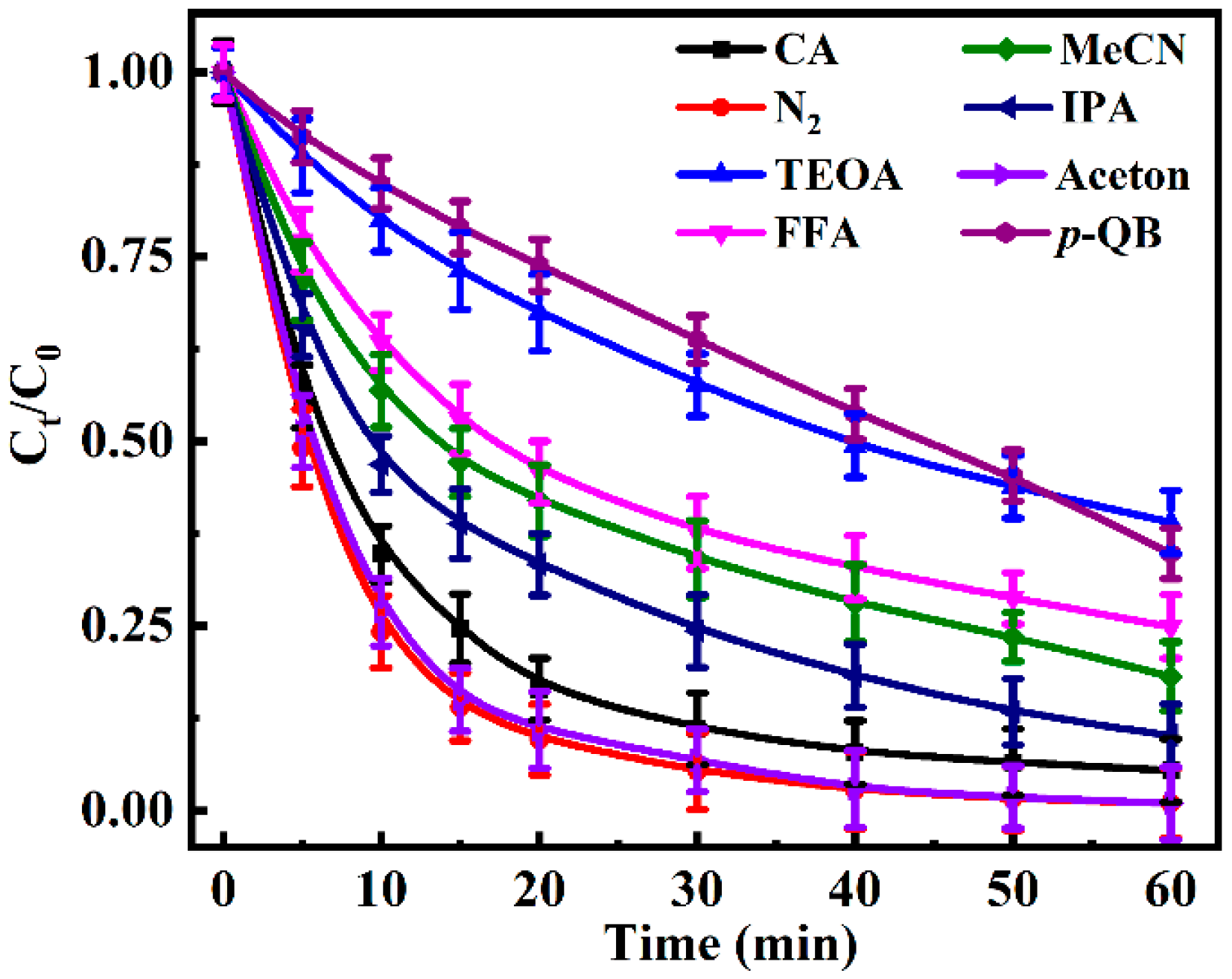
3.3.2. The Identification of the Reactive Species
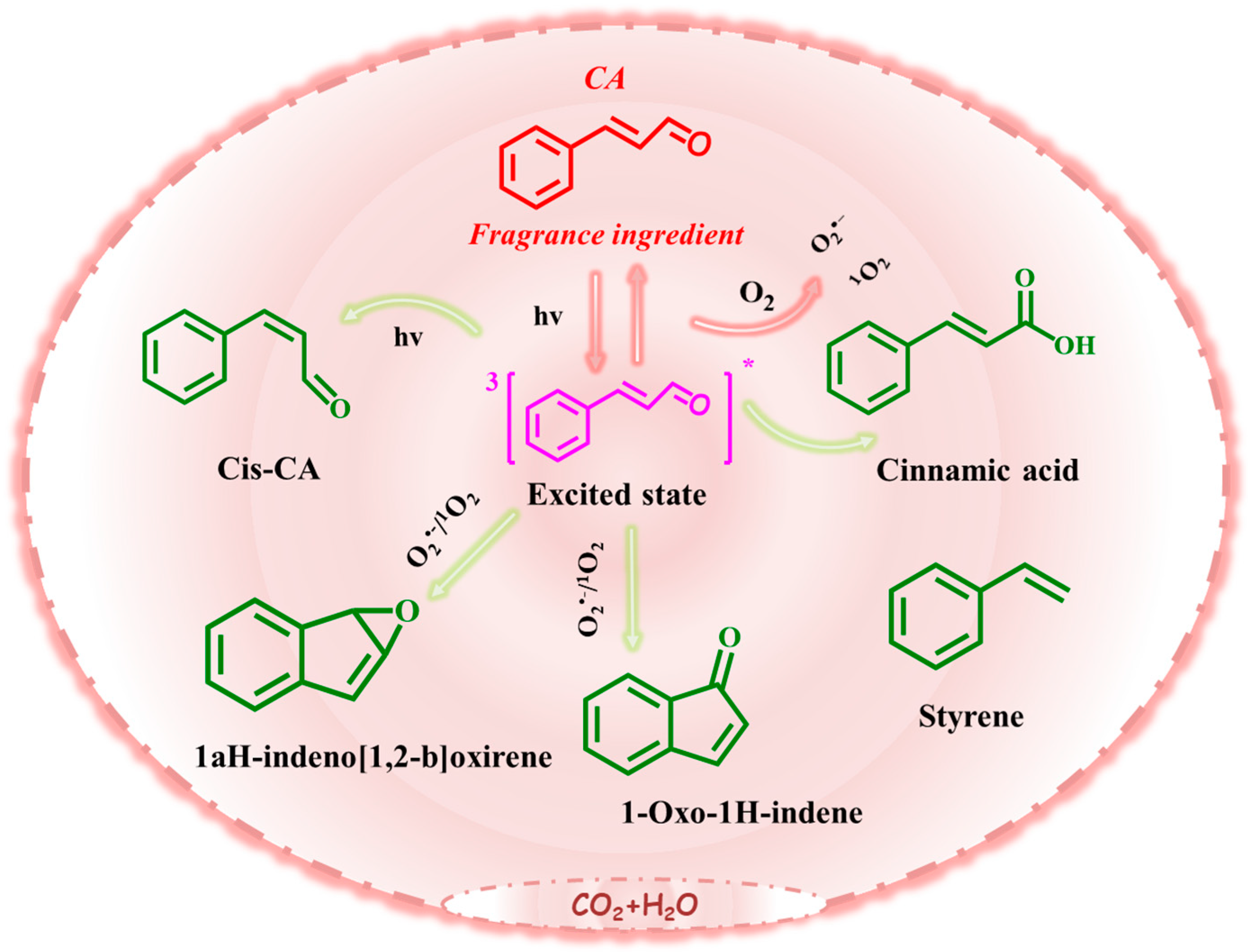
3.3.3. Photochemical Degradation Mechanism of CA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.T.; Tierney, P.A.; Pankow, J.F.; Talbot, P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019, 9, 2468. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.F.; Powers, S.M.; Hampton, S.E. An Evidence Synthesis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environment: Imbalances among Compounds, Sewage Treatment Techniques, and Ecosystem Types. Environ. Sci. Technol. 2019, 53, 12961–12973. [Google Scholar] [CrossRef]
- Sukakul, T.; Bruze, M.; Svedman, C. Fragrance Contact Allergy—A Review Focusing on Patch Testing. Acta Derm.-Venereol. 2024, 104, adv40332. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, M.Z.; Huang, L.Y.; Zhang, L.J.; Yu, X.J.; Liu, Y.; Li, J. Exploring Cinnamaldehyde: Preparation Methods, Biological Functions, Efficient Applications, and Safety. Food Rev. Int. 2024, 41, 615–642. [Google Scholar] [CrossRef]
- Wei, F.; Mortimer, M.; Cheng, H.; Sang, N.; Guo, L.-H. Parabens as chemicals of emerging concern in the environment and humans: A review. Sci. Total Environ. 2021, 778, 146150. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Homaei, A.; Sharifian, S. Need of the hour: To raise awareness on vicious fragrances and synthetic musks. Environ. Dev. Sustain. 2021, 23, 4764–4781. [Google Scholar] [CrossRef]
- Cocchiara, J.; Letizia, C.S.; Lalko, J.; Lapczynski, A.; Api, A.M. Fragrance material review on cinnamaldehyde. Food Chem. Toxicol. 2005, 43, 867–923. [Google Scholar] [CrossRef]
- Buerge, I.J.; Buser, H.R.; Muller, M.D.; Poiger, T. Behavior of the polycyclic musks HHCB and AHTN in lakes, two potential anthropogenic markers for domestic wastewater in surface waters. Environ. Sci. Technol. 2003, 37, 5636–5644. [Google Scholar] [CrossRef]
- Reeder, M.J. Allergic Contact Dermatitis to Fragrances. Dermatol. Clin. 2020, 8, 371–377. [Google Scholar] [CrossRef]
- Steinemann, A. Fragranced consumer products: Effects on asthmatics. Air Qual. Atmos. Health 2018, 11, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Swales, N.J.; Caldwell, J. Studies on trans-cinnamaldehyde II: Mechanisms of cytotoxicity in rat isolated hepatocytes. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 1996, 10, 37–42. [Google Scholar] [CrossRef]
- Sanyal, R.; Darroudi, F.; Parzefall, W.; Nagao, M.; Knasmuller, S. Inhibition of the genotoxic effects of heterocyclic amines in human derived hepatoma cells by dietary bioantimutagens. Mutagenesis 1997, 12, 297–303. [Google Scholar] [CrossRef]
- Mereto, E.; Brambilla-Campart, G.; Ghia, M.; Martelli, A.; Brambilla, G. Cinnamaldehyde-induced micronuclei in rodent liver. Mutat. Res. 1994, 322, 1–8. [Google Scholar] [CrossRef]
- Behar, R.Z.; Luo, W.; Lin, S.C.; Wang, Y.; Valle, J.; Pankow, J.F.; Talbot, P. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob. Control 2016, 25, ii94–ii102. [Google Scholar] [CrossRef] [PubMed]
- Tumová, J.; Sauer, P.; Golovko, O.; Ucun, O.K.; Grabic, R.; Máchová, J.; Kroupová, H.K. Effect of polycyclic musk compounds on aquatic organisms: A critical literature review supplemented by own data. Sci. Total Environ. 2019, 651, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Wang, L.; Zheng, M.G.; Lou, Y.H.; Shi, L. Determination and environmental risk assessment of synthetic musks in the water and sediments of the Jiaozhou Bay wetland, China. Environ. Sci. Pollut. Res. 2018, 25, 4915–4923. [Google Scholar] [CrossRef]
- Martin, C.; Moeder, M.; Daniel, X.; Krauss, G.; Schlosser, D. Biotransformation of the Polycyclic Musks HHCB and AHTN and Metabolite Formation by Fungi Occurring in Freshwater Environments. Environ. Sci. Technol. 2007, 41, 5395–5402. [Google Scholar] [CrossRef]
- Dearman, R.J.; Hilton, J.; Evans, P.; Harvey, P.; Basketter, D.A.; Kimber, I. Temporal stability of local lymph node assay responses to hexyl cinnamic aldehyde. J. Appl. Toxicol. 1998, 18, 281–284. [Google Scholar] [CrossRef]
- Karrow, N.A.; Leffel, E.K.; Guo, T.L.; Zhang, L.X.; McCay, J.A.; Germolec, D.R.; White, K.L., Jr. Dermal exposure to cinnamaldehyde alters lymphocyte subpopulations, number of interferon-gamma-producing cells, and expression of B7 costimulatory molecules and cytokine messenger RNAs in auricular lymph nodes of B6C3F1 mice. Am. J. Contact Dermat. Off. J. Am. Contact Dermat. Soc. 2001, 12, 6–17. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, S.L.; Zhang, C.; Yuan, E.D.; Wei, Q.Y.; Zeng, Z. Antioxidative Activities of Natural Hydroxy-Bearing Cinnamaldehydes and Cinnamic Acids: A Comparative Study. Trop. J. Pharm. Res. 2013, 12, 1017–1022. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.Y.; Zhou, Q.X.; Zhou, Q.Q.; Zhang, Y.; Zhu, L.F. Polycyclic musks in the environment: A review of their concentrations and distribution, ecological effects and behavior, current concerns and future prospects. Crit. Rev. Environ. Sci. Technol. 2021, 51, 323–377. [Google Scholar] [CrossRef]
- Ozaki, N.; Tanaka, T.; Kindaichi, T.; Ohashi, A. Photodegradation of fragrance materials and triclosan in water: Direct photolysis and photosensitized degradation. Environ. Technol. Innov. 2021, 23, 101766. [Google Scholar] [CrossRef]
- Canterino, M.; Marotta, R.; Temussi, F.; Zarrelli, A. Photochemical behaviour of musk tibetene—A chemical and kinetic investigation. Environ. Sci. Pollut. Res. 2008, 15, 182–187. [Google Scholar] [CrossRef]
- Gao, Y.P.; Li, G.Y.; Qin, Y.X.; Ji, Y.M.; Mai, B.X.; An, T.C. New theoretical insight into indirect photochemical transformation of fragrance nitro-musks: Mechanisms, eco-toxicity and health effects. Environ. Int. 2019, 129, 68–75. [Google Scholar] [CrossRef]
- Sokol, A.; Ratkiewicz, A.; Tomaszewska, I.; Karpinska, J. Kinetics and Mechanistic Studies of Photochemical and Oxidative Stability of Galaxolide. Water 2021, 13, 1813. [Google Scholar] [CrossRef]
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Emberger, M. Study of the photodegradation of a fragrance ingredient for aquatic environmental fate assessment. Chemosphere 2017, 173, 485–493. [Google Scholar] [CrossRef]
- Ahn, J.; Avonto, C.; Chittiboyina, A.G.; Khan, I.A. Is Isoeugenol a Prehapten? Characterization of a Thiol-Reactive Oxidative Byproduct of Isoeugenol and Potential Implications for Skin Sensitization. Chem. Res. Toxicol. 2020, 33, 948–954. [Google Scholar] [CrossRef]
- Fang, H.; Gao, Y.; Li, G.; An, J.; Wong, P.K.; Fu, H.; Yao, S.; Nie, X.; An, T. Advanced Oxidation Kinetics and Mechanism of Preservative Propylparaben Degradation in Aqueous Suspension of TiO2 and Risk Assessment of Its Degradation Products. Environ. Sci. Technol. 2013, 47, 2704–2712. [Google Scholar] [CrossRef]
- Borba, J.; Braga, R.C.; Alves, V.M.; Muratov, E.N.; Andrade, C.H. Pred-Skin: A web portal for accurate prediction of human skin sensitizers. Chem. Res. Toxicol. 2021, 34, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhang, H.L.; Zhang, Q.C.; Wen, H.M.; Cui, X.B.; Peng, G.P.; Shan, C.X.; Chai, C.; Li, W.; Zuo, C.B.; et al. Chemical Profile Analysis of Ling-Gui-Zhu-Gan Decoction by LC-QTOF MS and Simultaneous Determination of Nine Major Components Using QAMS Method. Chromatographia 2020, 83, 1371–1389. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, M.; Chittiboyina, A.G.; Vukmanovic, S.; Khan, I.A. Chemical stability and in chemico reactivity of 24 fragrance ingredients of concern for skin sensitization risk assessment. Toxicol. In Vitro Int. J. Publ. Assoc. Bibra 2018, 46, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Arribas, M.P.; Soro, P.; Silvestre, J.F. Allergic contact dermatitis to fragrances: Part 2. Actas Dermo-Sifiliogr. 2013, 104, 29–37. [Google Scholar] [CrossRef]
- Gao, Y.; Niu, X.; Qin, Y.; Guo, T.; Ji, Y.; Li, G.; An, T. Unexpected culprit of increased estrogenic effects: Oligomers in the photodegradation of preservative ethylparaben in water. Water Res. 2020, 176, 115745. [Google Scholar] [CrossRef]
- Sabrina Halladja, A.T.H.; Aguer, J.; Boulkamh, A.A.; Richard, C. Inhibition of Humic Substances Mediated Photooxygenation of Furfuryl Alcohol by 2,4,6-Trimethylphenol. Evidence for Reactivity of the Phenol with Humic Triplet Excited States. Environ. Sci. Technol. 2007, 41, 6066–6073. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, L.; Zhang, Y.; Ferronato, C.; Brigante, M.; Mailhot, G.; Yang, X.; Chovelon, J.M. Photochemical degradation of sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid in different water matrices. Water Res. 2013, 47, 5865–5875. [Google Scholar] [CrossRef] [PubMed]
- Broglia, M.F.; Bertolotti, S.G.; Previtali, C.M. Excited states quenching of phenosafranine dye by electron donors. J. Photochem. Photobiol. A Chem. 2005, 170, 261–265. [Google Scholar] [CrossRef]
- Latch, D.E.; Packer, J.L.; Stender, B.L.; Vanoverbeke, J.; Arnold, W.A.; Mcneill, K. Aqueous photochemistry of triclosan: Formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ. Toxicol. Chem. 2005, 24, 517. [Google Scholar] [CrossRef]
- Xing, R.; Wu, L.; Fei, Z.; Wu, P. Mesopolymer modified with palladium phthalocyaninesulfonate as a versatile photocatalyst for phenol and bisphenol A degradation under visible light irradiation. J. Environ. Sci. 2013, 25, 1687–1695. [Google Scholar] [CrossRef]
- Li, M.Y.; Cline, C.S.; Koker, E.B.; Carmichael, H.H.; Chignell, C.F.; Bilski, P. Quenching of Singlet Molecular Oxygen (1O2) by Azide Anion in Solvent Mixtures. Photochem. Photobiol. 2001, 74, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, K.; Cao, C.; Wang, M.; Chen, Q.; Wang, S. The laser flash photolysis and UV photolytical degradation of 4-n-nonylphenol. Sci. Sin. 2012, 42, 175–181. [Google Scholar]
- Horspool, W.M.; Song, P.S. CRC Handbook of Organic Photochemistry and Photobiology; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Werner, J.J.; Chintapalli, M.; Lundeen, R.A.; Wammer, K.H.; Arnold, W.A.; McNeill, K. Environmental photochemistry of tylosin: Efficient, reversible photoisomerization to a less-active isomer, followed by photolysis. J. Agric. Food Chem. 2007, 55, 7062–7068. [Google Scholar] [CrossRef]
- Shi, H.W.; Li, J.; Wang, T.; Rudolph, M.; Hashmi, A.S.K. Catalyst- and additive-free sunlight-induced autoxidation of aldehydes to carboxylic acids. Green Chem. 2022, 24, 5835–5841. [Google Scholar] [CrossRef]
- Xuan, J.; Zhang, Z.G.; Xiao, W.J. Visible-Light-Induced Decarboxylative Functionalization of Carboxylic Acids and Their Derivatives. Angew. Chem.-Int. Ed. 2015, 54, 15632–15641. [Google Scholar] [CrossRef]

| Name (Abbreviation) | Formula | Retention Time (min) | [M + H]+ | Fragment Ions | Structure |
|---|---|---|---|---|---|
| Cinnamyl aldehyde (CA) | C9H8O | 14.4 | 133.064 | 115.054 |  |
| Cis-Cinnamyl aldehyde (Cis-CA) | C9H8O | 4.0 | 133.064 | 115.054 | 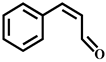 |
| Cinnamic acid | C9H8O2 | 8.2 | 149.059 | 131.048 | 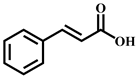 |
| Styrene | C8H8 | 2.9 | 105.069 | 79.054 | 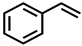 |
| 1aH-indeno [1,2-b]oxirene | C9H6O | 6.3 | 131.048 | 103.053 |  |
| 1-Oxo-1H-indene | C9H6O | 9.7 | 131.048 | 103.054 | 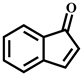 |
| Compounds | Irritation (p) | Skin Sensitization (%) | ||
|---|---|---|---|---|
| Eye Irritation | Skin Irritation | LLNA (In Vivo) | HRIPT/HMT (Human) | |
| CA | 75.0 | 69.0 | (+) 99.8 | (+) 99.0 |
| Cis-CA | 75.0 | 69.0 | (+) 99.8 | (+) 99.0 |
| Cinnamic acid | 94.0 | 83.0 | (+) 98.2 | (+) 86.0 |
| Styrene | 68.0 | 73.0 | (+) 99.7 | (+) 93.3 |
| 1aH-indeno [1,2-b]oxirene | 39.0 | 36.0 | (+) 89.6 | (+) 97.7 |
| 1-Oxo-1H-indene | 53.0 | 81.0 | (+) 99.4 | (+) 99.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Wu, J.; Chen, Y.; Luo, N.; Gao, Y. Overlooked Photochemical Risk of Antimicrobial Fragrances: Formation of Potent Allergens and Their Mechanistic Pathways. Toxics 2025, 13, 386. https://doi.org/10.3390/toxics13050386
Niu X, Wu J, Chen Y, Luo N, Gao Y. Overlooked Photochemical Risk of Antimicrobial Fragrances: Formation of Potent Allergens and Their Mechanistic Pathways. Toxics. 2025; 13(5):386. https://doi.org/10.3390/toxics13050386
Chicago/Turabian StyleNiu, Xiaolin, Junji Wu, Yi Chen, Na Luo, and Yanpeng Gao. 2025. "Overlooked Photochemical Risk of Antimicrobial Fragrances: Formation of Potent Allergens and Their Mechanistic Pathways" Toxics 13, no. 5: 386. https://doi.org/10.3390/toxics13050386
APA StyleNiu, X., Wu, J., Chen, Y., Luo, N., & Gao, Y. (2025). Overlooked Photochemical Risk of Antimicrobial Fragrances: Formation of Potent Allergens and Their Mechanistic Pathways. Toxics, 13(5), 386. https://doi.org/10.3390/toxics13050386









