Development of Neutral pH-Responsive Microgels by Tuning Cross-Linking Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
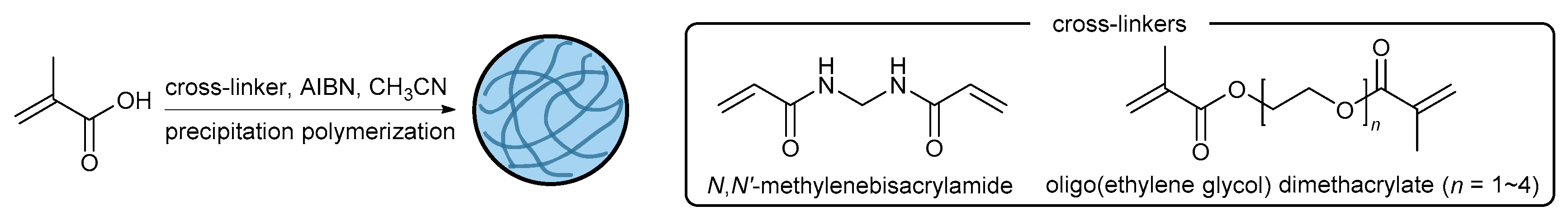
2.2. Synthesis
2.3. Nuclear Magnetic Resonance (NMR) Relaxometry
2.4. Magnetic Resonance Imaging (MRI)
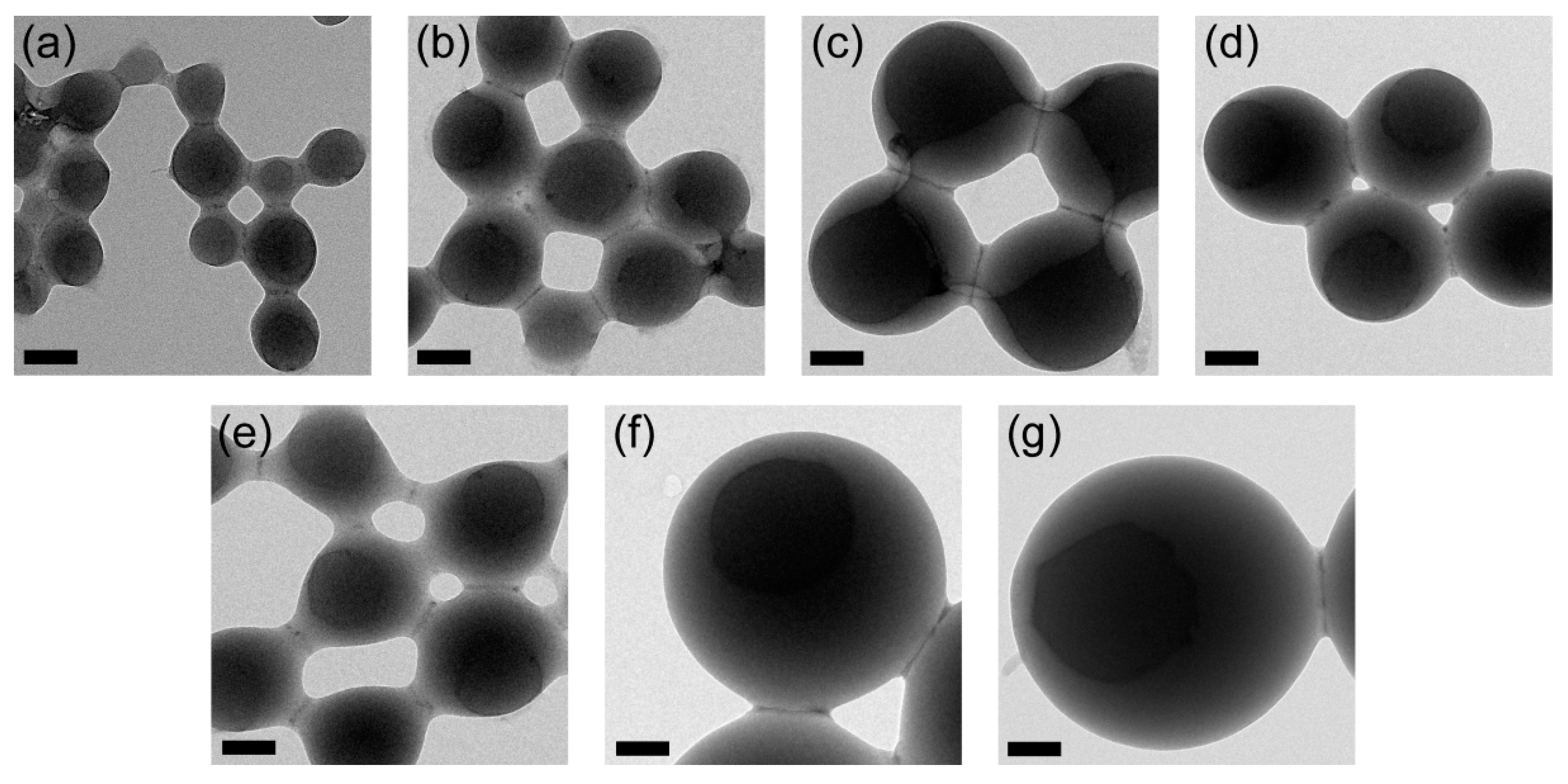
2.5. Transmission Electron Microscopy (TEM)
3. Results and Discussion
3.1. Synthesis of Cross-Linked Polymer Microgels
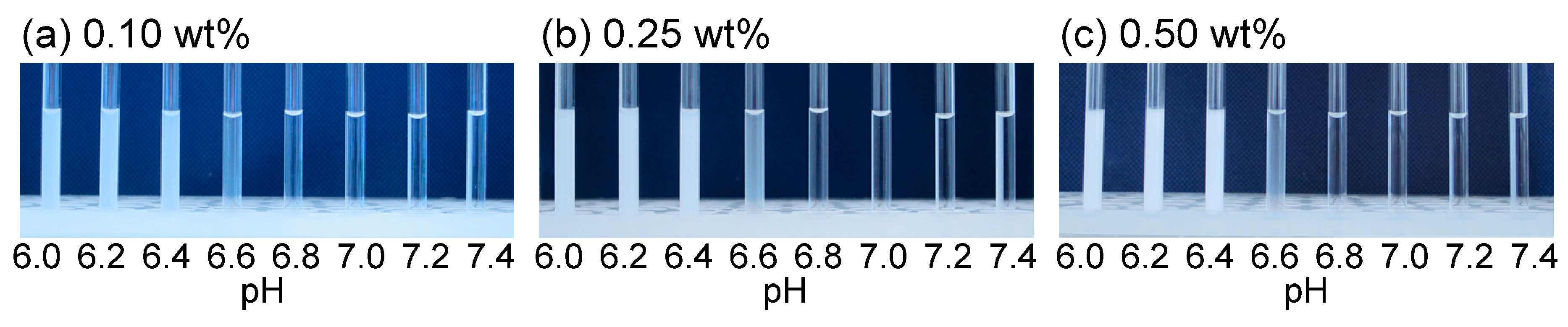
3.2. TEM Images and Volume Phase Transition Behaviors
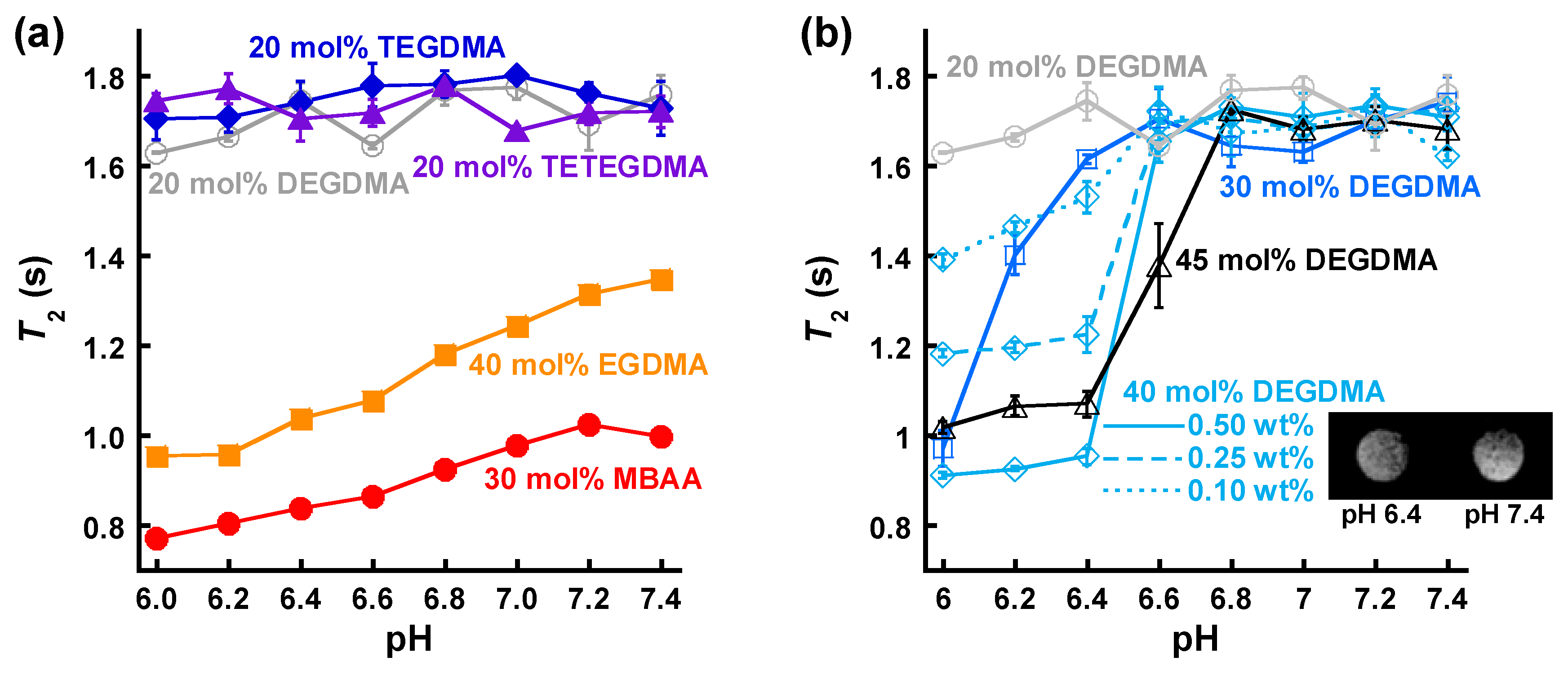
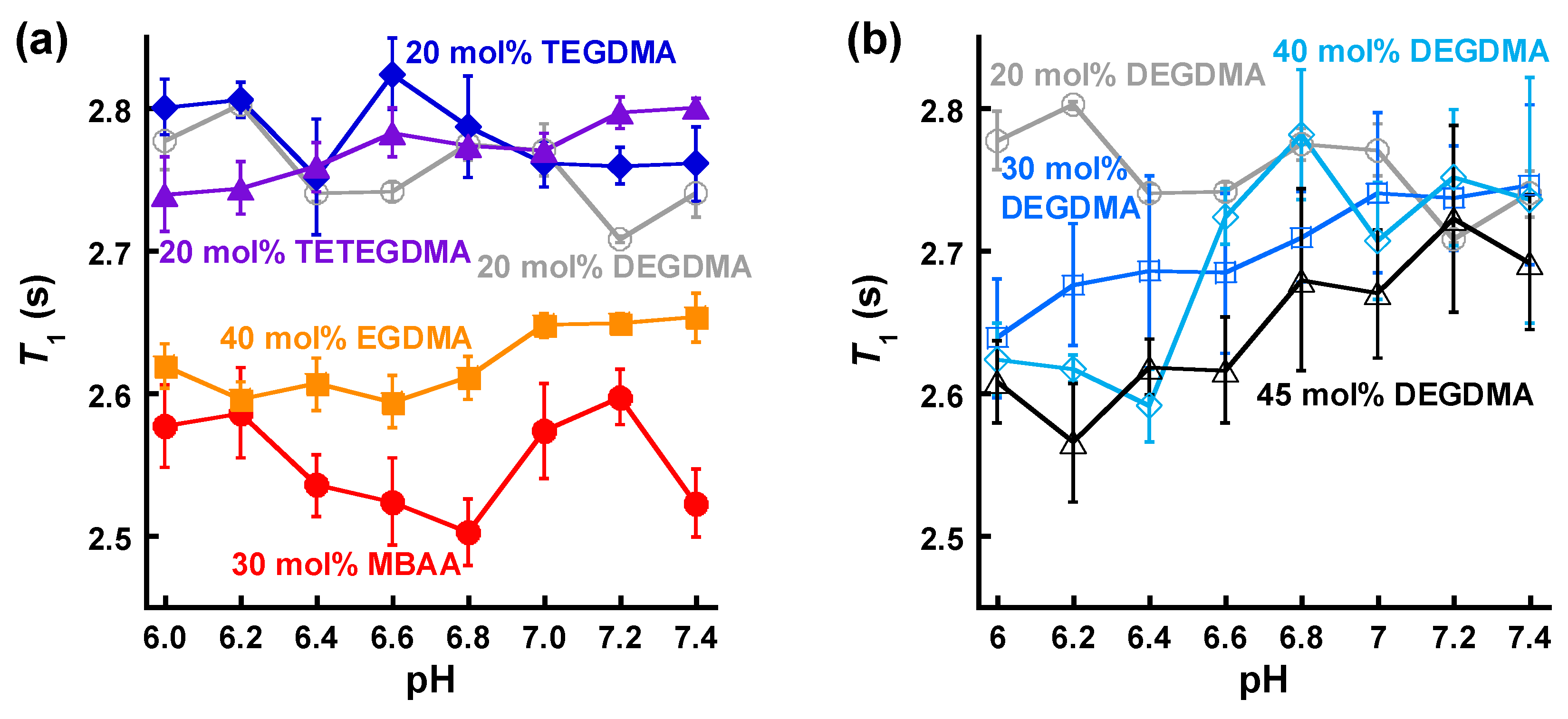
3.3. NMR Relaxometric Properties at Neutral pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gillies, R.J.; Liu, Z.; Bhujwalla, Z. 31P-MRS measurements of extracellular pH of tumors using 3- aminopropylphosphonate. Am. J. Physiol. Cell Physiol. 1994, 267, C195–C203. [Google Scholar] [CrossRef]
- van Sluis, R.; Bhujwalla, Z.M.; Raghunand, N.; Ballesteros, P.; Alvarez, J.; Cerdán, S.; Galons, J.-P.; Gillies, R.J. In vivo imaging of extracellular pH using 1H MRSI. Magn. Reson. Med. 1999, 41, 743–750. [Google Scholar] [CrossRef]
- Vermathen, P.; Capizzano, A.A.; Maudsley, A.A. Administration and 1H MRS detection of histidine in human brain: Application to in vivo pH measurement. Magn. Reson. Med. 2000, 43, 665–675. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, K.; Sherry, A.D. A novel pH-sensitive MRI contrast agent. Angew. Chem. Int. Ed. 1999, 38, 3192–3194. [Google Scholar] [CrossRef]
- Tóth, É.; Bolskar, R.D.; Borel, A.; González, G.; Helm, L.; Merbach, A.E.; Sitharaman, B.; Wilson, L.J. Water-soluble gadofullerenes: Toward high-relaxivity, pH-responsive MRI contrast agents. J. Am. Chem. Soc. 2005, 127, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Fedeli, F.; Sanino, A.; Terreno, E. A R2/R1 ratiometric procedure for a concentration-independent, pH-responsive, Gd(III)-based MRI agent. J. Am. Chem. Soc. 2006, 128, 11326–11327. [Google Scholar] [CrossRef]
- Ali, M.M.; Woods, M.; Caravan, P.; Opina, A.C.L.; Spiller, M.; Fettinger, J.C.; Sherry, A.D. Synthesis and relaxometric studies of a dendrimer-based pH-responsive MRI contrast agent. Chem. Eur. J. 2008, 14, 7250–7258. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Mizukami, S.; Kikuchi, K. Switchable MRI contrast agents based on morphological changes of pH-responsive polymers. Bioorg. Med. Chem. 2012, 20, 769–774. [Google Scholar] [CrossRef]
- Mi, P.; Kokuryo, D.; Cabral, H.; Wu, H.; Terada, Y.; Saga, T.; Aoki, I.; Nishiyama, N.; Kataoka, K. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat. Nanotechnol. 2016, 11, 724–730. [Google Scholar] [CrossRef]
- Botár, R.; Molnár, E.; Trencsényi, G.; Kiss, J.; Kálmán, F.K.; Tircsó, G. Stable and inert Mn(II)-based and pH-responsive contrast agents. J. Am. Chem. Soc. 2020, 142, 1662–1666. [Google Scholar] [CrossRef]
- Raghunand, N.; Howison, C.; Sherry, A.D.; Zhang, S.; Gillies, R.J. Renal and systemic pH imaging by contrast-enhanced MRI. Magn. Reson. Med. 2003, 49, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Raghunand, N.; Garcia-Martin, M.L.; Gatenby, R.A. pH Imaging: A review of pH measurement methods and applications in cancers. IEEE Eng. Med. Biol. Mag. 2004, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Ando, I.; Fujishige, S.; Kubota, K. Molecular motion and 1H NMR relaxation of aqueous poly(N-isopropylacrylamide) solution under high pressure. J. Polym. Sci. B Polym. Phys. 1991, 29, 963–968. [Google Scholar] [CrossRef]
- Sierra-Martín, B.; Romero-Cano, M.S.; Cosgrove, T.; Vincent, B.; Fernández-Barbero, A. Solvent relaxation of swelling PNIPAM microgels by NMR. Colloids Surf. A Physicochem. Eng. Asp. 2005, 270–271, 296–300. [Google Scholar] [CrossRef]
- Okada, S.; Mizukami, S.; Matsumura, Y.; Yoshioka, Y.; Kikuchi, K. A nanospherical polymer as an MRI sensor without paramagnetic or superparamagnetic species. Dalton Trans. 2013, 42, 15864–15867. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Stöver, H.D.H. Mono- or narrow disperse poly(methacrylate-co-divinylbenzene) microspheres by precipitation polymerization. J. Polym. Sci. A Polym. Chem. 1999, 37, 2899–2907. [Google Scholar] [CrossRef]
- Goh, E.C.C.; Stöver, H.D.H. Cross-linked poly(methacrylic acid-co-poly(ethylene oxide) methyl ether methacrylate) microspheres and microgels prepared by precipitation polymerization: A morphology study. Macromolecules 2002, 35, 9983–9989. [Google Scholar] [CrossRef]
- Bai, F.; Yang, X.; Huang, W. Preparation of narrow or monodisperse poly(ethyleneglycol dimethacrylate) microspheres by distillation-precipitation polymerization. Eur. Polym. J. 2006, 42, 2088–2097. [Google Scholar] [CrossRef]
- Bai, F.; Huang, B.; Yang, X.; Huang, W. Synthesis of monodisperse poly(methacrylic acid) microspheres by distillation-precipitation polymerization. Eur. Polym. J. 2007, 43, 3923–3932. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Wang, Y. Preparation of monodisperse hydrophilic polymer microspheres with N,N′-methylenediacrylamide as crosslinker by distillation precipitation polymerization. Polym. Int. 2007, 56, 905–913. [Google Scholar] [CrossRef]
- Li, G.; Yang, X.; Wang, B.; Wang, J.; Yang, X. Monodisperse temperature-responsive hollow polymer microspheres: Synthesis, characterization and biological application. Polymer 2008, 49, 3436–3443. [Google Scholar] [CrossRef]
- Scranton, A.B.; Bowman, C.N.; Klier, J.; Peppas, N.A. Polymerization reaction dynamics of ethylene glycol methacrylates and dimethacrylates by calorimetry. Polymer 1992, 33, 1683–1689. [Google Scholar] [CrossRef]
- Witte, J.; Kyrey, T.; Lutzki, J.; Dahl, A.M.; Houston, J.; Radulescu, A.; Pipich, V.; Stingaciu, L.; Kühnhammer, M.; Witt, M.U.; et al. A comparison of the network structure and inner dynamics of homogeneously and heterogeneously crosslinked PNIPAM microgels with high crosslinker content. Soft Matter 2019, 15, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Leyte, J.C.; Mandel, M. Potentiometric behavior of polymethacrylic acid. J. Polym. Sci. A 1964, 2, 1879–1891. [Google Scholar] [CrossRef]
- Kennan, R.P.; Richardson, K.A.; Zhong, J.; Maryanski, M.J.; Gore, J.C. The effects of cross-link density and chemical exchange on magnetization transfer in polyacrylamide gels. J. Magn. Reson. B 1996, 110, 267–277. [Google Scholar] [CrossRef]
- Gochberg, D.F.; Kennan, R.P.; Maryanski, M.J.; Gore, J.C. The role of specific side groups and pH in magnetization transfer in polymers. J. Magn. Reson. 1998, 131, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Calucci, L.; Forte, C.; Gerges, I.; Ranucci, E. Effect of pH on water proton NMR relaxation in agmatine-containing poly(amidoamine) hydrogels. Langmuir 2009, 25, 2449–2455. [Google Scholar] [CrossRef]
- Koenig, S.H.; Brown, R.D.; Ugolini, R. Magnetization transfer in cross-linked bovine serum albumin solutions at 200 MHz: A model for tissue. Magn. Reson. Med. 1993, 29, 311–316. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ulak, F.S.; Batun, M.S. Proton T1 and T2 relaxivities of serum proteins. Magn. Reson. Imaging 2004, 22, 683–688. [Google Scholar] [CrossRef]
- Yilmaz, A.; Budak, H.; Ulak, F.S. Determination of the effective correlation time modulating 1H NMR relaxation processes of bound water in protein solutions. Magn. Reson. Imaging 2008, 26, 254–260. [Google Scholar] [CrossRef]
- Calucci, L.; Forte, C.; Ranucci, E. Water/polymer interactions in poly(amidoamine) hydrogels by 1H nuclear magnetic resonance relaxation and magnetization transfer. J. Chem. Phys. 2008, 129, 064511. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, X.; Zheng, Z.; Cheng, X.; Hu, X.; Peng, Y. Magnetic separation of polymer hybrid iron oxide nanoparticles triggered by temperature. Chem. Commun. 2006, 26, 2765–2767. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Preparation and controlled self-assembly of Janus magnetic nanoparticles. J. Am. Chem. Soc. 2007, 129, 12878–12889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuan, J.; Wei, Y. Core-shell structural iron oxide hybrid nanoparticles: From controlled synthesis to biomedical applications. J. Mater. Chem. 2011, 21, 2823–2840. [Google Scholar] [CrossRef]
- Paquet, C.; de Haan, H.W.; Leek, D.M.; Lin, H.-Y.; Xiang, B.; Tian, G.; Kell, A.; Simard, B. Clusters of superparamagnetic iron oxide nanoparticles encapsulated in a hydrogel: A particle architecture generating a synergistic enhancement of the T2 relaxation. ACS Nano 2011, 5, 3104–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, S.; Mizukami, S.; Sakata, T.; Matsumura, Y.; Yoshioka, Y.; Kikuchi, K. Ratiometric MRI sensors based on core-shell nanoparticles for quantitative pH imaging. Adv. Mater. 2014, 26, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
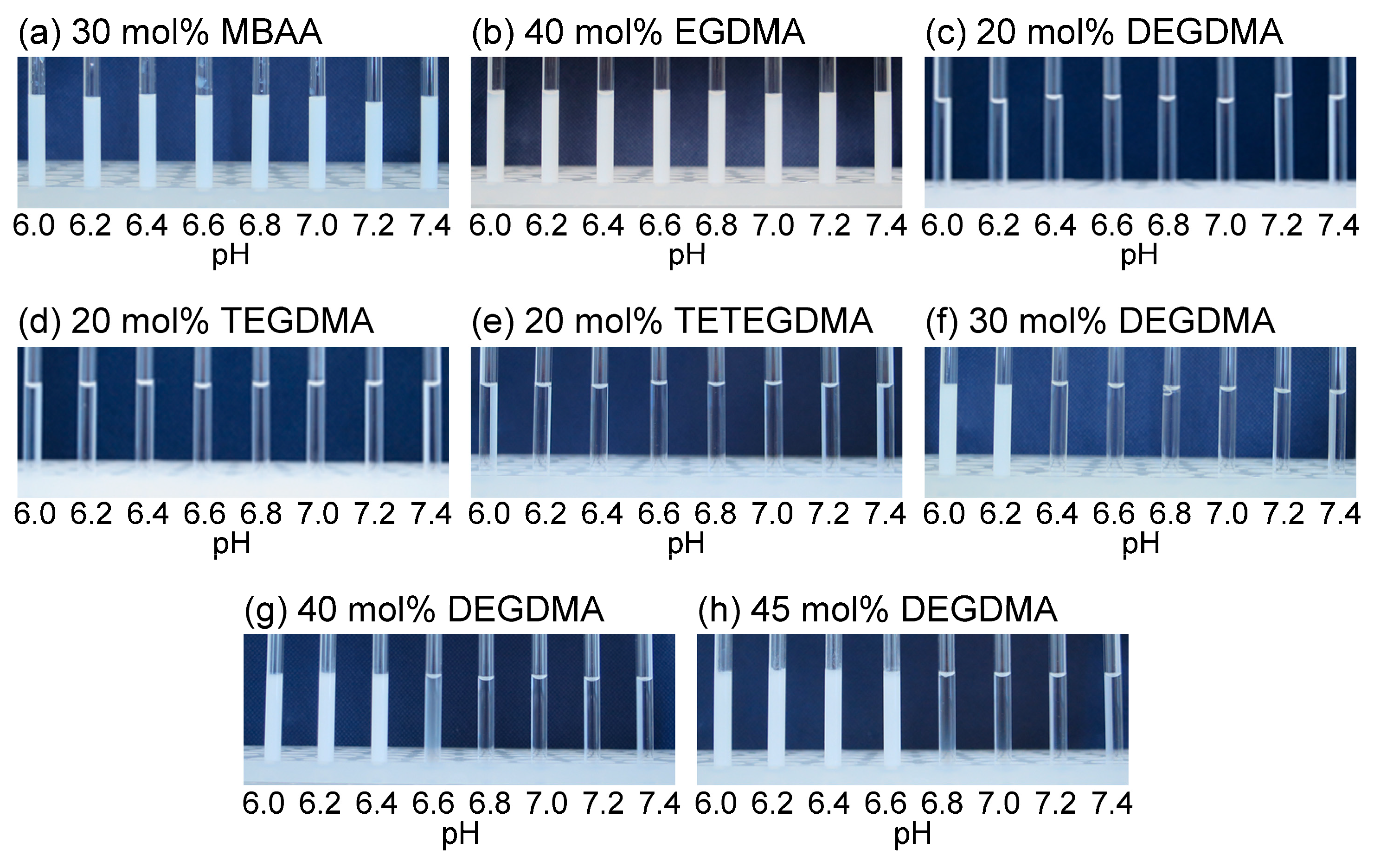
| Group | Cross-Linker and Repeating Unit (n) | Cross-Linking Degree (mol%) |
|---|---|---|
| 1 | MBAA | 30 |
| 1 | EGDMA (n = 1) | 40 |
| 1, 2 | DEGDMA (n = 2) | 20 |
| 1 | TEGDMA (n = 3) | 20 |
| 1 | TETEGDMA (n = 4) | 20 |
| 2 | DEGDMA (n = 2) | 30 |
| 2 | DEGDMA (n = 2) | 40 |
| 2 | DEGDMA (n = 2) | 45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, S.; Takayasu, S.; Tomita, S.; Suzuki, Y.; Yamamoto, S. Development of Neutral pH-Responsive Microgels by Tuning Cross-Linking Conditions. Sensors 2020, 20, 3367. https://doi.org/10.3390/s20123367
Okada S, Takayasu S, Tomita S, Suzuki Y, Yamamoto S. Development of Neutral pH-Responsive Microgels by Tuning Cross-Linking Conditions. Sensors. 2020; 20(12):3367. https://doi.org/10.3390/s20123367
Chicago/Turabian StyleOkada, Satoshi, Satoko Takayasu, Shunsuke Tomita, Yoshio Suzuki, and Shinya Yamamoto. 2020. "Development of Neutral pH-Responsive Microgels by Tuning Cross-Linking Conditions" Sensors 20, no. 12: 3367. https://doi.org/10.3390/s20123367





