An Analysis of a Compact Label-Free Guiding-Wave Biosensor Based on a Semiconductor-Clad Dielectric Strip Waveguide
Abstract
1. Introduction
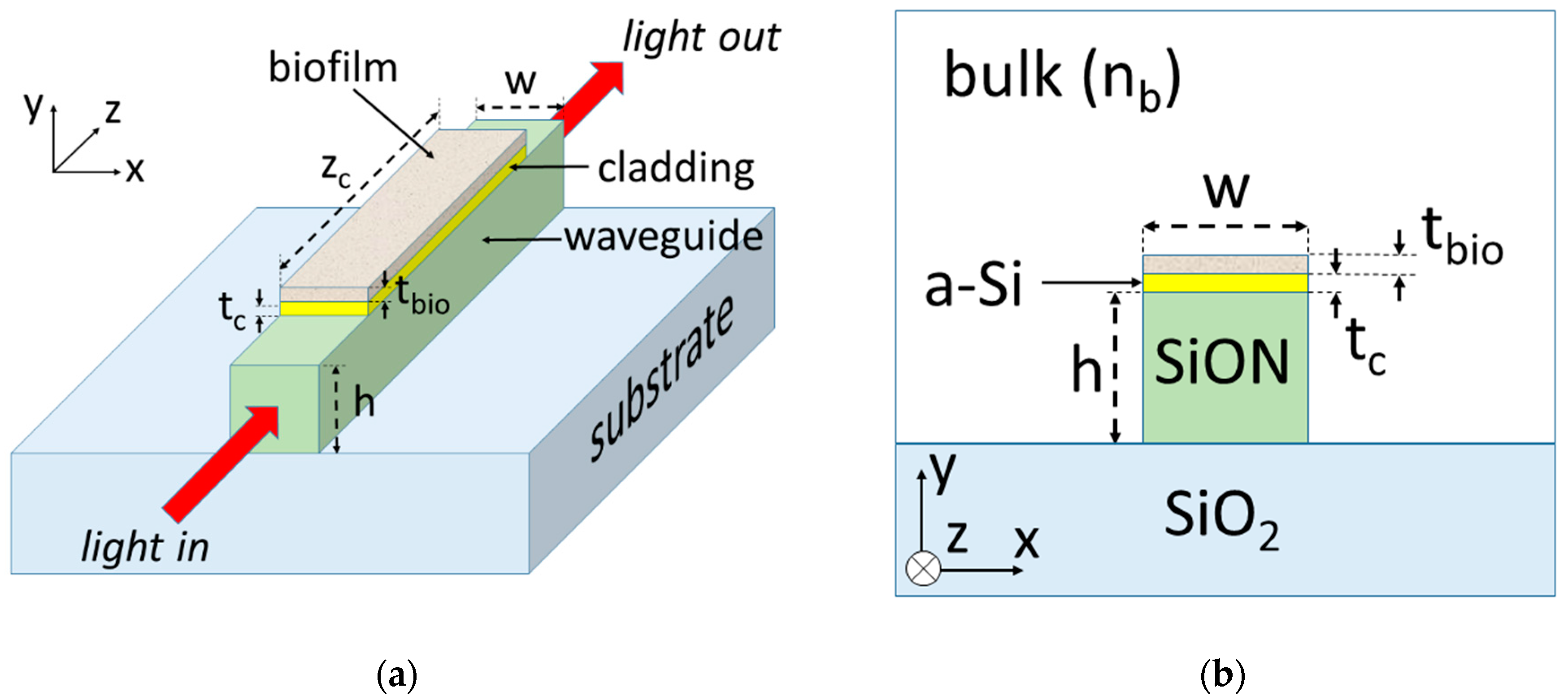
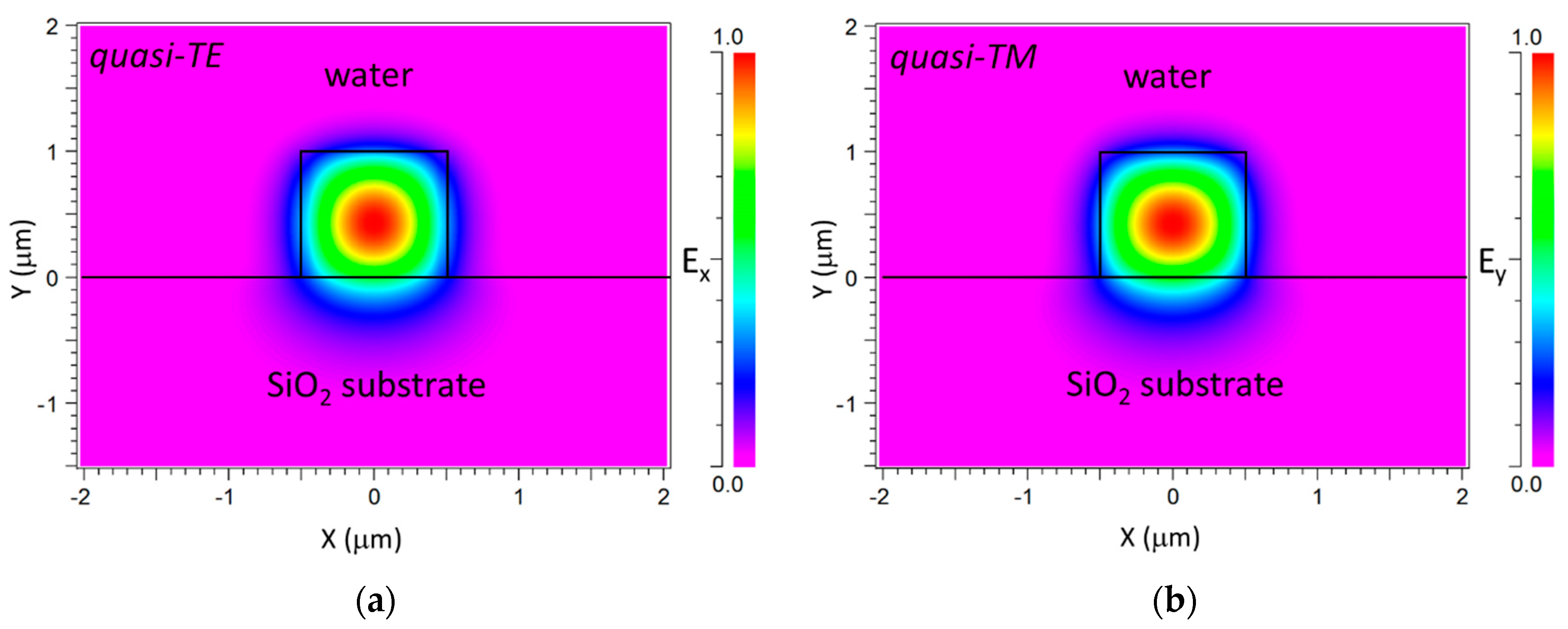
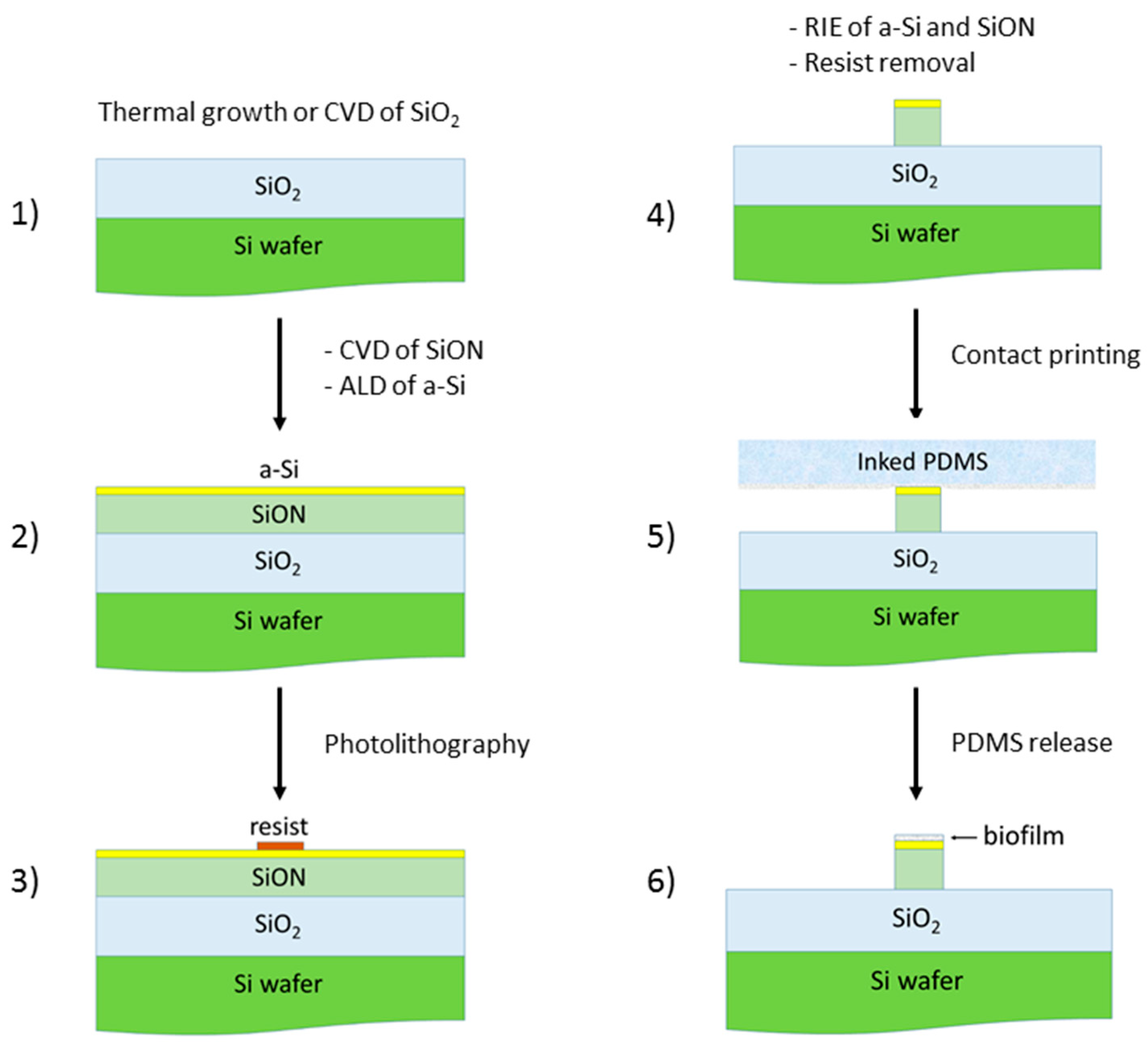
2. Device Configuration and Modeling
3. Results
3.1. Bulk Sensitivity
3.2. Biofilm Sensing
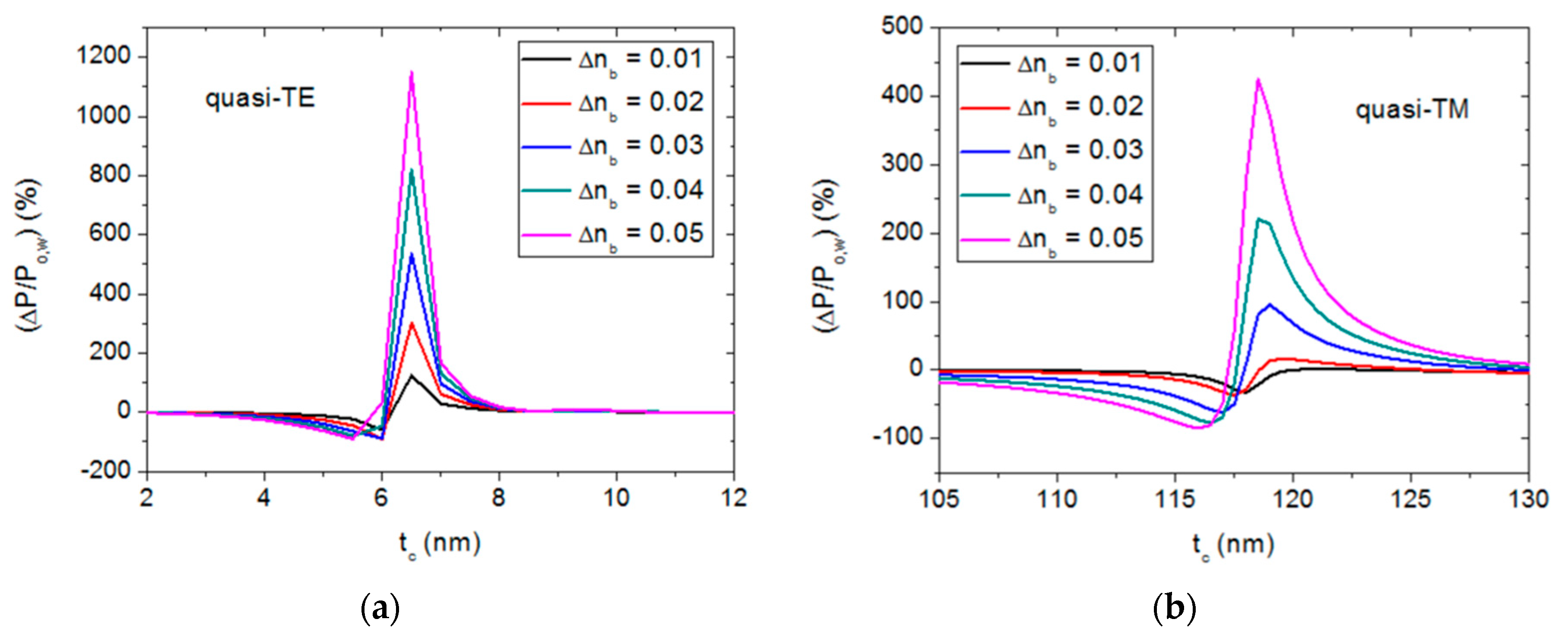
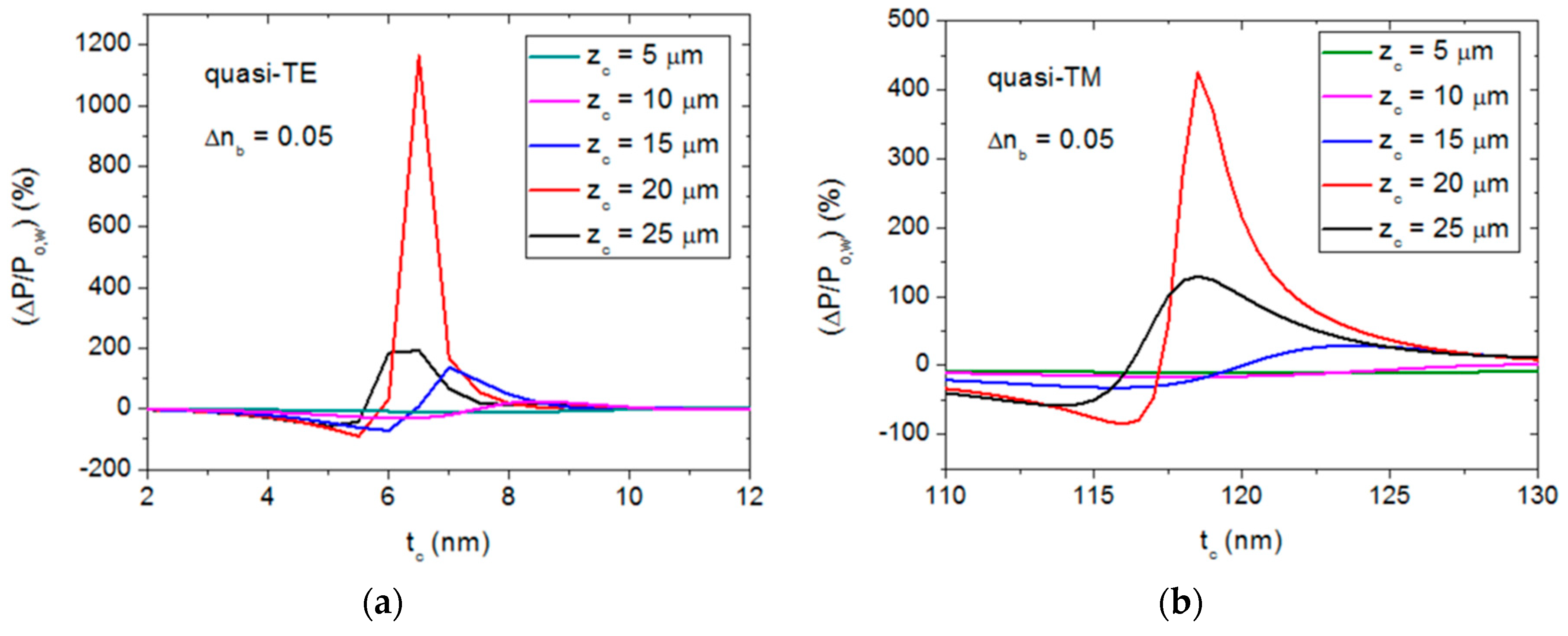
3.3. Material and Dimension Tolerance
4. Discussion
Funding
Conflicts of Interest
References
- Zinoviev, K.; Carrascosa, L.G.; del Río, J.S.; Sepúlveda, B.; Domínguez, C.; Lechuga, L.M. Silicon photonic biosensors for lab-on-a-chip applications. Adv. Opt. Technol. 2008, 2008, 1–6. [Google Scholar] [CrossRef]
- Washburn, A.L.; Bailey, R.C. Photonics-on-a-chip: Recent advances in integrated waveguides as enabling detection elements for real-world, lab-on-a-chip biosensing applications. Analyst 2011, 136, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Gavela, A.F.; García, D.G.; Ramirez, J.C.; Lechuga, L.M. Last advances in silicon-based optical biosensors. Sensors 2016, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef] [PubMed]
- Heideman, R.G.; Kooyman, R.P.H.; Greve, J. Performance of a highly sensitive optical waveguide Mach-Zehnder interferometer immunosensor. Sens. Actuators B 1993, 10, 209–217. [Google Scholar] [CrossRef]
- Weisser, M.; Tovar, G.; Mittler-Neher, S.; Knoll, W.; Brosinger, F.; Freimuth, H.; Lacher, M.; Ehrfeld, W. Specific bio-recognition reactions observed with an integrated Mach–Zehnder interferometer. Biosens. Bioelectron. 1999, 14, 405–411. [Google Scholar] [CrossRef]
- Schipper, E.; Brugman, A.; Dominguez, C.; Lechuga, L.; Kooyman, R.; Greve, J. The realization of an integrated Mach–Zehnder waveguide immunosensor in silicon technology. Sens. Actuators B Chem. 1997, 40, 147–153. [Google Scholar] [CrossRef]
- Liu, Q.; Tu, X.; Kim, K.W.; Kee, J.S.; Shin, Y.; Han, K.; Yoon, Y.; Lo, G.; Park, M.K. Highly sensitive Mach-Zehnder interferometer biosensor based on silicon nitride slot waveguide. Sens. Actuators B Chem. 2013, 188, 681–688. [Google Scholar] [CrossRef]
- Brandenburg, A. Differential refractometry by an integrated-optical Young interferometer. Sens. Actuators B Chem. 1997, 39, 266–271. [Google Scholar] [CrossRef]
- Brandenburg, A.; Krauter, R.; Künzel, C.; Stefan, M.; Schulte, H. Interferometric sensor for detection of surface-bound bioreactions. Appl. Opt. 2000, 39, 6396–6405. [Google Scholar] [CrossRef]
- Zinoviev, K.E.; González-Guerrero, A.B.; Domínguez, C.; Lechuga, L.M. Integrated bimodal waveguide interferometric biosensor for label-free analysis. J. Lightwave Technol. 2011, 29, 1926–1930. [Google Scholar] [CrossRef]
- Krioukov, E.; Klunder, D.J.; Driessen, A.; Greve, J.; Otto, C. Sensor based on an integrated optical microcavity. Opt. Lett. 2002, 27, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, A.; Popat, K.C.; Aldridge, J.C.; Desai, T.A.; Hryniewicz, J.; Chbouki, N.; Little, B.E.; King, O.; Van, V.; Chu, S.; et al. Optical sensing of biomolecules using microring resonators. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 148–155. [Google Scholar] [CrossRef]
- De Vos, K.; Bartolozzi, I.; Schacht, E.; Bienstman, P.; Baets, R. Silicon-on-Insulator microring resonator for sensitive and label-free biosensing. Opt. Express 2007, 15, 7610–7615. [Google Scholar] [CrossRef]
- Barrios, C.A.; Bañuls, M.J.; Gonzalez-Pedro, V.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Maquieira, A.; Sohlström, H.; Holgado, M.; Casquel, R. Label-free optical biosensing with slot waveguides. Opt. Lett. 2008, 33, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Fauchet, P.M. Two-dimensional silicon photonic crystal based biosensing platform for protein detection. Opt. Express 2007, 15, 4530–4535. [Google Scholar] [CrossRef]
- Scullion, M.G.; Falco, A.D.; Krauss, T.F. Slotted photonic crystal cavities with integrated microfluidics for biosensing applications. Biosens. Bioelectron. 2011, 27, 101–105. [Google Scholar] [CrossRef]
- Carson, R.F. Periodic Coupling in Semiconductor-Clad Dielectric Optical Guided-Wave Devices. Ph.D. Thesis, The University of Virginia, Charlottesville, VA, USA, 1985. [Google Scholar]
- Carson, R.F.; Batchman, T.E. Multimode phenomena in semiconductor-clad dielectric optical waveguide structures. Appl. Opt. 1990, 19, 2769–2780. [Google Scholar]
- Kaminow, I.P.; Mammel, W.L.; Weber, H.P. Metal-Clad Optical Waveguides: Analytical and Experimental Study. Appl. Opt. 1974, 13, 396. [Google Scholar] [CrossRef]
- Rashliegh, S.C. Planar Metal-Clad Dielectric Optical Waveguides. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, May 1975. [Google Scholar]
- Slavik, R.; Homola, J.; Ctyroky, J.; Brynda, E. Novel spectral fiber optic sensor based on surface plasmon resonance. Sens. Actuators B 2001, 74, 106–111. [Google Scholar] [CrossRef]
- Zourob, M.; Goddard, N.J. Metal clad leaky waveguides for chemical and biosensing applications. Biosens. Bioelectron. 2005, 20, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Skivesen, N.; Horvath, R.; Thinggaard, S.; Larsen, N.B.; Pedersen, H.C. Deep-probe metal-clad waveguide biosensors. Biosens. Bioelectron. 2007, 22, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Andreev, A.; Pantchev, B.; Danesh, P.; Zafirova, B.; Karakoleva, E.; Vlaikova, E.; Alipieva, E. A refractometric sensor using index-sensitive mode resonance between single-mode fiber and thin film amorphous silicon waveguide. Sens. Actuators B 2005, 106, 484–488. [Google Scholar] [CrossRef]
- Socorro, A.B.; Corres, J.M.; Del Villar, I.; Arregui, F.J.; Matias, I.R. Fiber-optic biosensor based on lossy mode resonances. Sens. Actuators B 2012, 174, 263–269. [Google Scholar] [CrossRef]
- Paliwal, N.; John, J. Lossy Mode Resonance (LMR) Based Fiber Optic Sensors: A Review. IEEE Sens. J. 2015, 15, 5367–5371. [Google Scholar] [CrossRef]
- Del Villar, I.; Arregui, F.J.; Zamarreño, C.R.; Corres, J.M.; Bariain, C.; Goicoechea, J.; Elosua, C.; Hernaez, M.; Rivero, P.J.; Socorro, A.B.; et al. Optical sensors based on lossy-mode resonances. Sens. Actuators B 2017, 240, 174–185. [Google Scholar] [CrossRef]
- Pierce, D.T.; Spicer, W.E. Electronic Structure of Amorphous Si from Photoemission and Optical Studies. Phys. Rev. B 1972, 5, 3017–3029. [Google Scholar] [CrossRef]
- Bossi, D.E.; Hammer, J.M.; Shaw, J.M. Optical properties of silicon oxynitride dielectric waveguides. Appl. Opt. 1987, 26, 609–611. [Google Scholar] [CrossRef]
- Vorös, J. The density and refractive index of adsorbing protein layers. Biophys. J. 2004, 87, 553–561. [Google Scholar] [CrossRef]
- Rsoft Photonic Device Tools. Available online: https://www.synopsys.com/photonic-solutions/rsoft-photonic-device-tools.html (accessed on 9 April 2020).
- Perera, C.; Vernon, K.; Cheng, E.; Sathian, J.; Jaatinen, E.; Davis, T. Highly compact refractive index sensor based on stripe waveguides for lab-on-a-chip sensing applications. Beilstein J. Nanotechnol. 2016, 7, 751–757. [Google Scholar] [CrossRef]
- Rodionov, S.A.; Remnev, M.A.; Klimov, V.V. Refractive index sensor based on all-dielectric gradient metasurface. Sens. Bio-Sens. Res. 2019, 22, 100263. [Google Scholar] [CrossRef]
- Takashima, Y.; Kusaba, K.; Haraguchi, M.; Naoi, Y. Highly sensitive refractive index sensor using dual resonance in subwavelength grating/waveguide with normally incident optical geometry. IEEE Sens. J. 2019, 19, 6147–6153. [Google Scholar] [CrossRef]
- Shakoor, A.; Grande, M.; Grant, J.; Cumming, D.R.S. One dimensional silicon nitride grating refractive index sensor suitable for integration with CMOS detectors. IEEE Photonics J. 2017, 9, 6800711. [Google Scholar]
- Dahlin, A.B. Plasmonic Sensors: An Integrated View of Refractometric Detection; IOS Press: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Lin, V.S.-Y.; Motesharei, K.; Dancil, K.-P.S.; Sailor, M.J.; Ghadiri, M.R. A porous silicon-based optical interferometric biosensor. Science 1997, 278, 840–843. [Google Scholar] [CrossRef]
- Imai, S.; Iizuka, T.; Sugiura, O.; Matsumura, M. Atomic layer epitaxy of Si using atomic H. Thin Solid Films 1993, 225, 168–172. [Google Scholar] [CrossRef]
- Hasunuma, E.; Sugahara, S.; Hoshino, S.; Imai, S.; Ikeda, K.; Matsumura, M. Gas-phase-reaction-controlled atomic-layer-epitaxy of silicon. J. Vac. Sci. Technol. A 1998, 16, 679–684. [Google Scholar] [CrossRef]
- Ikeda, K.; Yanase, J.; Sugahara, S.; Matsumura, M. Atomic-Layer-Epitaxy of Si. J. Korean Phys. Soc. 2001, 39, S447–S458. [Google Scholar]
- Bernard, A.; Renault, J.P.; Michel, B.; Bosshard, H.R.; Delamarche, E. Microcontact printing of proteins. Adv. Mater. 2000, 12, 1067–1070. [Google Scholar] [CrossRef]
- LaGraff, J.R.; Chu-LaGraff, Q. Scanning force microscopy and fluorescence microscopy of microcontact printed antibodies and antibody fragments. Langmuir 2006, 22, 4685–4693. [Google Scholar] [CrossRef]




| RI Sensor Configuration | Sensitivity (%/RIU) | LOD (RIU) | LOD × Lsens (RIU mm) | Footprint (μm2) |
|---|---|---|---|---|
| Metasurface [34] | 4450 | (2 × 10−4) b | N/A c | N/A c |
| Grating/waveguide [35] | 1306 | (7.6 × 10−4) b | N/A c | N/A c |
| 1-D grating [36] | 1700 | (5 × 10−4) b | N/A c | N/A c |
| M–Z interferometer [8] | 5.4 × 10−6 | 3.8 × 10−5 | 3.4 × 106 | |
| Bimodal waveguide [11] | 2.5 × 10−7 | 3.7 × 10−6 | 4.5 × 106 | |
| This work | 12,372 a | (8 × 10−5) b | 1.6 × 10−6 | 20 |
| Biosensor Configuration | LOD (ng/cm2) | LOD × Lsens (ng cm−2 mm) | Footprint (μm2) |
|---|---|---|---|
| M–Z interferometer [6] | 2 | 24 | 3 × 106 |
| M–Z interferometer [8] | 0.22 | 1.54 | 3.4 × 106 |
| Young interferometer [10] | 0.075 | 0.9 | 1.8 × 107 |
| This work | 3 a | 0.06 | 20 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo Barrios, C. An Analysis of a Compact Label-Free Guiding-Wave Biosensor Based on a Semiconductor-Clad Dielectric Strip Waveguide. Sensors 2020, 20, 3368. https://doi.org/10.3390/s20123368
Angulo Barrios C. An Analysis of a Compact Label-Free Guiding-Wave Biosensor Based on a Semiconductor-Clad Dielectric Strip Waveguide. Sensors. 2020; 20(12):3368. https://doi.org/10.3390/s20123368
Chicago/Turabian StyleAngulo Barrios, Carlos. 2020. "An Analysis of a Compact Label-Free Guiding-Wave Biosensor Based on a Semiconductor-Clad Dielectric Strip Waveguide" Sensors 20, no. 12: 3368. https://doi.org/10.3390/s20123368
APA StyleAngulo Barrios, C. (2020). An Analysis of a Compact Label-Free Guiding-Wave Biosensor Based on a Semiconductor-Clad Dielectric Strip Waveguide. Sensors, 20(12), 3368. https://doi.org/10.3390/s20123368





