A Multichannel Pattern-Recognition-Based Protein Sensor with a Fluorophore-Conjugated Single-Stranded DNA Set
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedures
2.3. Fluorescence Spectroscopy Measurements
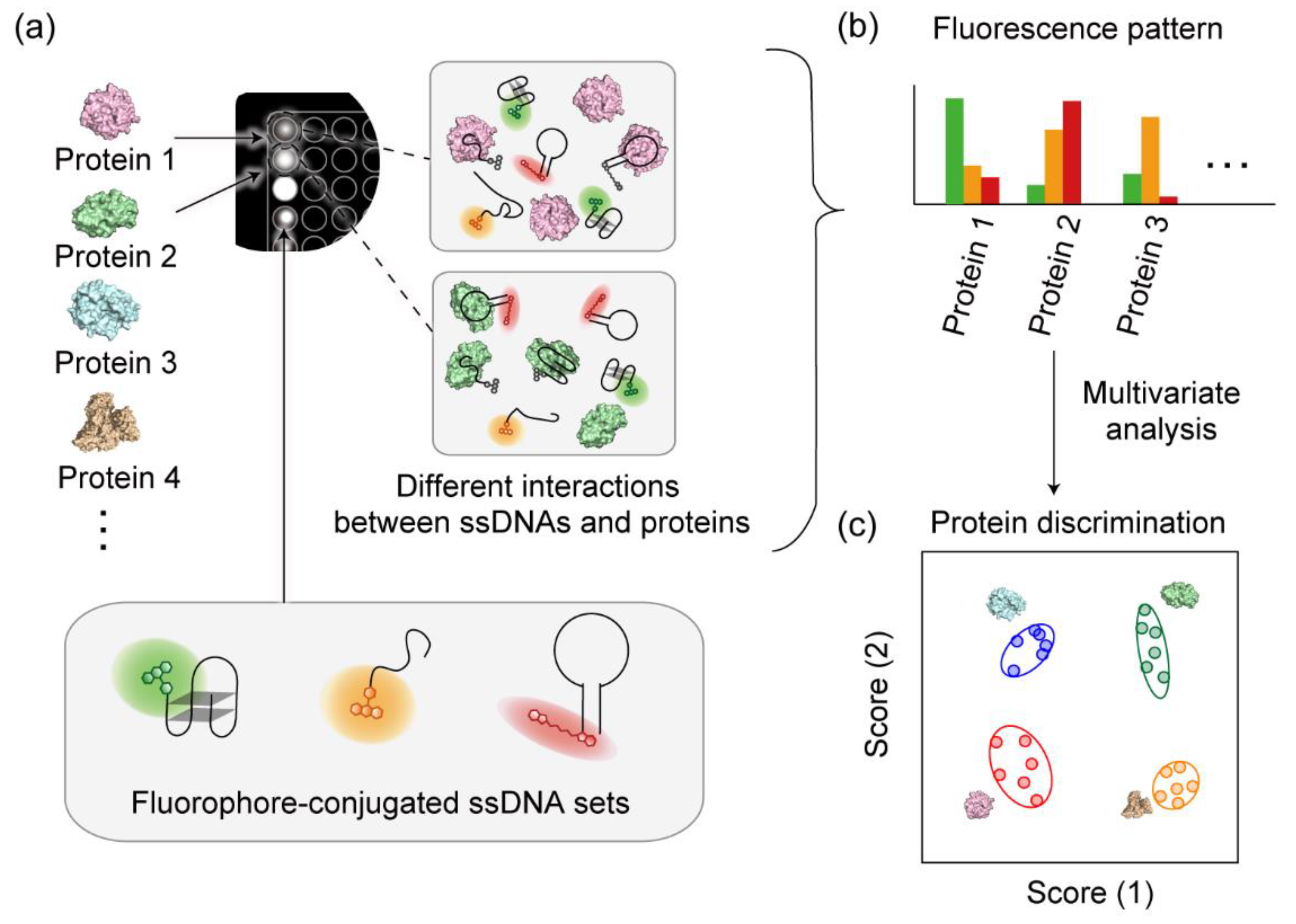
2.4. Pattern-Recognition-Based Sensing and Statistical Analysis
3. Results and Discussion
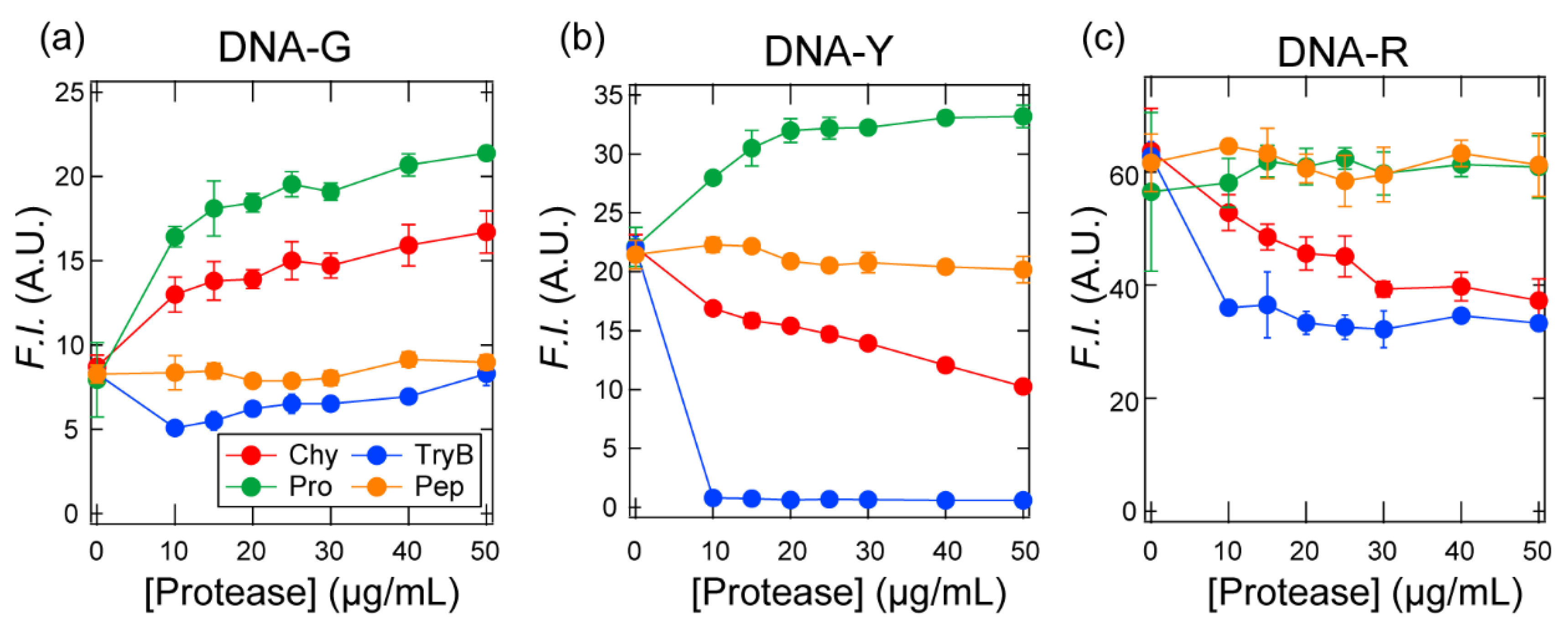
3.1. Design and Characterization of the Fluorophore-Conjugated ssDNA Set
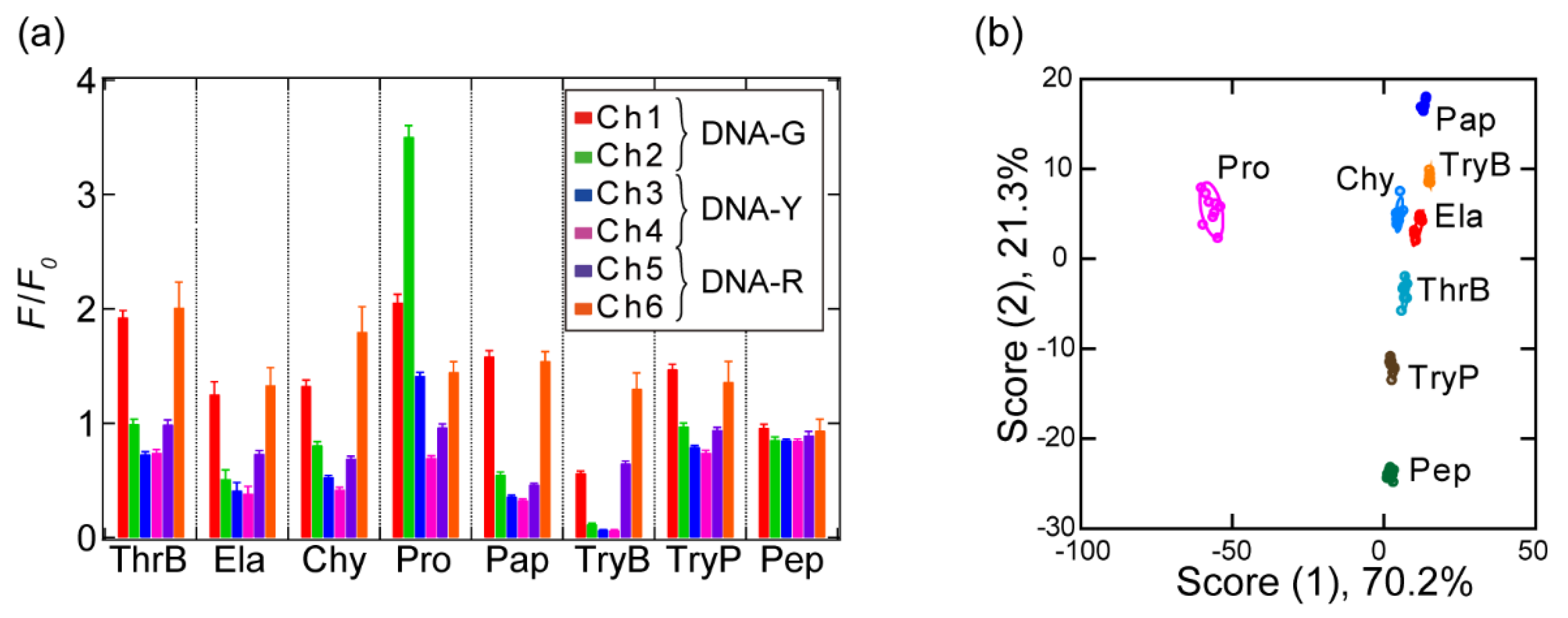
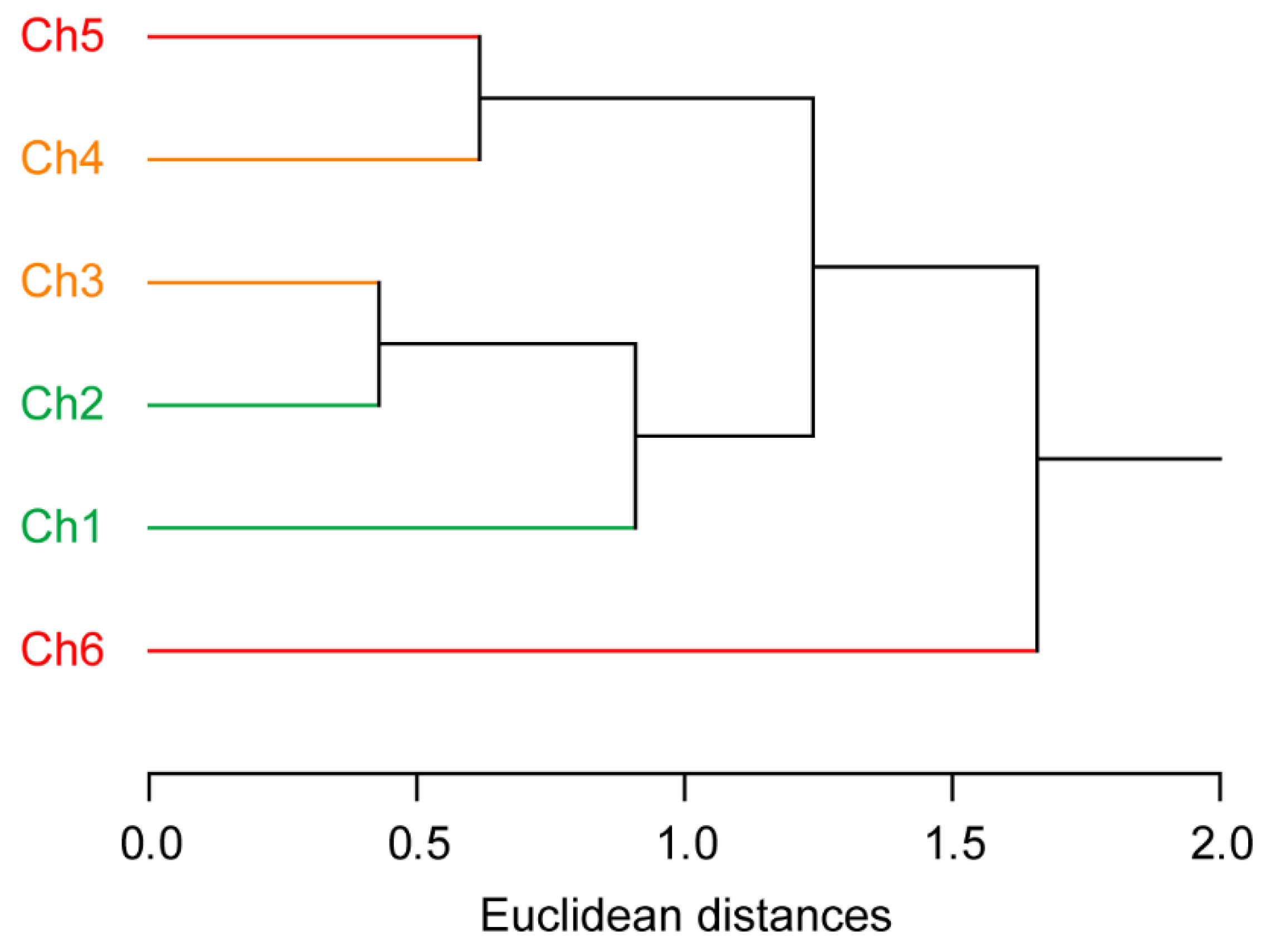
3.2. Pattern-Recognition-Based Protease Sensing Using a Multichannel ssDNA Sensor
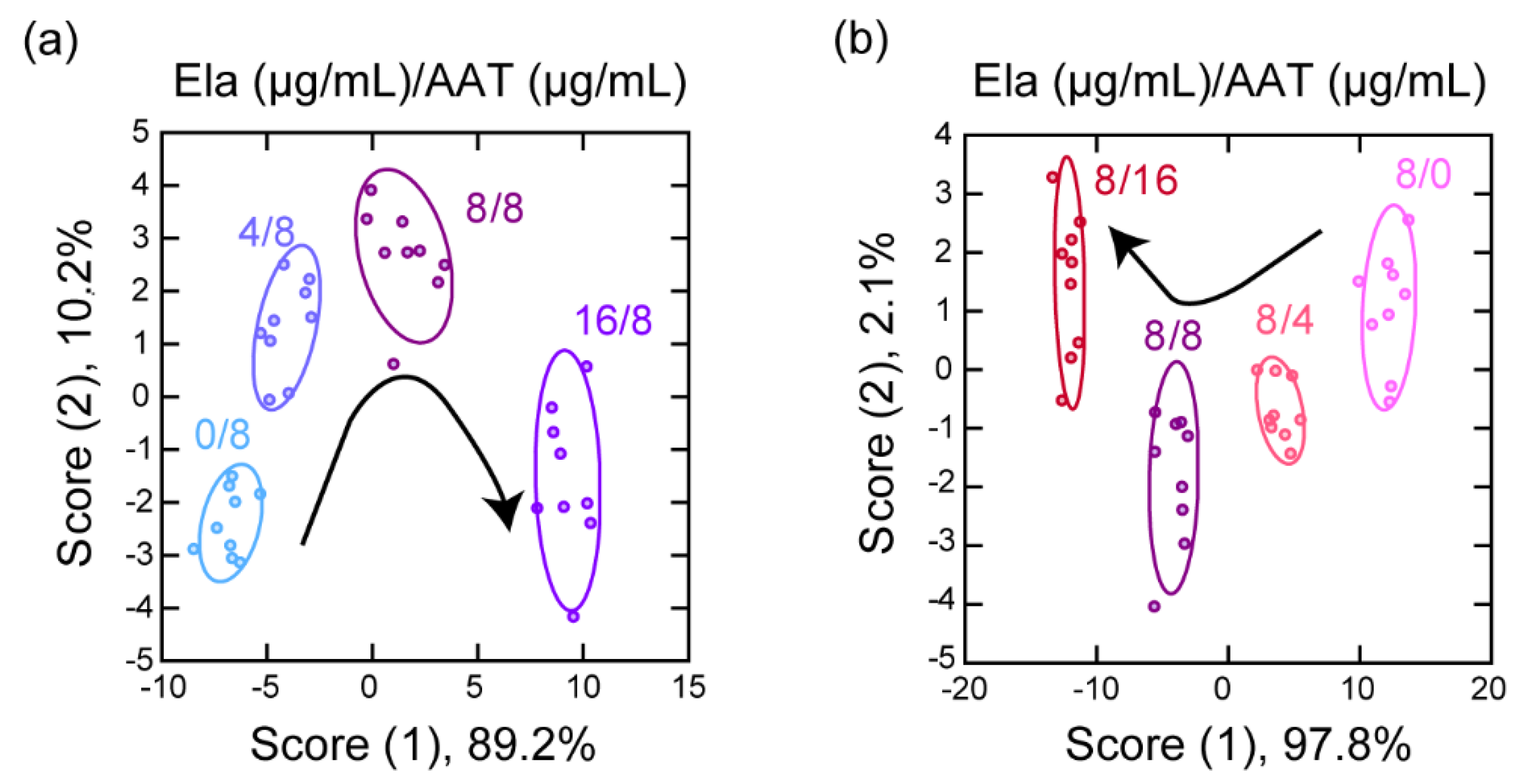
3.3. Detection of a Protease and Inhibitor Mixture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease detection with molecular biomarkers: From chemistry of body fluids to nature-inspired chemical sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Solier, C.; Langen, H. Antibody-based proteomics and biomarker research—Current status and limitations. Proteomics 2014, 14, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Yan, J.; Cai, S.; He, Q.; Peng, D.; Liu, Z.; Liu, Y. Cancer protein biomarker discovery based on nucleic acid aptamers. Int. J. Biol. Macromol. 2019, 132, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Hamachi, I. Protein recognition using synthetic small-molecular binders toward optical protein sensing in vitro and in live cells. Chem. Soc. Rev. 2015, 44, 4454–4471. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Akkerdaas, J.H.; Van Ree, R. Cross-reactivity of IgE antibodies to allergens. Allergy 2001, 56, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Fineman, M.S.; Mace, K.F.; Diamant, M.; Darsow, T.; Cirincione, B.B.; Booker Porter, T.K.; Kinninger, L.A.; Trautmann, M.E. Clinical relevance of anti-exenatide antibodies: Safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes. Metab. 2012, 14, 546–554. [Google Scholar] [CrossRef]
- Manimala, J.C.; Roach, T.A.; Li, Z.; Gildersleeve, J.C. High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology 2007, 17, 17C–23C. [Google Scholar] [CrossRef] [PubMed]
- Sugai, H.; Tomita, S.; Kurita, R. Pattern-recognition-based sensor arrays for cell characterization: From materials and data analyses to biomedical applications. Anal. Sci. 2020, 36, 923–934. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Peveler, W.J.; Rotello, V.M. Array-based “chemical nose” sensing in diagnostics and drug discovery. Angew. Chem. Int. Ed. 2019, 58, 5190–5200. [Google Scholar] [CrossRef]
- Freudenberg, J.; Hinkel, F.; Jänsch, D.; Bunz, U.H.F. Chemical tongues and noses based upon conjugated polymers. Top. Curr. Chem. 2017, 375, 67. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Ishihara, S.; Kurita, R. Biomimicry recognition of proteins and cells using a small array of block copolymers appended with amino acids and fluorophores. ACS Appl. Mater. Interfaces 2019, 11, 6751–6758. [Google Scholar] [CrossRef] [PubMed]
- Sugai, H.; Tomita, S.; Ishihara, S.; Kurita, R. Fingerprint-based protein identification in cell culture medium using environment-sensitive turn-on fluorescent polymer. Sens. Mater. 2019, 31, 1–11. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Golmohammadi, H.; Abbasi-Moayed, S.; Nejad, M.A.F.; Fahimi-Kashani, N.; Jafarinejad, S.; Shahrajabian, M.; Hormozi-Nezhad, M.R. Nanoparticle-based optical sensor arrays. Nanoscale 2017, 9, 16546–16563. [Google Scholar] [CrossRef]
- Xu, S.; Su, Z.; Zhang, Z.; Nie, Y.; Wang, J.; Ge, G.; Luo, X. Rapid synthesis of nitrogen doped carbon dots and their application as a label free sensor array for simultaneous discrimination of multiple proteins. J. Mater. Chem. B 2017, 5, 8748–8753. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Y.; Sun, X.; Wang, Z.; Luo, X. A multicoloured Au NCs based cross-reactive sensor array for discrimination of multiple proteins. J. Mater. Chem. B 2017, 5, 4207–4213. [Google Scholar] [CrossRef]
- Rana, S.; Le, N.D.B.; Mout, R.; Saha, K.; Tonga, G.Y.; Bain, R.E.S.; Miranda, O.R.; Rotello, C.M.; Rotello, V.M. A multichannel nanosensor for instantaneous readout of cancer drug mechanisms. Nat. Nanotechnol. 2015, 10, 65–69. [Google Scholar] [CrossRef]
- Rana, S.; Le, N.D.B.; Mout, R.; Duncan, B.; Elci, S.G.; Saha, K.; Rotello, V.M. A multichannel biosensor for rapid determination of cell surface glycomic signatures. ACS Cent. Sci. 2015, 1, 191–197. [Google Scholar] [CrossRef]
- Rout, B.; Unger, L.; Armony, G.; Iron, M.A.; Margulies, D. Medication detection by a combinatorial fluorescent molecular sensor. Angew. Chem. Int. Ed. 2012, 51, 12477–12481. [Google Scholar] [CrossRef]
- Rout, B.; Milko, P.; Iron, M.A.; Motiei, L.; Margulies, D. Authorizing multiple chemical passwords by a combinatorial molecular keypad lock. J. Am. Chem. Soc. 2013, 135, 15330–15333. [Google Scholar] [CrossRef]
- Hatai, J.; Motiei, L.; Margulies, D. Analyzing amyloid beta aggregates with a combinatorial fluorescent molecular sensor. J. Am. Chem. Soc. 2017, 139, 2136–2139. [Google Scholar] [CrossRef] [PubMed]
- Pode, Z.; Peri-Naor, R.; Georgeson, J.M.; Georgeson, T.; Kiss, V.; Unger, T.; Markus, B.; Barr, H.M.; Motiei, L.; Margulies, D. Protein recognition with a pattern-generating fluorescent molecular probe. Nat. Nanotechnol. 2017, 12, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Peveler, W.J.; Roldan, A.; Hollingsworth, N.; Porter, M.J.; Parkin, I.P. Multichannel detection and differentiation of explosives with a quantum dot array. ACS Nano 2016, 10, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; He, H.; Yang, H.; Wei, W. Simultaneous quadruple-channel optical transduction of a nanosensor for multiplexed qualitative and quantitative analysis of lectins. Chem. Commun. 2018, 54, 7754–7757. [Google Scholar] [CrossRef] [PubMed]
- Peveler, W.J.; Landis, R.F.; Yazdani, M.; Day, J.W.; Modi, R.; Carmalt, C.J.; Rosenberg, W.M.; Rotello, V.M. A rapid and robust diagnostic for liver fibrosis using a multichannel polymer sensor array. Adv. Mater. 2018, 30, 1800634–1800639. [Google Scholar] [CrossRef] [PubMed]
- Le, N.D.B.; Singla, A.K.; Geng, Y.; Han, J.; Seehafer, K.; Prakash, G.; Moyano, D.F.; Downey, C.M.; Monument, M.J.; Itani, D.; et al. Simple and robust polymer-based sensor for rapid cancer detection using serum. Chem. Commun. 2019, 55, 11458–11461. [Google Scholar] [CrossRef] [PubMed]
- Ngernpimai, S.; Geng, Y.; Makabenta, J.M.; Landis, R.F.; Keshri, P.; Gupta, A.; Li, C.H.; Chompoosor, A.; Rotello, V.M. Rapid identification of biofilms using a robust multichannel polymer sensor array. ACS Appl. Mater. Interfaces 2019, 11, 11202–11208. [Google Scholar] [CrossRef]
- Saenger, W. Protein-nucleic acid interaction. In Principles of Nucleic Acid Structure; Charles, R.C., Ed.; Springer: New York, NY, USA, 1984; pp. 385–391. [Google Scholar]
- Sun, W.; Lu, Y.; Mao, J.; Chang, N.; Yang, J.; Liu, Y. Multidimensional sensor for pattern recognition of proteins based on DNA–gold nanoparticles conjugates. Anal. Chem. 2015, 87, 3354–3359. [Google Scholar] [CrossRef]
- Tan, L.; Chen, Z.; Zhao, Y.; Wei, X.; Li, Y.; Zhang, C.; Wei, X.; Hu, X. Dual channel sensor for detection and discrimination of heavy metal ions based on colorimetric and fluorescence response of the AuNPs-DNA conjugates. Biosens. Bioelectron. 2016, 85, 414–421. [Google Scholar] [CrossRef]
- Das Saha, N.; Sasmal, R.; Meethal, S.K.; Vats, S.; Gopinathan, P.V.; Jash, O.; Manjithaya, R.; Gagey-Eilstein, N.; Agasti, S.S. Multichannel DNA sensor array fingerprints cell states and identifies pharmacological effectors of catabolic processes. ACS Sens. 2019, 4, 3124–3132. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Comprehensive screen of metal oxide nanoparticles for DNA adsorption, fluorescence quenching, and anion discrimination. ACS Appl. Mater. Interfaces 2015, 7, 24833–24838. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Liu, J. DNA-functionalized nanoceria for probing oxidation of phosphorus compounds. Langmuir 2018, 34, 15871–15877. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, J.; Lv, M.; Wang, J.; Gao, J.; Lu, J.; Li, Y.; Huang, Q.; Hu, J.; Fan, C. A graphene-based sensor array for high-precision and adaptive target identification with ensemble aptamers. J. Am. Chem. Soc. 2012, 134, 13843–13849. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, W.; Wang, Y.; Yang, X.; Wang, K.; Wang, Q.; Wang, P.; Chang, Y.; Tan, Y. Discrimination of hemoglobins with subtle differences using an aptamer based sensing array. Chem. Commun. 2015, 51, 8304–8306. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Ishihara, S.; Kurita, R. A multi-fluorescent DNA/graphene oxide conjugate sensor for signature-based protein discrimination. Sensors 2017, 17, 2194. [Google Scholar] [CrossRef]
- Tomita, S.; Matsuda, A.; Nishinami, S.; Kurita, R.; Shiraki, K. One-step identification of antibody degradation pathways using fluorescence signatures generated by cross-reactive DNA-based arrays. Anal. Chem. 2017, 89, 7818–7822. [Google Scholar] [CrossRef]
- Hizir, M.S.; Robertson, N.M.; Balcioglu, M.; Alp, E.; Rana, M.; Yigit, M.V. Universal sensor array for highly selective system identification using two-dimensional nanoparticles. Chem. Sci. 2017, 8, 5735–5745. [Google Scholar] [CrossRef]
- Ran, X.; Pu, F.; Wang, Z.; Ren, J.; Qu, X. DNA-MnO2 nanosheets as washing- and label-free platform for array-based differentiation of cell types. Anal. Chim. Acta 2019, 1056, 1–6. [Google Scholar] [CrossRef]
- Tomita, S.; Sugai, H.; Mimura, M.; Ishihara, S.; Shiraki, K.; Kurita, R. Optical fingerprints of proteases and their inhibited complexes provided by differential cross-reactivity of fluorophore-labeled single-stranded DNA. ACS Appl. Mater. Interfaces 2019, 11, 47428–47436. [Google Scholar] [CrossRef]
- Lienqueo, M.E.; Mahn, A.; Asenjo, J.A. Mathematical correlations for predicting protein retention times in hydrophobic interaction chromatography. J. Chromatogr. A 2002, 978, 71–79. [Google Scholar] [CrossRef]
- Fraczkiewicz, R.; Braun, W. Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J. Comput. Chem. 1998, 19, 319–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Görner, H. Photoprocesses of xanthene dyes bound to lysozyme or serum albumin. Photochem. Photobiol. 2009, 85, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ahsan, S.S.; Santiago-Berrios, M.B.; Abruña, H.D.; Webb, W.W. Mechanisms of quenching of alexa fluorophores by natural amino acids. J. Am. Chem. Soc. 2010, 132, 7244–7245. [Google Scholar] [CrossRef] [PubMed]
- Marmé, N.; Knemeyer, J.-P.; Sauer, M.; Wolfrum, J. Inter- and intramolecular fluorescence quenching of organic dyes by tryptophan. Bioconjug. Chem. 2003, 14, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Verdoes, M.; Verhelst, S.H.L. Detection of protease activity in cells and animals. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, P. Role of imbalance between neutrophil elastase and α1-antitrypsin in cancer development and progression. Lancet Oncol. 2004, 5, 182–190. [Google Scholar] [CrossRef]
- Sato, T.; Takahashi, S.; Mizumoto, T.; Harao, M.; Akizuki, M.; Takasugi, M.; Fukutomi, T.; Yamashita, J. Neutrophil elastase and cancer. Surg. Oncol. 2006, 15, 217–222. [Google Scholar] [CrossRef]
- McElvaney, N.G. Alpha-1 antitrypsin therapy in cystic fibrosis and the lung disease associated with alpha-1 antitrypsin deficiency. Ann. Am. Thorac. Soc. 2016, 13, S191–S196. [Google Scholar]
- Li, Z.; Suslick, K.S. A hand-held optoelectronic nose for the identification of liquors. ACS Sens. 2018, 3, 121–127. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Zhang, J.; Huo, D.; Hou, C.; Zhou, J.; Luo, H. Metal ions regulated Ag NPRs etching colorimetric sensor array for discrimination of Chinese Baijiu. Sens. Actuators B Chem. 2019, 297, 126715. [Google Scholar] [CrossRef]
- Wu, S.; Han, Y.; Wang, L.; Li, J.; Sun, Z.; Zhang, M.; Liu, P.; Li, G. Sensor array fabricated with nanoscale metal-organic frameworks for the histopathological examination of colon cancer. Anal. Chem. 2019, 91, 10772–10778. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Olivares, D.; Kaoud, T.S.; Jose, J.; Ellington, A.; Dalby, K.N.; Anslyn, E.V. Differential sensing of MAP kinases using SOX-peptides. Angew. Chem. Int. Ed. 2014, 53, 14064–14068. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence (5′ to 3′) | Structure |
|---|---|---|
| DNA-G | GGTTGGTGTGGTTGG-FAM | G-quadruplex |
| DNA-Y | TTTTTTTTTTTTTTTTTT-TAMRA | Simple repeated |
| DNA-R | Cy5-ACGGCATGGTGGGCGTCGT | Stem-loop |
| Analyte | Abbreviation | Source | Molecular Weight (×103) | Isoelectric Point | Φsurface |
|---|---|---|---|---|---|
| Protease | |||||
| Pepsin | Pep | Porcine stomach mucosa | 35 | 3.2 | 0.308 |
| Thrombin | ThrB | Bovine plasma | 38 | 7.1 | 0.293 |
| Elastase | Ela | Porcine pancreas | 26 | 8.5 | 0.325 |
| α-Chymotrypsin | Chy | Bovine pancreas | 25 | 8.8 | 0.285 |
| Proteinase K | Pro | Tritirachium album | 29 | 8.9 | 0.287 |
| Papain | Pap | Papaya latex | 23 | 9.6 | 0.307 |
| Trypsin | TryB | Bovine pancreas | 24 | 10.1 | 0.290 |
| Trypsin | TryP | Porcine pancreas | 23 | 10.2 | 0.312 |
| Protease inhibitor | |||||
| α1-Antitrypsin | AAT | Human plasma | 44 | 5.4 | 0.263 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, M.; Sugai, H.; Tomita, S.; Kurita, R. A Multichannel Pattern-Recognition-Based Protein Sensor with a Fluorophore-Conjugated Single-Stranded DNA Set. Sensors 2020, 20, 5110. https://doi.org/10.3390/s20185110
Okada M, Sugai H, Tomita S, Kurita R. A Multichannel Pattern-Recognition-Based Protein Sensor with a Fluorophore-Conjugated Single-Stranded DNA Set. Sensors. 2020; 20(18):5110. https://doi.org/10.3390/s20185110
Chicago/Turabian StyleOkada, Mari, Hiroka Sugai, Shunsuke Tomita, and Ryoji Kurita. 2020. "A Multichannel Pattern-Recognition-Based Protein Sensor with a Fluorophore-Conjugated Single-Stranded DNA Set" Sensors 20, no. 18: 5110. https://doi.org/10.3390/s20185110
APA StyleOkada, M., Sugai, H., Tomita, S., & Kurita, R. (2020). A Multichannel Pattern-Recognition-Based Protein Sensor with a Fluorophore-Conjugated Single-Stranded DNA Set. Sensors, 20(18), 5110. https://doi.org/10.3390/s20185110






