Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys
Abstract
1. Introduction
2. Animal Models for Dengue Virus Infection
3. Non-Human Primates
4. Viremia Kinetics in Non-Human Primates
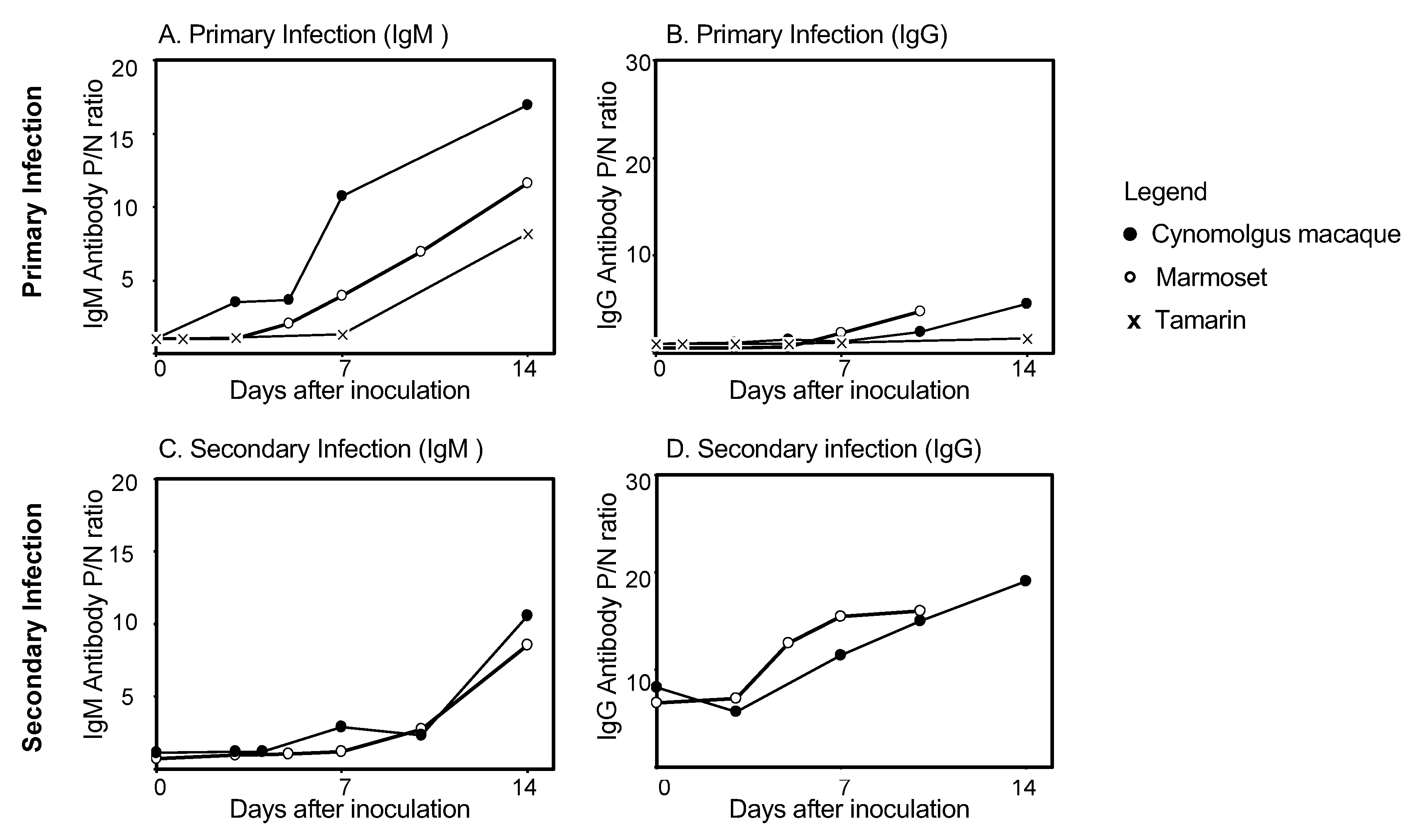
5. Antibody Responses in Non-Human Primates
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- WHO Dengue: Guidelines for diagnosis, treatment, prevention and control. Prev. Control 2009, 1.
- Zompi, S.; Harris, E. Animal models of dengue virus infection. Viruses 2012, 4, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Farrar, J.J.; van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Satoshi, K.; Yasuyuki, K.; Meng Ling, M.; Akira, K.; Masayuki, O.; Koh, S.; Tetsuro, K.; Kei, Y.; Yoshihiro, F.; Momoko, M.; et al. Autochthonous Dengue Fever, Tokyo, Japan, 2014. Emerg. Infect. Dis. J. 2015, 21, 517. [Google Scholar]
- Gjenero-Margan, I.; Aleraj, B.; Krajcar, D.; Lesnikar, V.; Klobučar, A.; Pem-Novosel, I.; Kurečić-Filipović, S.; Komparak, S.; Martić, R.; Duričić, S.; et al. Autochthonous dengue fever in Croatia, August- September 2010. Eurosurveillance 2011, 16, 19805. [Google Scholar]
- Succo, T.; Leparc-Goffart, I.; Ferre, J.-B.; Roiz, D.; Broche, B.; Maquart, M.; Noel, H.; Catelinois, O.; Entezam, F.; Caire, D.; et al. Autochthonous dengue outbreak in Nimes, South of France, July to September 2015. Eurosurveillance 2016, 21, 30240. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2017, 16, 712–723. [Google Scholar] [CrossRef]
- Twiddy, S.S.; Farrar, J.J.; Vinh Chau, N.; Wills, B.; Gould, E.A.; Gritsun, T.; Lloyd, G.; Holmes, E.C. Phylogenetic relationships and differential selection pressures among genotypes of dengue-2 virus. Virology 2002, 298, 63–72. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Montoya, M.; Gresh, L.; Balmaseda, A.; Harris, E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc. Natl. Acad. Sci. USA 2016, 113, 728–733. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Moi, M.L.; Ami, Y.; Muhammad Azami, N.A.; Shirai, K.; Yoksan, S.; Suzaki, Y.; Kitaura, K.; Lim, C.-K.; Saijo, M.; Suzuki, R.; et al. Marmosets (Callithrix jacchus) as a non-human primate model for evaluation of candidate dengue vaccines: Induction and maintenance of specific protective immunity against challenges with clinical isolates. J. Gen. Virol. 2017, 98, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Moi, M.L.; Takasaki, T.; Omatsu, T.; Nakamura, S.; Katakai, Y.; Ami, Y.; Suzaki, Y.; Saijo, M.; Akari, H.; Kurane, I.; et al. Demonstration of marmosets (Callithrix jacchus) as a non-human primate model for secondary dengue virus infection: High levels of viraemia and serotype cross-reactive antibody responses consistent with secondary infection of humans. J. Gen. Virol. 2014, 95, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Ip, P.-P.; Liao, F. Resistance to Dengue Virus Infection in Mice Is Potentiated by CXCL10 and Is Independent of CXCL10-Mediated Leukocyte Recruitment. J. Immunol. 2010, 184, 5705–5714. [Google Scholar] [CrossRef] [PubMed]
- Tuiskunen, A.; Wahlström, M.; Bergström, J.; Buchy, P.; Leparc-Goffart, I.; Lundkvist, Å. Phenotypic characterization of patient dengue virus isolates in BALB/c mice differentiates dengue fever and dengue hemorrhagic fever from dengue shock syndrome. Virol. J. 2011, 8, 398. [Google Scholar] [CrossRef]
- Christofferson, R.C.; McCracken, M.K.; Johnson, A.-M.; Chisenhall, D.M.; Mores, C.N. Development of a transmission model for dengue virus. Virol. J. 2013, 10, 127. [Google Scholar] [CrossRef]
- Marchette, N.J.; Halstead, S.B.; Falkler, W.A.; Stenhouse, A.; Nash, D. Studies on the Pathogenesis of Dengue Infection in Monkeys. III. Sequential Distribution of Virus in Primary and Heterologous Infections. J. Infect. Dis. 1973, 128, 23–30. [Google Scholar] [CrossRef]
- Freire, M.S.; Marchevsky, R.S.; Almeida, L.F.C.; Yamamura, A.M.Y.; Caride, E.C.; Brindeiro, P.A.; Motta, M.C.A.; Nogueira, R.M.R.; Kubelka, C.F.; Bonaldo, M.C.; et al. Wild dengue virus types 1, 2 and 3 viremia in rhesus monkeys. Memorias do Instituto Oswaldo Cruz 2007, 102, 203–208. [Google Scholar] [CrossRef]
- Koraka, P.; Benton, S.; van Amerongen, G.; Stittelaar, K.J.; Osterhaus, A.D.M.E. Characterization of humoral and cellular immune responses in cynomolgus macaques upon primary and subsequent heterologous infections with dengue viruses. Microbes Infect. 2007, 9, 940–946. [Google Scholar] [CrossRef]
- Omatsu, T.; Moi, M.L.; Takasaki, T.; Nakamura, S.; Katakai, Y.; Tajima, S.; Ito, M.; Yoshida, T.; Saito, A.; Akari, H.; et al. Changes in hematological and serum biochemical parameters in common marmosets (Callithrix jacchus) after inoculation with dengue virus. J. Med. Primatol 2012, 41, 289–296. [Google Scholar] [CrossRef]
- Omatsu, T.; Moi, M.L.; Hirayama, T.; Takasaki, T.; Nakamura, S.; Tajima, S.; Ito, M.; Yoshida, T.; Saito, A.; Katakai, Y.; et al. Common marmoset (Callithrix jacchus) as a primate model of dengue virus infection: Development of high levels of viraemia and demonstration of protective immunity. J. Gen. Virol. 2011, 92, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.K.; Watanabe, S.; Kavishna, R.; Alonso, S.; Vasudevan, S.G. Animal models for studying dengue pathogenesis and therapy. Antiviral Res. 2015, 123, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, A.; Konishi, E. A simple method for evaluating dengue vaccine effectiveness in mice based on levels of viremia caused by intraperitoneal injection of infected culture cells. Vaccine 2009, 27, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; de Queiroz Prado, R.; Almeida Xavier, E.; Cristina de Oliveira, N.; da Matta Guedes, P.M.; da Silva, J.S.; Moraes Figueiredo, L.T.; Aquino, V.H. Imunocompetent Mice Model for Dengue Virus Infection. Sci. World J. 2012, 2012, 525947. [Google Scholar] [CrossRef]
- Talarico, L.B.; Batalle, J.P.; Byrne, A.B.; Brahamian, J.M.; Ferretti, A.; García, A.G.; Mauri, A.; Simonetto, C.; Hijano, D.R.; Lawrence, A.; et al. The Role of Heterotypic DENV-specific CD8+T Lymphocytes in an Immunocompetent Mouse Model of Secondary Dengue Virus Infection. EBioMedicine 2017, 20, 202–216. [Google Scholar] [CrossRef]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification. PLOS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef]
- Fuchs, J.; Chu, H.; O’Day, P.; Pyles, R.; Bourne, N.; Das, S.C.; Milligan, G.N.; Barrett, A.D.T.; Partidos, C.D.; Osorio, J.E. Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice. Vaccine 2014, 32, 6537–6543. [Google Scholar] [CrossRef]
- Brewoo, J.N.; Kinney, R.M.; Powell, T.D.; Arguello, J.J.; Silengo, S.J.; Partidos, C.D.; Huang, C.Y.-H.; Stinchcomb, D.T.; Osorio, J.E. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax). Vaccine 2012, 30, 1513–1520. [Google Scholar] [CrossRef]
- Sarathy, V.V.; White, M.; Li, L.; Kaiser, J.A.; Campbell, G.A.; Milligan, G.N.; Bourne, N.; Barrett, A.D.T. Characterization of a murine model of non-lethal, symptomatic dengue virus infection. Sci. Rep. 2018, 8, 4900. [Google Scholar] [CrossRef]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A Protective Role for Dengue Virus-Specific CD8(+) T Cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Shresta, S. Mouse models of dengue virus infection and disease. Antiviral Res. 2008, 80, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Orozco, S.; Schmid, M.A.; Parameswaran, P.; Lachica, R.; Henn, M.R.; Beatty, R.; Harris, E. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J. Gen. Virol. 2012, 93, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Rico-Hesse, R. Humanized Mice Show Clinical Signs of Dengue Fever according to Infecting Virus Genotype. J. Virol. 2009, 83, 8638–8645. [Google Scholar] [CrossRef]
- Bente, D.A.; Melkus, M.W.; Garcia, J.V.; Rico-Hesse, R. Dengue Fever in Humanized NOD/SCID Mice. J. Virol. 2005, 79, 13797–13799. [Google Scholar] [CrossRef]
- Watanabe, Y.; Takahashi, T.; Okajima, A.; Shiokawa, M.; Ishii, N.; Katano, I.; Ito, R.; Ito, M.; Minegishi, M.; Minegishi, N.; et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/γcnull (NOG) mice (hu-HSC NOG mice). Int. Immunol. 2009, 21, 843–858. [Google Scholar] [CrossRef]
- Akkina, R. New generation humanized mice for virus research: Comparative aspects and future prospects. Virology 2013, 435, 14–28. [Google Scholar] [CrossRef]
- Cox, J.; Mota, J.; Sukupolvi-Petty, S.; Diamond, M.S.; Rico-Hesse, R. Mosquito Bite Delivery of Dengue Virus Enhances Immunogenicity and Pathogenesis in Humanized Mice. J. Virol. 2012, 86, 7637–7649. [Google Scholar] [CrossRef]
- Kuruvilla, J.G.; Troyer, R.M.; Devi, S.; Akkina, R. Dengue virus infection and immune response in humanized RAG2-/-γc -/- (RAG-hu) mice. Virology 2007, 369, 143–152. [Google Scholar] [CrossRef]
- Frias-Staheli, N.; Dorner, M.; Marukian, S.; Billerbeck, E.; Labitt, R.N.; Rice, C.M.; Ploss, A. Utility of Humanized BLT Mice for Analysis of Dengue Virus Infection and Antiviral Drug Testing. J. Virol. 2014, 88, 2205–2218. [Google Scholar] [CrossRef]
- Choi, B.; Chun, E.; Kim, M.; Kim, S.Y.; Kim, S.-T.; Yoon, K.; Lee, K.-Y.; Kim, S.J. Human T cell development in the liver of humanized NOD/SCID/IL-2Rγnull (NSG) mice generated by intrahepatic injection of CD34+ human (h) cord blood (CB) cells. Clin. Immunol. 2011, 139, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Bente, D.A.; Rico-Hesse, R. Models of dengue virus infection. Drug Discov. Today. Dis. Models 2006, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Falzaran, D.; Bente, D.A. Animal models for viral haemorrhagic fever. Clin. Microbiol. Infect. 2015. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Onlamoon, N.; Hsiao, H.-M.; Perng, G.C.; Villinger, F.; Adachi, A.; Suzuki, Y. Can non-human primates serve as models for investigating dengue disease pathogenesis? Front. Microbiol. 2013, 4, 305. [Google Scholar] [CrossRef]
- Onlamoon, N.; Noisakran, S.; Hsiao, H.-M.; Duncan, A.; Villinger, F.; Ansari, A.A.; Perng, G.C. Dengue virus–induced hemorrhage in a nonhuman primate model. Blood 2010, 115, 1823–1834. [Google Scholar] [CrossRef]
- Cassetti, M.C.; Durbin, A.; Harris, E.; Rico-Hesse, R.; Roehrig, J.; Rothman, A.; Whitehead, S.; Natarajan, R.; Laughlin, C. Report of an NIAID workshop on dengue animal models. Vaccine 2010, 28, 4229–4234. [Google Scholar] [CrossRef]
- Kato, F.; Ishida, Y.; Kawakami, A.; Takasaki, T.; Saijo, M.; Miura, T.; Hishiki, T. Evaluation of Macaca radiata as a non-human primate model of Dengue virus infection. Sci. Rep. 2018, 8, 3421. [Google Scholar] [CrossRef]
- Valdé, S.I.; Zaro, G.L.; Castro, J.; Odoyo, D.N.; Hitler, R.; Munene, E.; Romero, Y.; Ochola, L.; Cosme, K.; Kariuki, T.; et al. Olive baboons: A non-human primate model for testing dengue virus type 2 replication. Int. J. Infect. Dis. 2013, 17, e1176–e1181. [Google Scholar] [CrossRef]
- Estes, J.D.; Wong, S.W.; Brenchley, J.M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 2018, 18, 390–404. [Google Scholar] [CrossRef]
- Ferreira, M.S.; de Castro, P.H.G.; Silva, G.A.; Casseb, S.M.M.; Dias Júnior, A.G.; Rodrigues, S.G.; Azevedo, R.d.S.d.S.; Silva, M.F.C.e.; Zauli, D.A.G.; Araújo, M.S.S.; et al. Callithrix penicillata: A feasible experimental model for dengue virus infection. Immunol. Lett. 2014, 158, 126–133. [Google Scholar] [CrossRef]
- Durbin, A.P.; Whitehead, S.S. The Dengue Human Challenge Model: Has the Time Come to Accept This Challenge? J. Infect. Dis. 2012, 207, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Eckels, K.H.; Putnak, J.R.; Lyons, A.G.; Thomas, S.J.; Vaughn, D.W.; Gibbons, R.V.; Fernandez, S.; Gunther, V.J.; Mammen, M.P., Jr.; et al. Experimental Dengue Virus Challenge of Human Subjects Previously Vaccinated With Live Attenuated Tetravalent Dengue Vaccines. J. Infect. Dis. 2012, 207, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Gunther, V.J.; Putnak, R.; Eckels, K.H.; Mammen, M.P.; Scherer, J.M.; Lyons, A.; Sztein, M.B.; Sun, W. A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine 2011, 29, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Cassetti, M.C.; Thomas, S.J. Dengue Human Infection Model: Introduction. J. Infect. Dis. 2014, 209, S37–S39. [Google Scholar] [CrossRef][Green Version]
- Mammen, M.P.; Lyons, A.; Innis, B.L.; Sun, W.; McKinney, D.; Chung, R.C.Y.; Eckels, K.H.; Putnak, R.; Kanesa-thasan, N.; Scherer, J.M.; et al. Evaluation of Dengue Virus strains for human challenge studies. Vaccine 2014, 32, 1488–1494. [Google Scholar] [CrossRef]
- Whitehorn, J.; Van, V.C.N.; Simmons, C.P. Dengue Human Infection Models Supporting Drug Development. J. Infect. Dis. 2014, 209, S66–S70. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. Ethical issues associated with vector-borne diseases. In Proceedings of the WHO scoping meeting, Geneva, switzerland, 23–24 February 2017. [Google Scholar]
- Lin, Y.L.; Liao, C.L.; Chen, L.K.; Yeh, C.T.; Liu, C.I.; Ma, S.H.; Huang, Y.Y.; Huang, Y.L.; Kao, C.L.; King, C.C. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J. Virol. 1998, 72, 9729–9737. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Yan, Y.-S.; Weng, Y.-W.; Huang, H.-L.; Li, S.-Q.; He, S.; Zhang, J.-M. High-level expression of recombinant dengue virus type 2 envelope domain III protein and induction of neutralizing antibodies in BALB/C mice. J. Virol. Methods 2007, 143, 125–131. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Shresta, S. Mouse models to study dengue virus immunology and pathogenesis. Front. Immunol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Huang, K.-J.; Li, S.-Y.J.; Chen, S.-C.; Liu, H.-S.; Lin, Y.-S.; Yeh, T.-M.; Liu, C.-C.; Lei, H.-Y. Manifestation of thrombocytopenia in dengue-2-virus-infected mice. J. Gen. Virol. 2000, 81, 2177–2182. [Google Scholar] [CrossRef]
- Cui, L.; Hou, J.; Fang, J.; Lee, Y.H.; Costa, V.V.; Wong, L.H.; Chen, Q.; Ooi, E.E.; Tannenbaum, S.R.; Chen, J.; et al. Serum Metabolomics Investigation of Humanized Mouse Model of Dengue Virus Infection. J. Virol. 2017, 91, e00386-17. [Google Scholar] [CrossRef] [PubMed]
- Sariol, C.A.; White, L.J. Utility, Limitations, and Future of Non-Human Primates for Dengue Research and Vaccine Development. Front. Immunol. 2014, 5, 452. [Google Scholar] [CrossRef]
- Lavinder, C.H.; Francis, E. The Etiology of Dengue. An Attempt to Produce the Disease in the Rhesus Monkey by the Inoculation of Defibrinated Blood. J. Infect. Dis. 1914, 15, 341–346. [Google Scholar] [CrossRef]
- Widjaja, S.; Winoto, I.; Sturgis, J.; Maroef, C.N.; Listiyaningsih, E.; Tan, R.; Pamungkas, J.; Blair, P.J.; Sajuthi, D.; Porter, K.R. Macaca nemestrina and dengue virus infectivity: A potential model for evaluating dengue vaccine candidates. Microbiol. Indones. 2010, 4, 1. [Google Scholar] [CrossRef]
- Yoshida, T.; Omatsu, T.; Saito, A.; Katakai, Y.; Iwasaki, Y.; Kurosawa, T.; Hamano, M.; Higashino, A.; Nakamura, S.; Takasaki, T.; et al. Dynamics of cellular immune responses in the acute phase of dengue virus infection. Arch. Virol. 2013, 158, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Whitehead, S.S.; Durbin, A.P. Dengue human infection models to advance dengue vaccine development. Vaccine 2015, 33, 7075–7082. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Whitehead, S.S.; Pierce, K.K.; Tibery, C.M.; Grier, P.L.; Hynes, N.A.; Larsson, C.J.; Sabundayo, B.P.; Talaat, K.R.; Janiak, A.; et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016, 8, 330ra36. [Google Scholar] [CrossRef]
- Bravo, J.R.; Guzmán, M.G.; Kouri, G.P. Why dengue haemorrhagic fever in Cuba? I. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 816–820. [Google Scholar] [CrossRef]
- Voevodin, A.F.; Marx, P.A. Classification of Nonhuman Primates. Simian Virol. 2009, 1–38. [Google Scholar] [CrossRef]
- Glazko, G.V.; Nei, M. Estimation of Divergence Times for Major Lineages of Primate Species. Mol. Biol. Evol. 2003, 20, 424–434. [Google Scholar] [CrossRef]
- Disotell, T.R. The phylogeny of Old World monkeys. Evol. Anthropol. Issues News Rev. 1996, 5, 18–24. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Rylands, A.B.; Wilson, D.E. The mammals of the world, part 3: Primates. Barcelona Lynx Edicions 2013. [Google Scholar]
- Liedigk, R.; Kolleck, J.; Böker, K.O.; Meijaard, E.; Md-Zain, B.M.; Abdul-Latiff, M.A.B.; Ampeng, A.; Lakim, M.; Abdul-Patah, P.; Tosi, A.J.; et al. Mitogenomic phylogeny of the common long-tailed macaque (Macaca fascicularis fascicularis). BMC Genomics 2015, 16, 222. [Google Scholar] [CrossRef]
- Van Esch, E.; Cline, J.M.; Buse, E.; Wood, C.E.; De Rijk, E.P.C.T.; Weinbauer, G.F. Summary comparison of female reproductive system in human and the cynomolgus monkey (Macaca fascicularis). Toxicol. Pathol. 2008, 36, 171S–172S. [Google Scholar] [CrossRef]
- Drevon-Gaillot, E.; Perron-Lepage, M.-F.; Clément, C.; Burnett, R.; Perron, M.-F.; Drevon-Gaillot, E.; Clément, C.; Porret-Blanc, G.; Burnett, R. A review of background findings in Cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Toxicol. Lett. 2006, 58, S307. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.H.; Barnett, D.K.; Colman, R.J.; Yamamoto, M.E.; Schultz-Darken, N.J. Aspects of Common Marmoset Basic Biology and Life History Important for Biomedical Research. Comp. Med. 2003, 53, 339–350. [Google Scholar] [PubMed]
- Carrion, R.; Patterson, J.L. An animal model that reflects human disease: The common marmoset (Callithrix jacchus). Curr. Opin. Virol. 2012, 2, 357–362. [Google Scholar] [CrossRef]
- Abbott, D.H.; Hearn, J.P. Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J. Reprod. Fertil. 1978, 53, 155–166. [Google Scholar] [CrossRef]
- Mansfield, K. Marmoset Models Commonly Used in Biomedical Research. Comp. Med. 2003, 53, 383–392. [Google Scholar]
- Fujii, Y.; Kitaura, K.; Matsutani, T.; Shirai, K.; Suzuki, S.; Takasaki, T.; Kumagai, K.; Kametani, Y.; Shiina, T.; Takabayashi, S.; et al. Immune-Related Gene Expression Profile in Laboratory Common Marmosets Assessed by an Accurate Quantitative Real-Time PCR Using Selected Reference Genes. PLoS ONE 2013, 8, e56296. [Google Scholar] [CrossRef]
- Ando, K.; Maeda, J.; Inaji, M.; Okauchi, T.; Obayashi, S.; Higuchi, M.; Suhara, T.; Tanioka, Y. Neurobehavioral protection by single dose l-deprenyl against MPTP-induced parkinsonism in common marmosets. Psychopharmacology 2008, 195, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Bright, H.; Carroll, A.R.; Watts, P.A.; Fenton, R.J. Development of a GB virus B marmoset model and its validation with a novel series of hepatitis C virus NS3 protease inhibitors. J. Virol. 2004, 78, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.P.; Aronson, J.F.; Tardif, S.D.; Patterson, J.L.; Brasky, K.M.; Geiger, R.; de la Garza, M.; Carrion, R.; Weaver, S.C. Common marmosets (Callithrix jacchus) as a nonhuman primate model to assess the virulence of eastern equine encephalitis virus strains. J. Virol. 2008, 82, 9035–9042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sussman, R.W.; Kinzey, W.G. The ecological role of the callitrichidae: A review. Am. J. Phys. Anthropol. 1984, 64, 419–449. [Google Scholar] [CrossRef]
- Takikawa, S.; Engle, R.E.; Faulk, K.N.; Emerson, S.U.; Purcell, R.H.; Bukh, J. Molecular evolution of GB virus B hepatitis virus during acute resolving and persistent infections in experimentally infected tamarins. J. Gen. Virol. 2010, 91, 727–733. [Google Scholar] [CrossRef]
- Wood, J.D.; Peck, O.C.; Tefend, K.S.; Stonerook, M.J.; Caniano, D.A.; Mutabagani, K.H.; Lhotak, S.; Sharma, H.M. Evidence that colitis is initiated by environmental stress and sustained by fecal factors in the cotton-top tamarin (Saguinus oedipus). Dig. Dis. Sci. 2000, 45, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lackner, A.A.; Carville, A.; Xia, D.; MacKey, J.; Lin, K.-C.; Mansfield, K.G.; Schauer, D.B.; Newman, J. V Enteropathogenic Escherichia coli and Ulcerative Colitis in Cotton-Top Tamarins (Saguinus oedipus). J. Infect. Dis. 2001, 184, 803–807. [Google Scholar]
- Hofmann, P.; Kahnt, K.; Matz-Rensing, K.; Brack, M.; Kaup, F.J. Three spontaneous lymphomas in a colony of cotton-top tamarins (Saguinus oedipus). J. Med. Primatol. 2001, 30, 322–327. [Google Scholar] [CrossRef]
- Lushbaugh, C.C.; Humason, G.L.; Swartzendruber, D.C.; Richter, C.B.; Gengozian, N. Spontaneous colonic adenocarcinoma in marmosets. Primates Med. 1978, 10, 119–134. [Google Scholar]
- Tobi, M.; Kim, M.; Zimmer, R.; Hatfield, J.; Kam, M.; Khoury, N.; Carville, A.; Lawson, M.J.; Schiemann, W.P.; Thomas, P. Colorectal cancer in the cotton top tamarin (Saguinus oedipus): How do they evade liver metastasis? Dig. Dis. Sci. 2011, 56, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue Viremia Titer, Antibody Response Pattern, and Virus Serotype Correlate with Disease Severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Yang, C.; Hsieh, S.; Juan, C.; Chang, S.; Yu, C.; Lin, L.; Huang, J.; King, C. Slower Rates of Clearance of Viral Load and Virus-Containing Immune Complexes in Patients with Dengue Hemorrhagic Fever. Clin. Infect. Dis. 2006, 43, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Endy, T.P.; Nisalak, A.; Chunsuttitwat, S.; Vaughn, D.W.; Green, S.; Ennis, F.A.; Rothman, A.L.; Libraty, D.H. Relationship of Preexisting Dengue Virus (DV) Neutralizing Antibody Levels to Viremia and Severity of Disease in a Prospective Cohort Study of DV Infection in Thailand. J. Infect. Dis. 2004, 189, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Guilarde, A.O.; Turchi, M.D.; Jr, J.B.S.; Feres, V.C.R.; Rocha, B.; Levi, J.E.; Souza, V.A.U.F.; Boas, L.S.V.; Pannuti, C.S.; Martelli, C.M.T. Dengue and Dengue Hemorrhagic Fever among Adults: Clinical Outcomes Related to Viremia, Serotypes, and Antibody Response. J. Infect. Dis. 2008, 197, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Endy, T.P. Critical issues in dengue vaccine development. Curr. Opin. Infect. Dis. 2011, 24, 442–450. [Google Scholar] [CrossRef]
- Ito, M.; Katakai, Y.; Ono, F.; Akari, H.; Mukai, R.; Takasaki, T.; Kotaki, A.; Kurane, I. Serotype-specific and cross-reactive neutralizing antibody responses in cynomolgus monkeys after infection with multiple dengue virus serotypes. Arch. Virol. 2011, 156, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Takasaki, T.; Yamada, K.; Nerome, R.; Tajima, S.; Kurane, I. Development and evaluation of fluorogenic TaqMan reverse transcriptase PCR assays for detection of dengue virus types 1 to 4. J. Clin. Microbiol 2004, 42, 5935–5937. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Omatsu, T.; Saito, A.; Katakai, Y.; Iwasaki, Y.; Iijima, S.; Kurosawa, T.; Hamano, M.; Nakamura, S.; Takasaki, T.; et al. CD16+ natural killer cells play a limited role against primary dengue virus infection in tamarins. Arch. Virol. 2012, 157, 363–368. [Google Scholar] [CrossRef]
- Koraka, P.; Benton, S.; van Amerongen, G.; Stittelaar, K.J.; Osterhaus, A.D.M.E. Efficacy of a live attenuated tetravalent candidate dengue vaccine in naïve and previously infected cynomolgus macaques. Vaccine 2007, 25, 5409–5416. [Google Scholar] [CrossRef]
- Bernardo, L.; Izquierdo, A.; Prado, I.; Rosario, D.; Alvarez, M.; Santana, E.; Castro, J.; Martínez, R.; Rodríguez, R.; Morier, L.; et al. Primary and Secondary Infections of Macaca fascicularis Monkeys with Asian and American Genotypes of Dengue Virus 2. Clin. Vaccine Immunol. 2008, 15, 439–446. [Google Scholar] [CrossRef][Green Version]
- Moi, M.L.; Omatsu, T.; Hirayama, T.; Nakamura, S.; Katakai, Y.; Yoshida, T.; Saito, A.; Tajima, S.; Ito, M.; Takasaki, T. Presence of viral genome in urine and development of hematuria and pathological changes in kidneys in common marmoset (Callithrix jacchus) after inoculation with dengue virus. Pathogens 2013, 2, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Siler, J.F.; Hall, M.W.; Hitchens, A.P. Dengue: Its History, Epidemiology, Mechanism of Transmission, Etiology, Clinical Manifestations, Immunity, and Prevention. Philipp. J. Sci 1926, 29, 1–304. [Google Scholar]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Rothman, A.L.; Ennis, F.A.; Nisalak, A. Dengue in the early febrile phase: Viremia and antibody responses. J. Infect. Dis. 1997, 176, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Rosen, L. A Simple Technique for Demonstrating Transmission of Dengue Virus by Mosquitoes without the Use of Vertebrate Hosts*. Am. J. Trop. Med. Hyg. 1976, 25, 146–150. [Google Scholar] [CrossRef]
- Osorio, J.E.; Brewoo, J.N.; Silengo, S.J.; Arguello, J.; Moldovan, I.R.; Tary-Lehmann, M.; Powell, T.D.; Livengood, J.A.; Kinney, R.M.; Huang, C.Y.-H.; et al. Efficacy of a Tetravalent Chimeric Dengue Vaccine (DENVax) in Cynomolgus Macaques. Am. J. Trop. Med. Hyg. 2011, 84, 978–987. [Google Scholar] [CrossRef]
- Ito, M.; Mukai, R.; Takasaki, T.; Kotaki, A.; Kurane, I. Antibody-dependent enhancement of dengue virus infection in vitro by undiluted sera from monkeys infected with heterotypic dengue virus. Arch. Virol. 2010, 155, 1617–1624. [Google Scholar] [CrossRef]
- Moi, M.L.; Takasaki, T.; Kurane, I. Human antibody response to dengue virus: Implications for dengue vaccine design. Trop. Med. Health 2016, 44, 1. [Google Scholar] [CrossRef]
- Azami, N.A.M.; Moi, M.L.; Ami, Y.; Suzaki, Y.; Lim, C.-K.; Taniguchi, S.; Saijo, M.; Takasaki, T.; Kurane, I. Genotype-specific and cross-reactive neutralizing antibodies induced by dengue virus infection: Detection of antibodies with different levels of neutralizing activities against homologous and heterologous genotypes of dengue virus type 2 in common marmose. Virol. J. 2018, 15, 51. [Google Scholar] [CrossRef]
- Imrie, A.; Meeks, J.; Gurary, A.; Sukhbaatar, M.; Truong, T.T.; Cropp, C.B.; Effler, P. Antibody to dengue 1 detected more than 60 years after infection. Viral. Immunol. 2007, 20, 672–675. [Google Scholar] [CrossRef]
| Type of Animal Model | Benefits of Use This Model | Limitations | References |
|---|---|---|---|
| Immunocompetent mice (C57BL/6 mice, BALB/c mice) |
|
| [22,23,24,25] |
| Interferon alpha/beta/gamma receptor knock-out mice) (AG129 mice) |
|
| [26,27,28,29,30] |
| IFN -/- mice (IFNAR-/- mice) |
|
| [31,32,33] |
| Humanized mice (hu-NSG mice, NOD/SCID mice, NOD-scidIL2Rγnull mice, RAG2-/-γc-/-mice, BLT-NOD/SCID mice) |
|
| [34,35,36,37,38,39,40,41] |
| Non-human primates (rhesus macaque, bonnet monkey, olive baboons, African green monkey) |
|
| [42,43,44,45,46,47,48,49,50] |
| Dengue human infection model (DHIM) |
|
| [51,52,53,54,55,56,57] |
| Type of Infection | Animal ID | Inoculated Virus | Dengue Viral RNA Copy Numbers (log10 Genome copies/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after Inoculation | |||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 7 | 10 | 14 | |||
| Primary | (A) Cynomolgus macaques | ||||||||||
| Group 1 | |||||||||||
| CM1 | DENV1 01-27 | NT | NT | NT | 2.8 | NT | - | - | - | - | |
| CM2 | NT | NT | NT | - | NT | - | - | - | - | ||
| Group 2 | |||||||||||
| CM3 | DENV2 DHF0663 | NT | NT | NT | 6.5 | NT | - | - | - | - | |
| CM4 | NT | NT | NT | 3.1 | NT | - | - | - | - | ||
| CM5 | NT | NT | NT | 4.1 | NT | - | - | - | - | ||
| CM6 | NT | NT | NT | 7.2 | NT | - | - | - | - | ||
| Group 3 | |||||||||||
| CM7 | DENV3 DSS1403 | NT | NT | NT | 2.5 | NT | - | - | - | - | |
| CM8 | NT | NT | NT | 3.6 | NT | - | - | - | - | ||
| (B) Marmosets 1 | |||||||||||
| Group 4 | |||||||||||
| M1 | DENV1 02-17 | - | NT | NT | 5.6 | NT | 5.7 | - | - | - | |
| M2 | - | NT | 7.0 | NT | NT | 6.5 | 7.7 | 6.0 | - | ||
| Group 5 | |||||||||||
| M3 | DENV2 DHF0663 | - | NT | NT | 7.2 | NT | 5.0 | - | NT | - | |
| M4 | - | NT | NT | 7.5 | NT | 6.8 | 5.4 | NT | - | ||
| M5 | - | NT | 4.5 | NT | 6.0 | NT | 4.0 | NT | - | ||
| M6 | - | NT | 5.0 | NT | 6.3 | NT | 4.2 | NT | - | ||
| Group 6 | |||||||||||
| M7 | DENV3 DSS1403 | - | NT | NT | - | NT | 4.7 | - | - | - | |
| M8 | - | NT | 4.9 | NT | 5.6 | NT | - | NT | - | ||
| (C) Tamarins 1 | |||||||||||
| Group 7 | |||||||||||
| T1 | DENV2 DHF0663 | - | 6.4 | NT | 6.1 | NT | 4.2 | - | NT | NT | |
| T2 | - | 7.3 | NT | 7.5 | NT | 6.3 | 4.2 | NT | NT | ||
| Group 8 | |||||||||||
| T3 | DENV2 DHF0663 | - | 5.3 | NT | 6.2 | NT | NT | 4.5 | - | - | |
| T4 | - | 4.7 | NT | 4.6 | NT | NT | 5.4 | - | - | ||
| T5 | - | 5.3 | NT | 6.3 | NT | NT | 6.2 | - | - | ||
| Secondary | (D) Cynomolgus macaques | ||||||||||
| Group 9 | |||||||||||
| CM1 | DENV2 DHF0663 | NT | NT | NT | 6.2 | NT | - | - | - | - | |
| CM2 | NT | NT | NT | 6.2 | NT | - | - | - | - | ||
| Group 10 | |||||||||||
| CM3 | DENV3 DSS1403 | NT | NT | NT | 3.0 | NT | - | - | - | - | |
| CM4 | NT | NT | NT | 2.8 | NT | - | - | - | - | ||
| (E) Marmoset 2 | |||||||||||
| Group 11 | |||||||||||
| M9 | DENV2 DHF0663 | - | - | NT | 6.7 | NT | NT | 4.5 | NT | - | |
| M10 | - | - | NT | 6.2 | NT | NT | - | NT | - | ||
| M11 | - | - | NT | 6.4 | NT | NT | 3.9 | NT | - | ||
| Group 12 | |||||||||||
| M12 | DENV3 DSS1403 | - | - | 7.0 | NT | NT | 6.5 | 5.2 | 4.7 | - | |
| M13 | - | - | 7.5 | NT | NT | 7.7 | 6.0 | 4.2 | - | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad Azami, N.A.; Takasaki, T.; Kurane, I.; Moi, M.L. Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys. Pathogens 2020, 9, 247. https://doi.org/10.3390/pathogens9040247
Muhammad Azami NA, Takasaki T, Kurane I, Moi ML. Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys. Pathogens. 2020; 9(4):247. https://doi.org/10.3390/pathogens9040247
Chicago/Turabian StyleMuhammad Azami, Nor Azila, Tomohiko Takasaki, Ichiro Kurane, and Meng Ling Moi. 2020. "Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys" Pathogens 9, no. 4: 247. https://doi.org/10.3390/pathogens9040247
APA StyleMuhammad Azami, N. A., Takasaki, T., Kurane, I., & Moi, M. L. (2020). Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys. Pathogens, 9(4), 247. https://doi.org/10.3390/pathogens9040247






