Efficacy of Crataegus Extract Mixture on Body Fat and Lipid Profiles in Overweight Adults: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Procedures
2.4. Study Products and Interventions
2.5. Efficacy Outcome Measures
2.6. Safety Outcomes
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Participants
3.2. Primary Efficacy Outcomes
3.3. Secondary Efficacy Outcomes
3.3.1. Body Fat Measured by BIA
3.3.2. Anthropometric Parameters
3.3.3. Serum Lipid Concentrations
3.3.4. Serum Concentrations of Free Fatty Acids and Adipokines
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. The Korea National Health and Nutrition Examination Survey 2021; Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2022. [Google Scholar]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; He, C.; He, W.; Yang, M.; Luo, X.; Li, C. Obesity and Bone Health: A Complex Link. Front. Cell Dev. Biol. 2020, 8, 600181. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Hendricks, E.J. Off-label drugs for weight management. Diabetes Metab. Syndr. Obes. 2017, 10, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Prevention of Obesity and Type 2 Diabetes with Aged Citrus Peel (Chenpi) Extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef]
- Wang, T.; An, Y.; Zhao, C.; Han, L.; Boakye-Yiadom, M.; Wang, W.; Zhang, Y. Regulation effects of Crataegus pinnatifida leaf on glucose and lipids metabolism. J. Agric. Food Chem. 2011, 59, 4987–4994. [Google Scholar] [CrossRef]
- Hu, M.; Zeng, W.; Tomlinson, B. Evaluation of a crataegus-based multiherb formula for dyslipidemia: A randomized, double-blind, placebo-controlled clinical trial. Evid. Based Complement. Alternat Med. 2014, 2014, 365742. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- Kang, S.; Song, S.; Lee, J.; Chang, H.; Lee, S. Clinical Investigations of the Effect of Citrus unshiu Peel Pellet on Obesity and Lipid Profile. Evid. Based Complement. Altern. Med. 2018, 2018, 4341961. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, Y.S.; Song, M.; Lee, M.; Park, J.; Kim, H. A Herbal Formula HT048, Citrus unshiu and Crataegus pinnatifida, Prevents Obesity by Inhibiting Adipogenesis and Lipogenesis in 3T3-L1 Preadipocytes and HFD-Induced Obese Rats. Molecules 2015, 20, 9656–9670. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jin, B.; Lee, S.H.; Song, M.; Bae, H.; Min, B.J.; Park, J.; Lee, D.; Kim, H. Herbal Formula HT048 Attenuates Diet-Induced Obesity by Improving Hepatic Lipid Metabolism and Insulin Resistance in Obese Rats. Molecules 2016, 21, 1424. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Dechelotte, P.; Coeffier, M. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef] [PubMed]
- Cruz Rivera, P.N.; Goldstein, R.L.; Polak, M.; Lazzari, A.A.; Moy, M.L.; Wan, E.S. Performance of bioelectrical impedance analysis compared to dual X-ray absorptiometry (DXA) in Veterans with COPD. Sci. Rep. 2022, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Bazzocchi, A.; Ponti, F.; Albisinni, U.; Battista, G.; Guglielmi, G. DXA: Technical aspects and application. Eur. J. Radiol. 2016, 85, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Ardavani, A.; Aziz, H.; Smith, K.; Atherton, P.J.; Phillips, B.E.; Idris, I. The Effects of Very Low Energy Diets and Low Energy Diets with Exercise Training on Skeletal Muscle Mass: A Narrative Review. Adv. Ther. 2021, 38, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Hewlings, S.; Kalman, D. Body Composition Changes in Weight Loss: Strategies and Supplementation for Maintaining Lean Body Mass, a Brief Review. Nutrients 2018, 10, 1876. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Knuth, N.D.; Huizenga, R.; Rood, J.C.; Ravussin, E.; Hall, K.D. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J. Clin. Endocrinol. Metab. 2012, 97, 2489–2496. [Google Scholar] [CrossRef]
- Kim, M.; Hong, M.; Kang, Y.; Choi, Y.; Kim, S.; Kim, J.; Cho, K.; Kim, W. The Correlation between Plasma Leptin Concentration and Adiposity in Obesity. Korean J. Fam. Med. 2003, 24, 360–364. [Google Scholar]
- Shin, J.; Nam, S.; Na, S.; Kim, E.; Kim, K.; Cha, B.; Song, Y.; Lim, S.; Lee, H.; Huh, K. Serum immunoreactive-leptin concentrations and its relation to adiposity and other biochemical parameters in Korean Males. Endocrinol. Metab. 1998, 13, 216–222. [Google Scholar]
- Shim, D.; Kim, S.; Kim, S.; Choi, Y.; Jeong, U.; Lee, H.; Choi, H.; Kim, J.; Lee, S. Serum leptin concentration in diabetic and nondiabetic Koreans. J. Obes. Metab. Syndr. 1999, 8, 102–108. [Google Scholar]
- Kim, D.; Kim, N.; Shin, D.; Kim, S.; Choi, K.; Kim, J.; Shin, C.; Lee, S.; Baik, S.; Choi, D. Plasma Leptin Concentration, Obesity, and Insulin Resistance in Healthy Korean Population. Diabetes Metab. J. 2002, 26, 100–111. [Google Scholar]
- Kim, J.; Shin, H.; Jeong, I.; Cho, S.; Min, S.; Lee, S.; Park, C.; Oh, K.; Hong, E.; Kim, H.; et al. The relationship of adiponectin, leptin and ghrelin to insulin resistance and cardiovascular risk factors in human obesity. Korean J. Med. 2005, 69, 631–641. [Google Scholar]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wang, M.; Wang, S.-Y.; Zhang, J.; Du, Y.-P.; Zhao, Y.; Zheng, X.-H.; Ma, B.-P. Phenolic compounds from the leaves of Crataegus pinnatifida Bge. var. major N.E.Br. And their lipid-lowering effects. Bioorg. Med. Chem. Lett. 2021, 47, 128211. [Google Scholar] [CrossRef]
- Hu, H.; Weng, J.; Cui, C.; Tang, F.; Yu, M.; Zhou, Y.; Shao, F.; Zhu, Y. The Hypolipidemic Effect of Hawthorn Leaf Flavonoids through Modulating Lipid Metabolism and Gut Microbiota in Hyperlipidemic Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 3033311. [Google Scholar] [CrossRef]
- Zeng, S.L.; Li, S.Z.; Lai, C.J.; Wei, M.Y.; Chen, B.Z.; Li, P.; Zheng, G.D.; Liu, E.H. Evaluation of anti-lipase activity and bioactive flavonoids in the Citri Reticulatae Pericarpium from different harvest time. Phytomedicine 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Jung, H.K.; Jeong, Y.S.; Park, C.-D.; Park, C.-H.; Hong, J.-H. Inhibitory effect of citrus peel extract on lipid accumulation of 3T3-L1 adipocytes. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 169–176. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, M.-H.; Bae, C.-S.; Choi, H.J.; Ma, E.H.; Park, S.-J.; Cho, S.-S.; Park, D.-H. Unripe Citrus unshiu peel inhibited pre-adipocyte’s differentiation via leptin-PPARγ/FAS pathway and pro-inflammatory cytokines’ release. J. Funct. Foods 2023, 107, 105681. [Google Scholar] [CrossRef]
- Wu, J.; Peng, W.; Qin, R.; Zhou, H. Crataegus pinnatifida: Chemical constituents, pharmacology, and potential applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, W.; Liu, Y.; Ye, X.; Chen, K.; Li, X.; Cao, Y. The chemistry, stability and health effects of phenolic compounds in cultivated hawthorn (Crataegus pinnatifida var. major): A review. Food Qual. Saf. 2023, 7, fyad067. [Google Scholar] [CrossRef]
- Tao, W.; Deqin, Z.; Yuhong, L.; Hong, L.; Zhanbiao, L.; Chunfeng, Z.; Limin, H.; Xiumei, G. Regulation effects on abnormal glucose and lipid metabolism of TZQ-F, a new kind of Traditional Chinese Medicine. J. Ethnopharmacol. 2010, 128, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, L.; Xing, Y.; Yang, S.; Li, H.; Cao, Y. Roles and Mechanisms of Hawthorn and Its Extracts on Atherosclerosis: A Review. Front. Pharmacol. 2020, 11, 118. [Google Scholar] [CrossRef]
- Nakajima, V.M.; Macedo, G.A.; Macedo, J.A. Citrus bioactive phenolics: Role in the obesity treatment. LWT-Food Sci. Technol. 2014, 59, 1205–1212. [Google Scholar] [CrossRef]
- Lu, K.; Yip, Y.M. Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus. Future Pharmacol. 2023, 3, 14–37. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Editorial Board of Zhong Hua Ben Cao. Zhong Hua Ben Cao; Shanghai Science and Technology Press: Shanghai, China, 1999. [Google Scholar]
- Tassell, M.C.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 2010, 4, 32–41. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Yuan, X.; Ren, X.; Pan, C. Study on utilizing the natural resources of Crataegus pinnatifida leaf. Heilongjiang J. Tradit. Chin. Med. 1991, 6, 44–48. [Google Scholar]
- Park, H.; Hwang, Y.H.; Choi, J.G.; Ma, J.Y. In vitro and in vivo evaluation of systemic and genetic toxicity of Citrus unshiu peel. J. Ethnopharmacol. 2018, 215, 120–123. [Google Scholar] [CrossRef]

| Inclusion and Exclusion Criteria |
|---|
| Inclusion criteria |
| Participants who met all of the following criteria were eligible for inclusion: |
| 1. Men and women aged ≥20 and ≤60 years; |
| 2. A body mass index from 25 to <30 kg/m2; |
| 3. Participants who voluntarily decided to participate and signed the informed consent. |
| Exclusion criteria |
| Participants were excluded if any of the following criteria applied: |
| 1. A systolic blood pressure of ≥160 mmHg or diastolic blood pressure of ≥100 mmHg; hypertensive patients taking diuretics; |
| 2. A fasting blood glucose level of ≥126 mg/dL or random blood glucose level of ≥200 mg/dL; participants with diabetes mellitus taking oral hypoglycemic drugs or insulin; |
| 3. Diseases of the heart, kidney, liver, or thyroid, or cerebrovascular diseases; |
| 4. Gallbladder diseases, gastrointestinal diseases, gout, or porphyria; |
| 5. Mental Disorders such as depression, schizophrenia, alcoholism, drug addiction, etc.; |
| 6. Taking anti-obesity drugs; |
| 7. Pregnant or breastfeeding; |
| 8. Attended weight loss program or consumed diet food within the last 30 days; |
| 9. Persons unable to exercise due to severe musculoskeletal disorders; |
| 10. Diagnosed with and treated for cancer within the last 5 years; |
| 11. Asthma and other allergic diseases; |
| 12. A history of surgery within the last 6 months; |
| 13. A history of drug or alcohol addiction; |
| 14. Persons who have participated in another clinical trial within the last 3 months; |
| 15. Illiterate persons or persons with a limited ability to read; |
| 16. Persons considered to be inadequate for participation due to other reasons. |
| Characteristic | CEM 840 mg/day (n = 34) | CEM 1200 mg/day (n = 35) | Placebo (n = 36) | Total (n = 105) |
|---|---|---|---|---|
| Age (years) | 31.0 (10.4) 1 | 31.3 (9.8) | 30.8 (9.2) | 31.0 (9.7) |
| Sex | ||||
| Male | 13 (38.2) | 16 (45.7) | 13 (36.1) | 42 (40.0) |
| Female | 21 (61.8) | 19 (54.3) | 23 (63.9) | 63 (60.0) |
| Marital status | ||||
| Married | 12 (35.3) | 15 (42.9) | 17 (47.2) | 42 (40.0) |
| Single | 22 (64.7) | 20 (57.1) | 19 (52.8) | 63 (60.0) |
| Regular exercise | ||||
| Yes | 15 (44.1) | 14 (40.0) | 17 (47.2) | 46 (43.8) |
| No | 19 (55.9) | 21 (60.0) | 19 (52.8) | 59 (56.2) |
| Smoking status | ||||
| Never | 26 (76.5) | 26 (74.3) | 28 (77.8) | 80 (76.2) |
| Ex-smoker | 3 (8.8) | 2 (5.7) | 1 (2.8) | 6 (5.7) |
| Current smoker | 5 (14.7) | 7 (20.0) | 7 (19.4) | 19 (18.1) |
| Drinking status | ||||
| Never | 11 (32.4) | 15 (42.9) | 14 (38.9) | 40 (38.0) |
| Drinker | 23 (67.6) | 20 (57.1) | 22 (61.1) | 65 (62.0) |
| DXA variables | ||||
| Fat percentage (%) | ||||
| Total body fat | 38.1 (7.3) | 37.6 (5.5) | 37.1 (7.5) | 37.6 (7.6) |
| Arms | 36.1 (10.5) | 35.2 (9.1) | 34.6 (10.5) | 35.3 (10.0) |
| Legs | 35.9 (10.1) | 34.8 (7.4) | 34.0 (8.5) | 34.9 (8.3) |
| Trunk | 41.9 (6.6) | 41.8 (5.0) | 41.5 (7.5) | 41.7 (6.3) |
| Android | 46.7 (7.2) | 47.8 (4.8) | 47.4 (7.5) | 47.3 (9.6) |
| Gynoid | 43.7 (10.4) | 43.0 (7.6) | 42.5 (9.3) | 41.7 (6.5) |
| Body fat mass (kg) | 26.3 (4.8) | 26.9 (3.1) | 26.5 (5.7) | 26.6 (4.6) |
| Fat-free mass (kg) | 45.1 (9.1) | 48.3 (9.2) | 48.0 (9.3) | 47.2 (9.2) |
| Lean body mass (kg) | 43.4 (9.0) | 45.3 (8.6) | 45.1 (8.8) | 47.8 (32.0) |
| BIA variables | ||||
| Body fat mass (kg) | 25.2 (4.5) | 25.1 (3.2) | 24.9 (5.3) | 25.1 (4.4) |
| Body fat percentage (%) | 34.4 (6.5) | 33.5 (5.9) | 33.0 (7.2) | 33.7 (6.5) |
| Anthropometric parameters | ||||
| Body weight (kg) | 73.9 (9.7) | 76.1 (9.3) | 76.3 (9.5) | 75.4 (9.4) |
| Body mass index (kg/m2) | 27.1 (1.6) | 27.1 (1.5) | 27.2 (1.5) | 27.1 (1.5) |
| Waist circumference (cm) | 92.3 (7.0) | 92.5 (6.4) | 91.4 (6.3) | 92.1 (6.5) |
| Hip circumference (cm) | 103.1 (4.5) | 103.8 (2.9) | 103.6 (5.1) | 103.5 (4.2) |
| Waist-to-hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) |
| Serum levels | ||||
| Triglycerides (mg/dL) | 133.1 (109.1) | 130.1 (76.5) | 102.9 (46.4) | 121.7 (80.9) |
| Total cholesterol (mg/dL) | 187.3 (35.8) | 188.5 (31.1) | 184.8 (33.3) | 186.9 (32.9) |
| HDL-C (mg/dL) | 55.8 (14.4) | 57.3 (11.6) | 56.7 (13.6) | 56.6 (13.1) |
| LDL-C (mg/dL) | 113.3 (29.7) | 115.3 (27.9) | 114.2 (29.1) | 114.3 (28.6) |
| VLDL-C (mg/dL) | 26.6 (21.8) | 26.0 (15.3) | 20.6 (9.3) | 24.3 (16.2) |
| Free fatty acids (µEq/L) | 668.3 (238.4) | 568.5 (135.1) | 586.8 (216.4) | 606.3 (202.8) |
| Leptin (ng/mL) | 16.5 (10.6) | 16.2 (10.3) | 14.6 (10.3) | 15.7 (10.3) |
| DXA Variables 1 | CEM 840 mg/day (n = 34) | CEM 1200 mg/day (n = 35) | Placebo (n = 36) | p Value 2 |
|---|---|---|---|---|
| Changes in fat percentages (%) | ||||
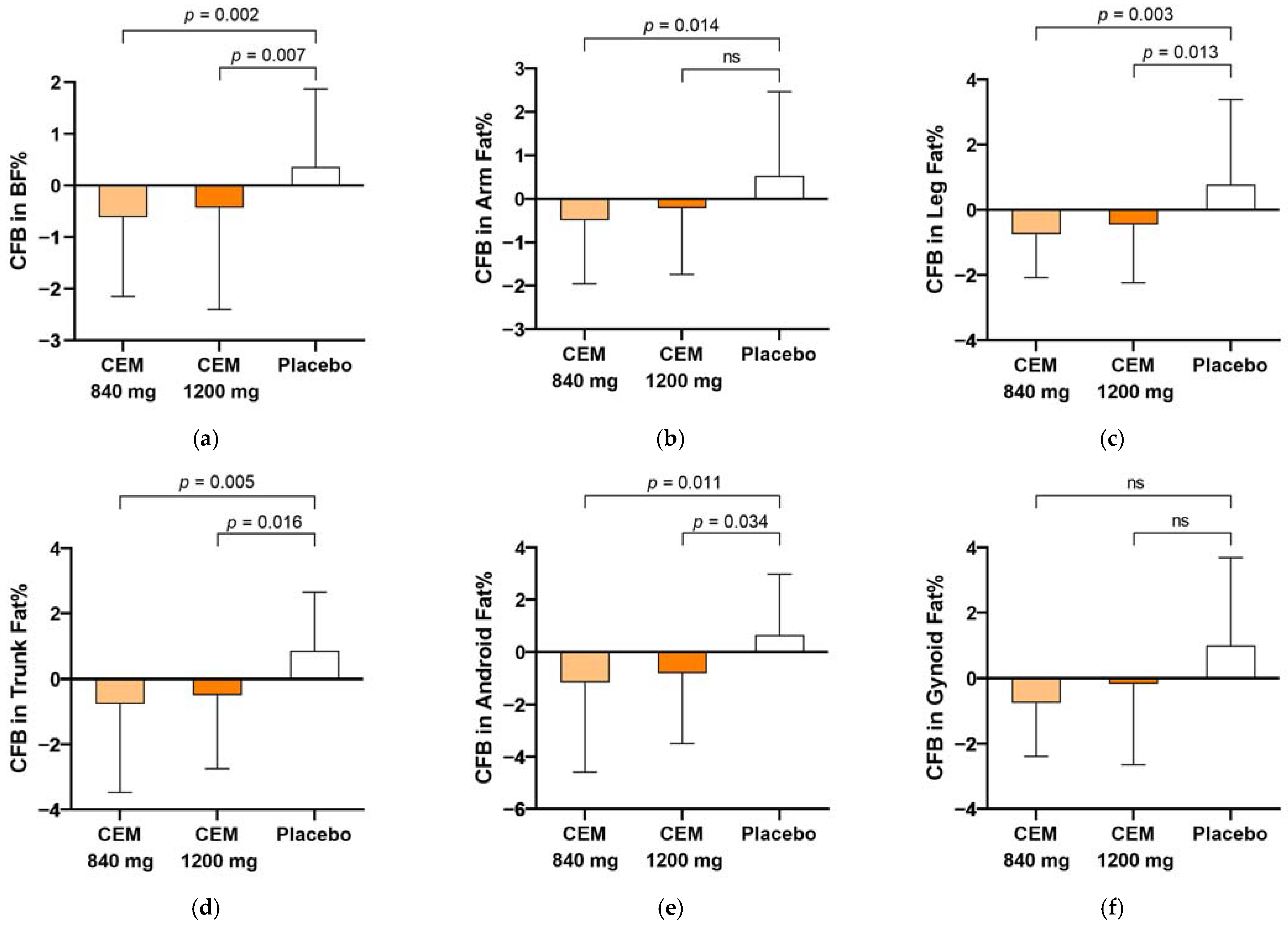
| Total body fat | −0.7 (1.8) 3,* | −0.4 (1.8) * | 0.8 (1.8) | 0.003 |
| Arms | −0.5 (1.5) * | −0.2 (1.5) | 0.5 (1.9) | 0.039 |
| Legs | −0.7 (1.3) * | −0.5 (1.8) * | 0.8 (2.6) | 0.006 |
| Trunk | −0.8 (2.7) * | −0.5 (2.3) * | 0.9 (1.8) | 0.010 |
| Android | −1.1 (3.4) * | −0.8 (2.7)* | 0.7 (2.3) | 0.025 |
| Gynoid | −0.7 (1.6) | −0.1 (2.5) | 1.0 (2.7) | 0.116 |
| Changes in measures | ||||
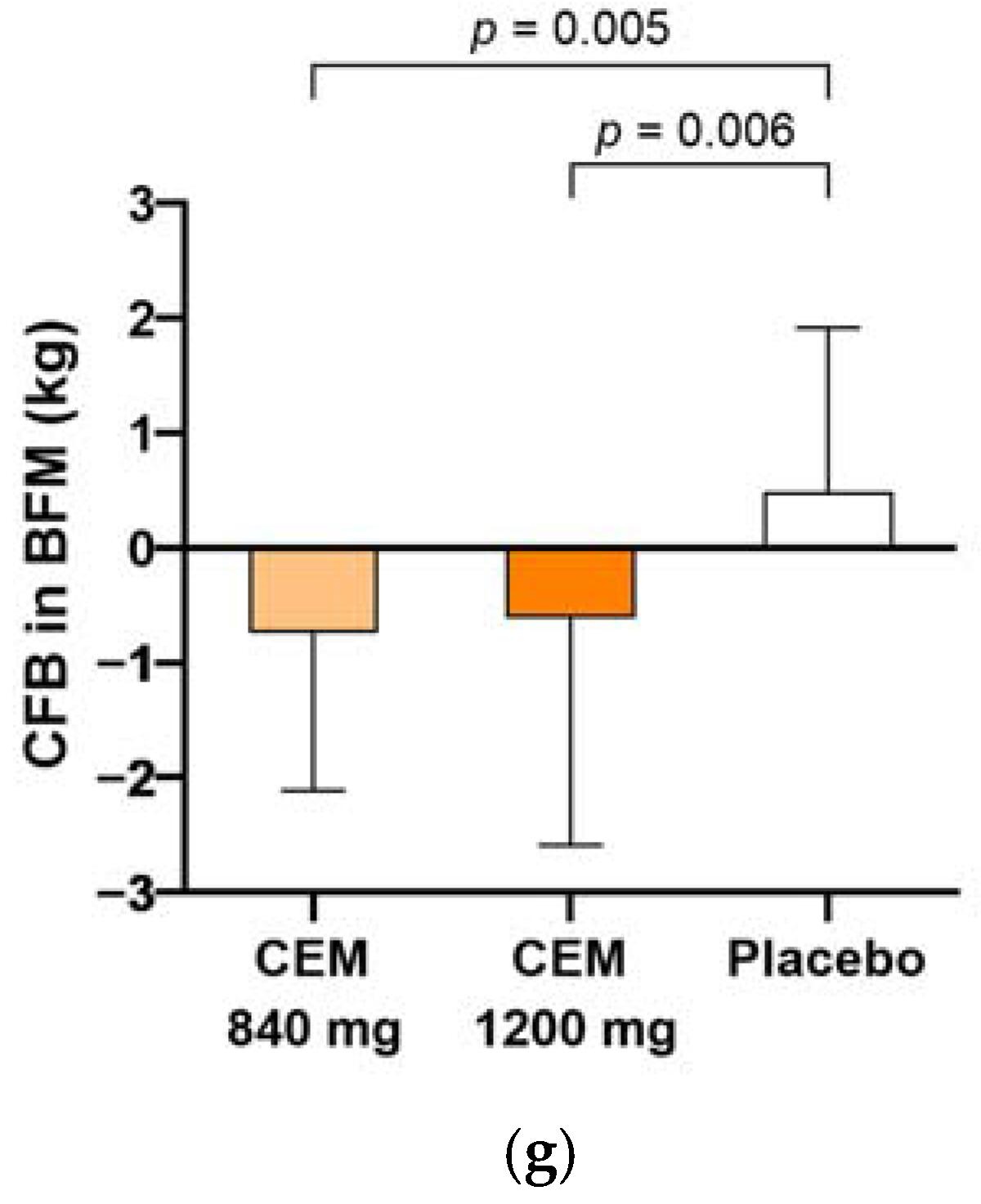
| Body fat mass (kg) | −0.6 (1.6) * | −0.6 (1.8) * | 0.8 (1.4) | 0.000 |
| Fat-free mass (kg) | 1.1 (5.3) | −0.2 (1.2) | −0.3 (2.0) | 0.177 |
| Lean body mass (kg) | 0.1 (0.9) | −0.2 (1.2) | 0.0 (2.4) | 0.770 |
| Endpoints 1 | CEM 840 mg/day (n = 34) | CEM 1200 mg/day (n = 35) | Placebo (n = 36) | p Value 2 |
|---|---|---|---|---|
| Changes in BIA variables | ||||
| Body fat mass (kg) | −0.7 (1.4) 3,* | −0.6 (2.0) * | 0.5 (1.4) | 0.003 |
| Body fat percentage (%) | −0.6 (1.5) | −0.4 (2.0) | 0.4 (1.5) | 0.556 |
| Changes in anthropometric parameters | ||||
| Body weight (kg) | −0.8 (1.8) * | −0.9 (2.2) * | 0.4 (1.9) | 0.020 |
| Body mass index (kg/m2) | −0.3 (0.7) * | −0.3 (0.9) * | 0.1 (0.7) | 0.019 |
| Waist circumference (cm) | −1.3 (3.3) | −1.3 (3.8) | 0.5 (3.5) | 0.066 |
| Hip circumference (cm) | −0.3 (1.7) | −1.0 (2.7) | −0.1 (2.6) | 0.343 |
| Waist-to-hip ratio | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.111 |
| Endpoints 1 |
CEM 840 mg/day (n = 34) |
CEM 1200 mg/day (n = 35) |
Placebo (n = 36) | p Value 2 |
|---|---|---|---|---|
| Changes in lipid profiles (mg/dL) | ||||
| Triglycerides | −18.6 (43.9) 3 | −19.4 (49.9) | 0.6 (32.5) | 0.092 |
| Total cholesterol | −3.4 (16.6) | −1.1 (21.8) | 1.8 (25.6) | 0.623 |
| HDL-C | −0.1 (5.8) | 1.4 (7.9) | 0.2 (8.0) | 0.674 |
| LDL-C | 0.1 (16.4) | 2.5 (19.7) | 4.1 (22.0) | 0.710 |
| VLDL-C | −3.7 (8.8) | −3.9 (10.0) | 0.1 (6.5) | 0.091 |
| Changes in measures | ||||
| Free fatty acids (µEq/L) | −33.3 (234.3) | −27.5 (161.5) | −23.1 (193.0) | 0.978 |
| Leptin (ng/mL) | 0.1 (4.9) | −2.8 (7.4) * | 2.2 (6.0) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Kim, D.-Y.; Lee, H.S.; Rhee, S.Y.; Lim, H. Efficacy of Crataegus Extract Mixture on Body Fat and Lipid Profiles in Overweight Adults: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 494. https://doi.org/10.3390/nu16040494
Song J, Kim D-Y, Lee HS, Rhee SY, Lim H. Efficacy of Crataegus Extract Mixture on Body Fat and Lipid Profiles in Overweight Adults: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(4):494. https://doi.org/10.3390/nu16040494
Chicago/Turabian StyleSong, Jungbin, Do-Yeon Kim, Han Songyi Lee, Sang Youl Rhee, and Hyunjung Lim. 2024. "Efficacy of Crataegus Extract Mixture on Body Fat and Lipid Profiles in Overweight Adults: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 4: 494. https://doi.org/10.3390/nu16040494





