Effects of Prebiotic Yeast Mannan on Gut Health and Sleep Quality in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Tablets
2.4. Bowel Habits and Analyses of Fecal Samples
2.5. Analyses of Sleep Quality
2.6. Diet Survey and Safety Evaluation
2.7. Ethics Committee
2.8. Statistical Analysis
3. Results
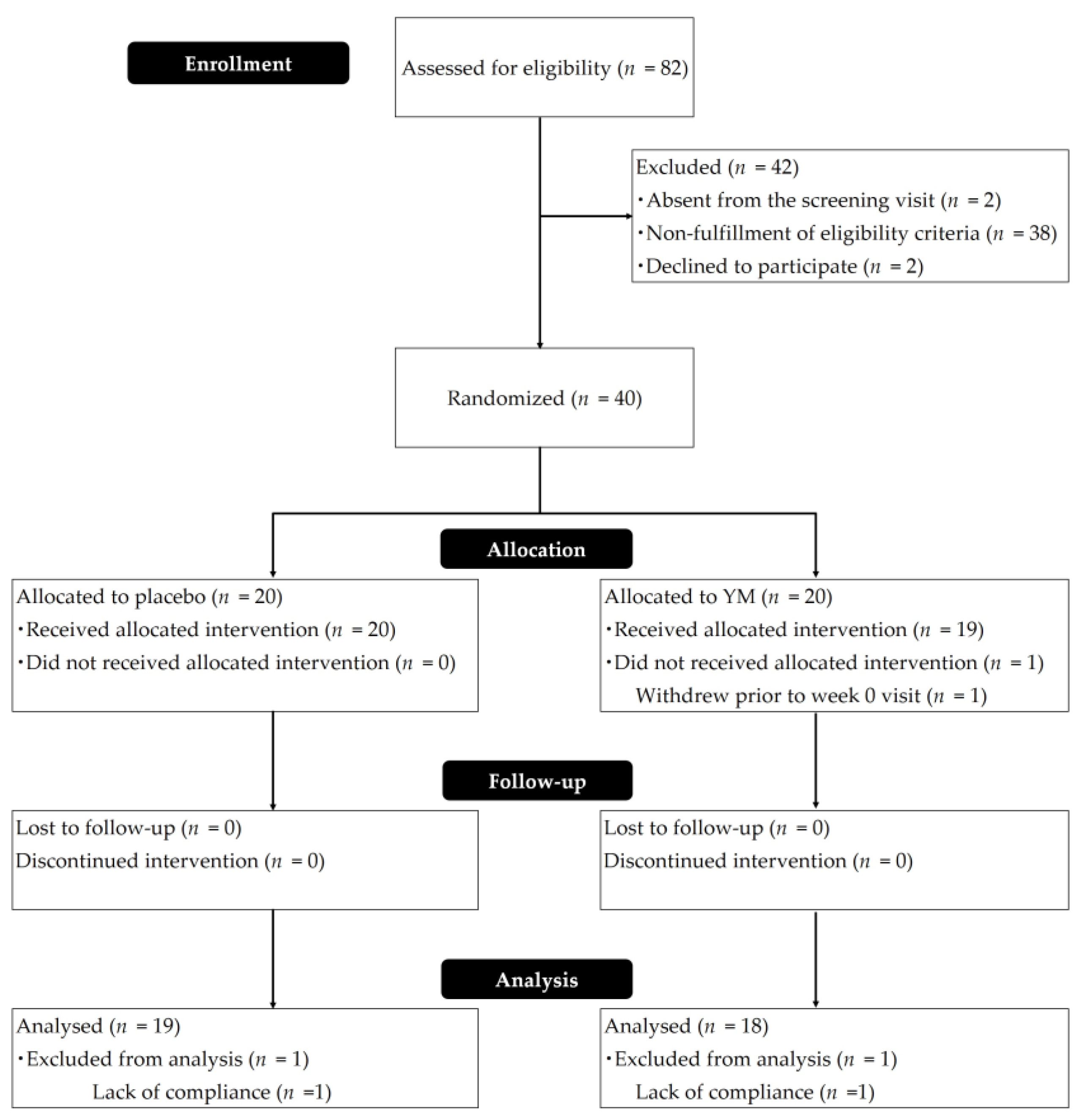
3.1. Demographics
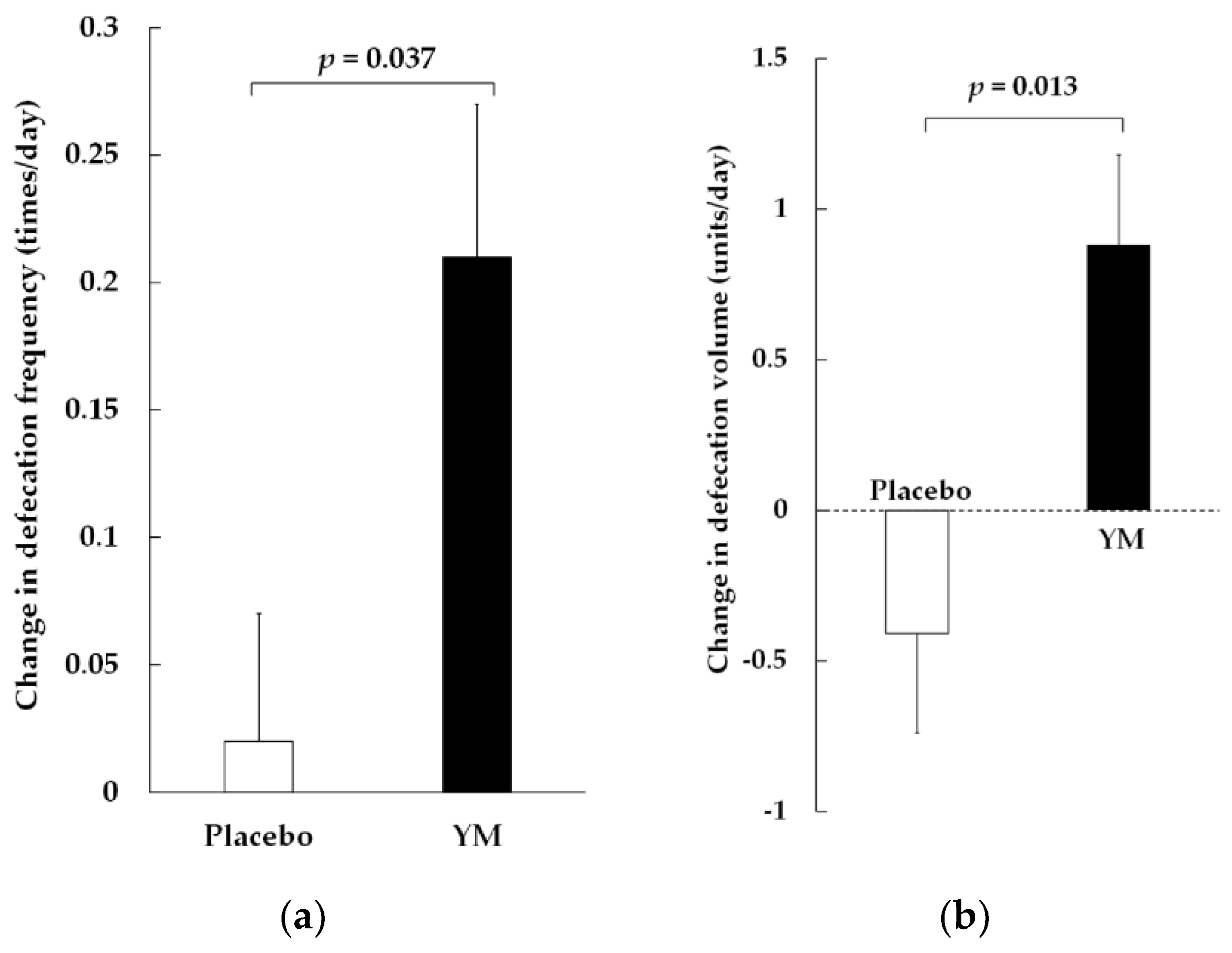
3.2. Bowel Movements, Stool Volume, and BSS
3.3. Sleep
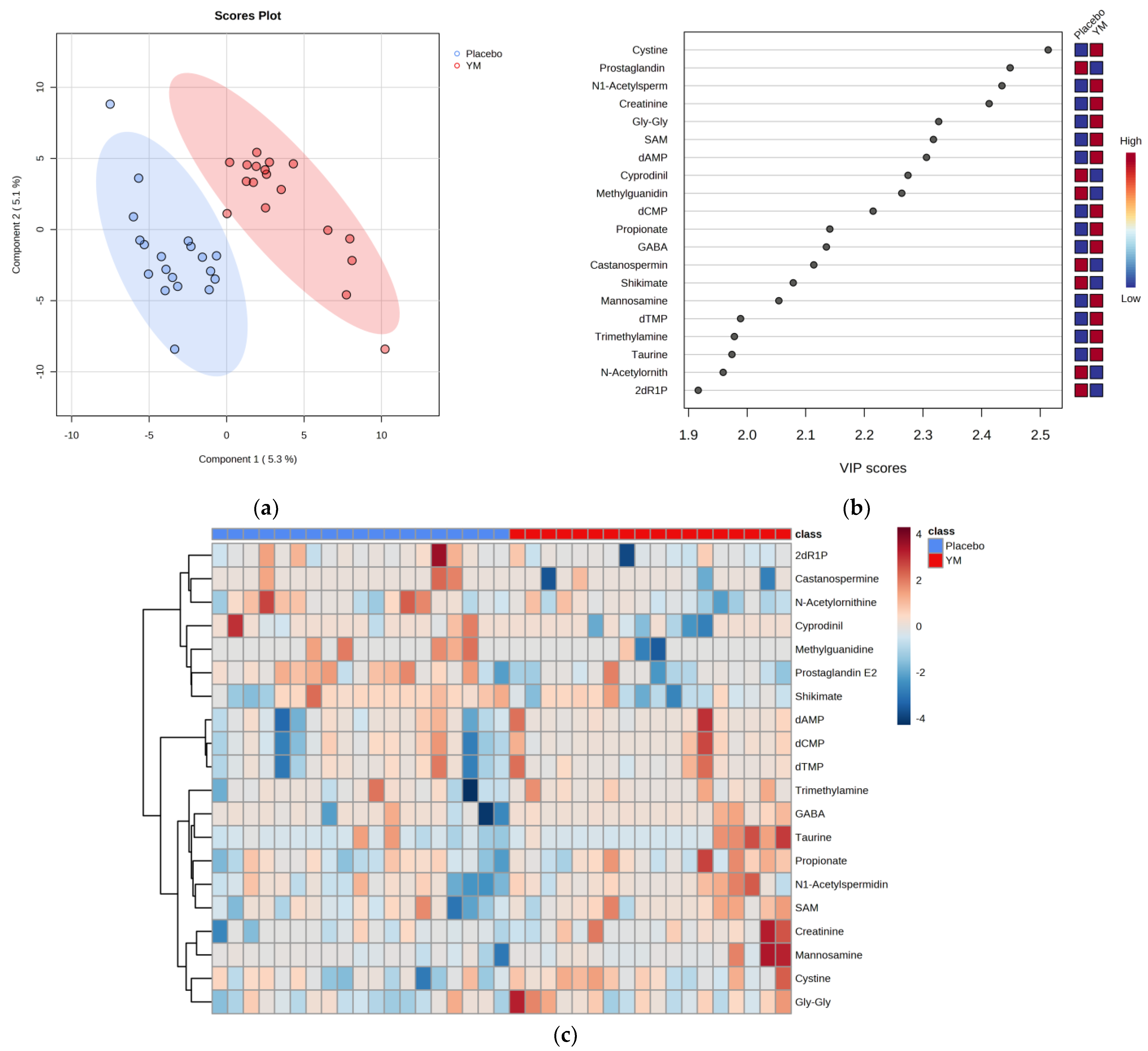
3.4. Fecal Properties
3.5. Relationship between Fecal Metabolites and Sleep
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pacheco, A.R.; Moel, M.; Segrè, D. Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat. Commun. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Halverson, T.; Alagiakrishnan, K. Gut microbes in neurocognitive and mental health disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef]
- Farias, D.P.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Prebiotics: Trends in food, health and technological applications. Trends Food Sci. Technol. 2019, 93, 23–35. [Google Scholar] [CrossRef]
- Oba, S.; Washida, K.; Shimada, Y.; Sunagawa, T.; Tanihiro, R.; Sugiyama, H.; Nakamura, Y. Yeast mannan increases Bacteroides thetaiotaomicron abundance and suppresses putrefactive compound production in in vitro fecal microbiota fermentation. Biosci. Biotechnol. Biochem. 2020, 84, 2174–2178. [Google Scholar] [CrossRef]
- Oba, S.; Sunagawa, T.; Tanihiro, R.; Awashima, K.; Sugiyama, H.; Odani, T.; Nakamura, Y.; Kondo, A.; Sasaki, D.; Sasaki, K. Prebiotic effects of yeast mannan, which selectively promotes Bacteroides thetaiotaomicron and Bacteroides ovatus in a human colonic microbiota model. Sci. Rep. 2020, 10, 17351. [Google Scholar] [CrossRef]
- Tanihiro, R.; Sakano, K.; Oba, S.; Nakamura, C.; Ohki, K.; Hirota, T.; Sugiyama, H.; Ebihara, S.; Nakamura, Y. Effects of yeast mannan which promotes beneficial Bacteroides on the intestinal environment and skin condition: A randomized, double-blind, placebo-controlled study. Nutrients 2020, 12, 3673. [Google Scholar] [CrossRef]
- Liu, H.Z.; Liu, L.; Hui, H.; Wang, Q. Structural characterization and antineoplastic activity of Saccharomyces cerevisiae mannoprotein. Int. J. Food Prop. 2015, 18, 359–371. [Google Scholar] [CrossRef]
- Kocourek, J.; Ballou, C.E. Method for fingerprinting yeast cell wall mannans. J. Bacteriol. 1969, 100, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Sanderson, I.R.; Muhammed, R.; Allen, S.; Tzivinikos, C.; Henderson, P.; Gervais, L.; Jeffery, I.B.; Mullins, D.P.; O’Herlihy, E.A.; et al. A double-blind, placebo-controlled trial to assess safety and tolerability of (Thetanix) Bacteroides thetaiotaomicron in adolescent Crohn’s disease. Clin. Transl. Gastroenterol. 2020, 12, e00287. [Google Scholar] [CrossRef] [PubMed]
- Billings, M.E.; Hale, L.; Johnson, D.A. Physical and social environment relationship with sleep health and disorders. Chest 2020, 157, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Orr, W.C.; Chen, C.L. Sleep and the gastrointestinal tract. Neurol. Clin. 2005, 23, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Liu, Q.Q.; Su, H.Z.; Zhang, H.P.; Fan, J.M.; Yang, J.H.; Hu, A.K.; Liu, Y.Q.; Chou, D.; Zeng, Y.M. Gut microbiota in obstructive sleep apneahypopnea syndrome: Disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 2019, 133, 905–917. [Google Scholar] [CrossRef]
- Lin, A.; Shih, C.T.; Huang, C.L.; Wu, C.C.; Lin, C.T.; Tsai, Y.C. Hypnotic effects of Lactobacillus fermentum PS150TM on pentobarbital-induced sleep in mice. Nutrients 2019, 11, 2409. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Saleh-Ghadimi, S.; Dehghan, P.; Sarmadi, B.; Maleki, P. Improvement of sleep by resistant dextrin prebiotic in type 2 diabetic women coincides with attenuation of metabolic endotoxemia: Involvement of gut brain axis. J. Sci. Food Agric. 2022, 102, 5229–5237. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.J.; Virjee, J.; Heaton, K.W. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990, 300, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. BioSyst. 2008, 4, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. BioSyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- MetaboAnalyst—Statistical, Functional and Integrative Analysis of Metabolomics Data. Available online: https://www.metaboanalyst.ca/ (accessed on 24 November 2023).
- Hatanaka, M.; Yamamoto, K.; Suzuki, N.; Iio, S.; Takara, T.; Morita, H.; Takimoto, T.; Nakamura, T. Effect of Bacillus subtilis C-3102 on loose stools in healthy volunteers. Benef. Microbes 2018, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Liu, C.; Summanen, P.; Xu, H.; Finegold, S. Application of quantitative real-time PCR for rapid identification of Bacteroides fragilis group and related organisms in human wound samples. Anaerobe 2011, 17, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.; Firmesse, O.; Gourmelon, M.; Bridonneau, C.; Tap, J.; Mondot, S.; Doré, J.; Corthier, G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol. Ecol. 2009, 68, 351–362. [Google Scholar] [CrossRef]
- Araki, T.; Uemura, T.; Yoshimoto, S.; Takemoto, A.; Noda, Y.; Izumi, S.; Sekitani, T. Wireless monitoring using a stretchable and transparent sensor sheet containing metal nanowires. Adv. Mater. 2020, 32, e1902684. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Matsumori, S.; Teramoto, K.; Iyori, H.; Soda, T.; Yoshimoto, S.; Mizutani, H. HARU Sleep: A deep learning-based sleep scoring system with wearable sheet-type frontal EEG sensors. IEEE Access 2022, 10, 13624–13632. [Google Scholar] [CrossRef]
- Aziz, I.; Whitehead, W.E.; Palsson, O.S.; Törnblom, H.; Simrén, M. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Choi, S.C.; Park, K.S.; Park, M.I.; Shin, J.E.; Lee, T.H.; Jung, K.W.; Koo, H.S.; Myung, S.-J.; Constipation Research Group of Korean Society of Neurogastroenterology and Motility. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. [Google Scholar]
- Yao, L.; Seaton, S.C.; Ndousse-Fetter, S.; Adhikari, A.A.; DiBenedetto, N.; Mina, A.I.; Banks, A.S.; Bry, L.; Devlin, A.S. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 2018, 7, e37182. [Google Scholar] [CrossRef]
- Porter, N.T.; Larsbrink, J. Investigation and alteration of organic acid synthesis pathways in the mammalian gut symbiont Bacteroides thetaiotaomicron. Microbiol. Spectr. 2022, 10, e02312-21. [Google Scholar] [CrossRef]
- Appleby, R.N.; Walters, J.R. The role of bile acids in functional GI disorders. Neurogastroenterol. Motil. 2014, 26, 1057–1069. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Wang, J.K.; Yao, S.K. Roles of gut microbiota and metabolites in pathogenesis of functional constipation. Evid.-Based Complement. Altern. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef]
- Ge, X.; Zhao, W.; Ding, C.; Tian, H.; Xu, L.; Wang, H.; Ni, L.; Jiang, J.; Gong, J.; Zhu, W.; et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci. Rep. 2017, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Cheng, L.; Cicala, M.; Ripetti, V.; Biancani, P.; Behar, J. Progesterone receptors and serotonin levels in colon epithelial cells from females with slow transit constipation. Neurogastroenterol. Motil. 2011, 23, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front. Neurosci. 2019, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Hale, L.; Moore, M.; Patel, N.P. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med. Rev. 2010, 14, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van de Wouw, M.; Drogos, L.; Vaghef-Mehrabani, E.; Reimer, R.A.; Tomfohr-Madsen, L.; Giesbrecht, G.F. Sleep and the gut microbiota in preschool-aged children. Sleep 2022, 45, zsac020. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Margret, A.A.; Mareeswari, R.; Arun Kumar, K.; Jerley, A.A. Relative profiling of L-tryptophan derivatives from selected edible mushrooms as psychoactive nutraceuticals to inhibit P-glycoprotein: A paradigm to contest blood-brain barrier. BioTechnologia 2021, 102, 55–64. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Han, C.J.; Jarrett, M.E.; Gu, H.; Djukovic, D.; Shulman, R.J.; Raftery, D.; Henderson, W.A.; Cain, K.C. Serum tryptophan metabolite levels during sleep in patients with and without irritable bowel syndrome (IBS). Biol. Res. Nurs. 2016, 18, 193–198. [Google Scholar] [CrossRef]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2020, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.I.; Shin, Y.Y.; Chung, S.E.; Shin, W.C. Safety and efficacy of gamma-aminobutyric acid from fermented rice germ in patients with insomnia symptoms: A randomized, double-blind trial. J. Clin. Neurol. 2018, 14, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Yamatsu, A.; Yamashita, Y.; Maru, I.; Yang, J.; Tatsuzaki, J.; Kim, M. The improvement of sleep by oral intake of GABA and Apocynum venetum leaf extract. J. Nutr. Sci. Vitaminol. 2015, 61, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Nielsen, C.U.; Carstensen, M.; Brodin, B. Carrier-mediated γ-aminobutyric acid transport across the basolateral membrane of human intestinal Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2012, 81, 458–462. [Google Scholar] [CrossRef]
- Ikeyama, N.; Murakami, T.; Toyoda, A.; Mori, H.; Iino, T.; Ohkuma, M.; Sakamoto, M. Microbial interaction between the succinate-utilizing bacterium Phascolarctobacterium faecium and the gut commensal Bacteroides thetaiotaomicron. MicrobiologyOpen 2020, 9, e1111. [Google Scholar] [CrossRef]
| Parameters | Placebo | YM |
|---|---|---|
| Age, years | 52.2 ± 7.3 | 48.1 ± 10.2 |
| Gender, n (%) | ||
| Male | 4 (21.1) | 6 (33.3) |
| Female | 15 (78.9) | 12 (66.7) |
| Alcohol drinking habits, n (%) | ||
| No | 9 (47.4) | 8 (44.4) |
| Yes | 10 (52.6) | 10 (55.6) |
| Smoking habit, n (%) | ||
| Non-smoker | 12 (63.2) | 12 (66.7) |
| Ex-smoker | 4 (21.1) | 3 (16.7) |
| (no smoking for > 6 months) | ||
| Smoker | 3 (15.8) | 3 (16.7) |
| Fiber intake (g/day) | 11.2 ± 3.3 | 12.5 ± 3.4 |
| Parameters (Unit) | Group | Pre-Treatment | Post-Treatment | Changes | |||
|---|---|---|---|---|---|---|---|
| Values | p-Values | Values | p-Values | Values | p-Values | ||
| TIB (min) | Placebo | 375.9 ± 13.9 | 0.355 | 375.5 ± 13.8 | 0.293 | −0.5 ± 8.5 | 0.003 ** |
| YM | 349.2 ± 18.9 | 404.3 ± 14.6 | 55.1 ± 18.1 | ||||
| SE (%) | Placebo | 75.8 ± 5.3 | 0.521 | 83.7 ± 2.1 | 0.239 | 7.9 ± 4.3 | 0.203 |
| YM | 77.7 ± 3.8 | 76.8 ± 3.5 | −0.9 ± 3.5 | ||||
| N3 duration (min) | Placebo | 44.0 ± 8.4 | 0.864 | 30.7 ± 5.5 | 0.022 * | −13.4 ± 9.9 | 0.152 |
| YM | 48.1 ± 8.4 | 54.8 ± 9.4 | 6.7 ± 8.1 | ||||
| SOL (min) | Placebo | 30.0 ± 8.0 | 0.913 | 17.9 ± 3.8 | 0.501 | −12.1 ± 9.0 | 0.239 |
| YM | 23.1 ± 4.3 | 23.8 ± 5.9 | 0.8 ± 4.7 | ||||
| N3 latency (min) | Placebo | 55.1 ± 9.8 | 0.696 | 98.3 ± 27.3 | 0.008 ** | 43.2 ± 25.7 | 0.017 * |
| YM | 65.1 ± 16.8 | 37.1 ± 11.1 | −28.1 ± 12.0 | ||||
| Parameters (Unit) | Placebo | YM | p-Values 1 | ||||
|---|---|---|---|---|---|---|---|
| Pre- Treatment | Post- Treatment | Changes | Pre- Treatment | Post- Treatment | Changes | ||
| Total bacteria (109 copies/g feces) | 155.4 ± 17.3 | 170.9 ± 20.5 | 15.5 ± 20.4 | 182.1 ± 57.9 | 172.3 ± 26.5 | −9.8 ± 52.4 | 0.649 |
| B. thetaiotaomicron (109 copies/g feces) | 0.49 ± 0.23 | 0.31 ± 0.15 | −0.18 ± 0.11 | 0.15 ± 0.05 | 0.31 ± 0.13 | 0.16 ± 0.12 | 0.046 * |
| Relative abundance of | 0.31 ± 0.12 | 0.17 ± 0.07 | −0.14 ± 0.08 | 0.09 ± 0.03 | 0.15 ± 0.05 | 0.07 ± 0.04 | 0.035 * |
| B. thetaiotaomicron (%) | |||||||
| Dependent Variables | Steps | Independent Variables | R2 | Adjusted R2 | B ± SE | β | p-Values |
|---|---|---|---|---|---|---|---|
| TIB | 1 | Propionate | 0.557 | 0.530 | 0.04 ± 0.01 | 0.747 | <0.0001 |
| N3 latency | 1 | GABA | 0.372 | 0.333 | −0.41 ± 0.13 | −0.610 | 0.007 |
| 2 | GABA | 0.641 | 0.593 | −0.46 ± 0.11 | −0.683 | 0.001 | |
| dTMP | −4.60 ± 1.37 | −0.524 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanihiro, R.; Yuki, M.; Sasai, M.; Haseda, A.; Kagami-Katsuyama, H.; Hirota, T.; Honma, N.; Nishihira, J. Effects of Prebiotic Yeast Mannan on Gut Health and Sleep Quality in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2024, 16, 141. https://doi.org/10.3390/nu16010141
Tanihiro R, Yuki M, Sasai M, Haseda A, Kagami-Katsuyama H, Hirota T, Honma N, Nishihira J. Effects of Prebiotic Yeast Mannan on Gut Health and Sleep Quality in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2024; 16(1):141. https://doi.org/10.3390/nu16010141
Chicago/Turabian StyleTanihiro, Reiko, Masahiro Yuki, Masaki Sasai, Akane Haseda, Hiroyo Kagami-Katsuyama, Tatsuhiko Hirota, Naoyuki Honma, and Jun Nishihira. 2024. "Effects of Prebiotic Yeast Mannan on Gut Health and Sleep Quality in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 16, no. 1: 141. https://doi.org/10.3390/nu16010141





