Examining the Association between Coffee Intake and the Risk of Developing Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis
Highlights
- This meta-analysis pooled data from 27 studies, encompassing over 543,000 participants, and found no significant association between overweight or obesity and IBS overall.
- A statistically significant association between obesity and IBS was observed in studies using the Rome IV criteria (OR 1.59; 95% CI 1.13–2.23), but not with earlier Rome criteria (Rome II or III).
- Excess body weight may not be the primary driver of IBS; obesity and IBS share psychosocial comorbidities like stress and depression, which may mediate the observed associations.
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Literature Retrieval
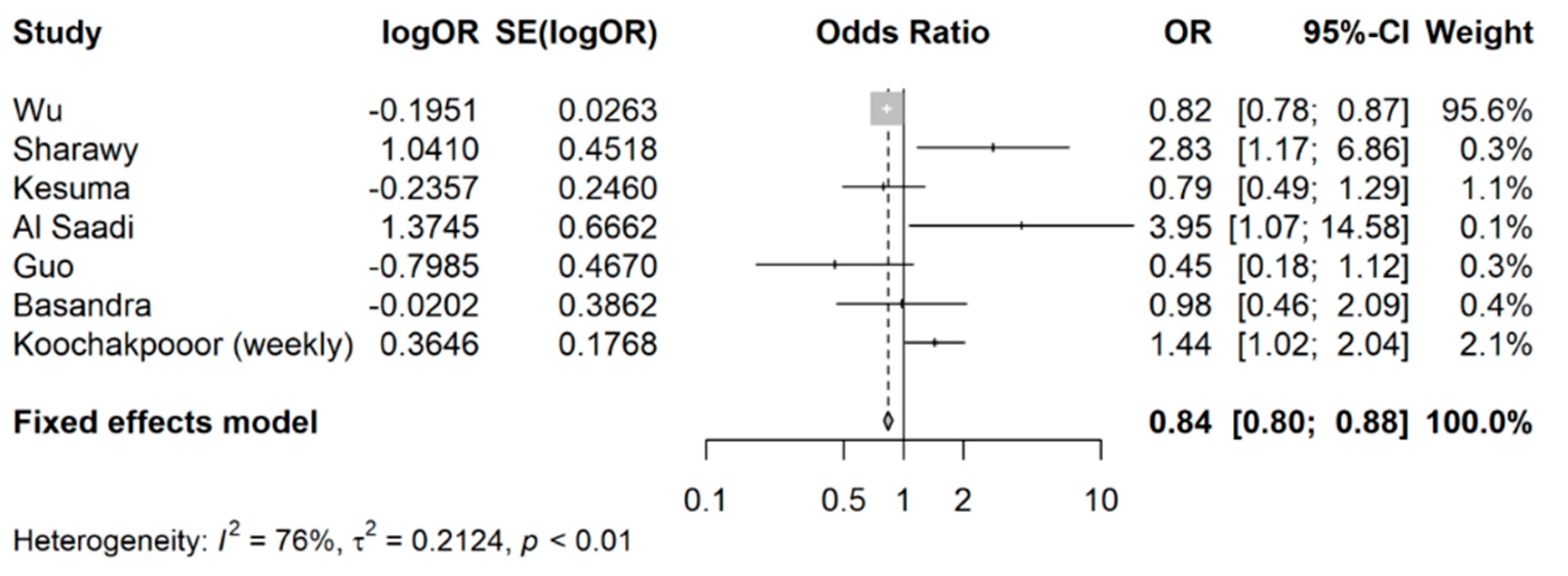
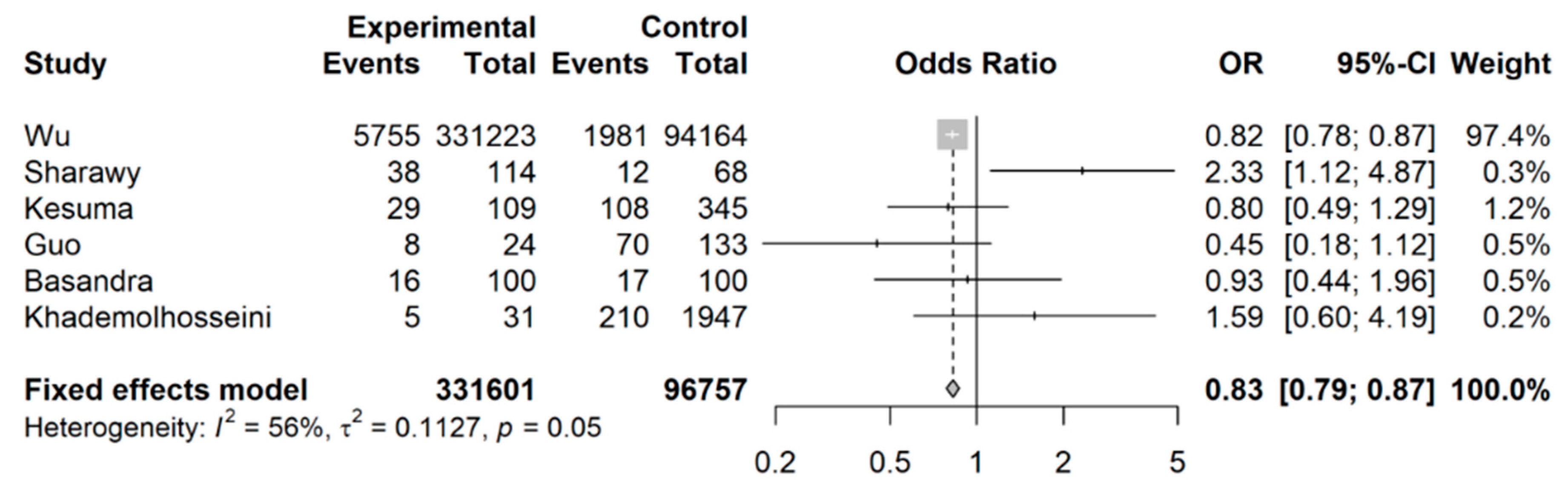
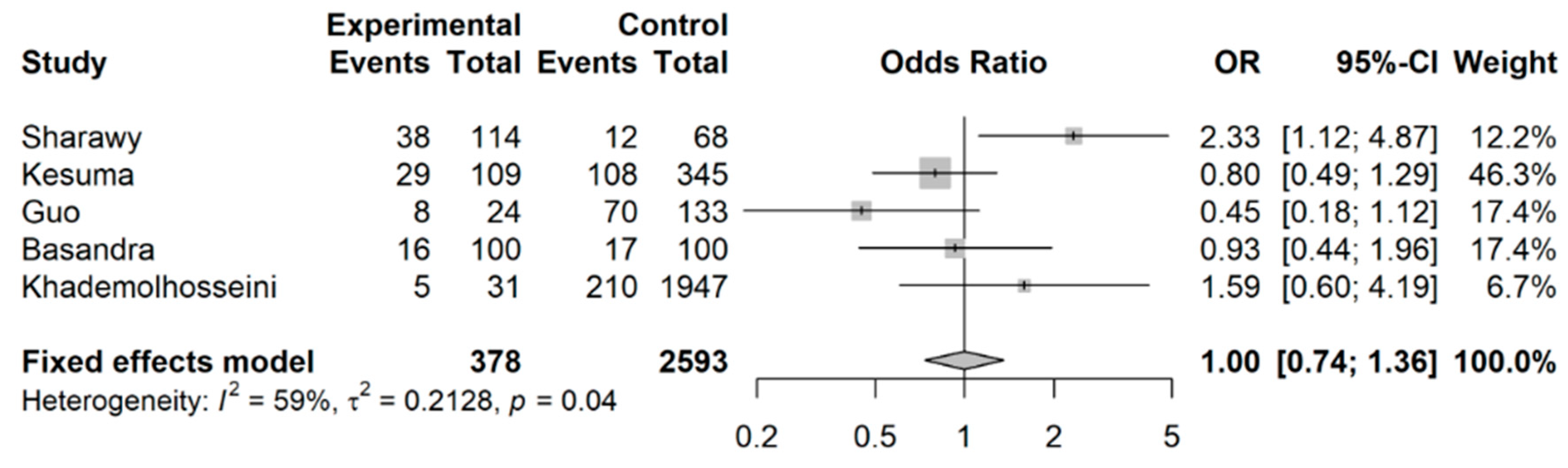
3.2. Coffee Intake and Risk of Developing IBS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef]
- Buono, J.L.; Carson, R.T.; Flores, N.M. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual. Life Outcomes 2017, 15, 35. [Google Scholar] [CrossRef]
- Frändemark, Å.; Törnblom, H.; Jakobsson, S.; Simrén, M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Am. J. Gastroenterol. 2018, 113, 1540–1549. [Google Scholar] [CrossRef]
- Pace, F.; Molteni, P.; Bollani, S.; Sarzi-Puttini, P.; Stockbrügger, R.; Porro, G.B.; Drossman, D.A. Inflammatory bowel disease versus irritable bowel syndrome: A hospital-based, case-control study of disease impact on quality of life. Scand. J. Gastroenterol. 2003, 38, 1031–1038. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef]
- Chey, W.D.; Hashash, J.G.; Manning, L.; Chang, L. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology 2022, 162, 1737–1745.e5. [Google Scholar] [CrossRef]
- Agakidis, C.; Kotzakioulafi, E.; Petridis, D.; Apostolidou, K.; Karagiozoglou-Lampoudi, T. Mediterranean Diet Adherence is Associated with Lower Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents. Nutrients 2019, 11, 1283. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Statista. Global Coffee Consumption 2012/13–2020/21. Statista. 9 March 2023. Available online: https://www.statista.com/statistics/292595/global-coffee-consumption (accessed on 16 October 2023).
- Safe, S.; Kothari, J.; Hailemariam, A.; Upadhyay, S.; Davidson, L.A.; Chapkin, R.S. Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2706. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Nehlig, A. Effects of Coffee on the Gastro-Intestinal Tract: A Narrative Review and Literature Update. Nutrients 2022, 14, 399. [Google Scholar] [CrossRef]
- Khademolhosseini, F.; Mehrabani, D.; Nejabat, M.; Beheshti, M.; Heydari, S.T.; Mirahmadizadeh, A.; Salehi, M.; Zare, N.; Saberi-Firoozi, M. Irritable bowel syndrome in adults over 35 years in Shiraz, southern Iran: Prevalence and associated factors. J. Res. Med. Sci. 2011, 16, 200–206. [Google Scholar]
- Basandra, S.; Bajaj, D. Epidemiology of Dyspepsia and Irritable Bowel Syndrome (IBS) in Medical Students of Northern India. J. Clin. Diagn. Res. 2014, 8, JC13–JC16. [Google Scholar]
- Guo, Y.B.; Zhuang, K.M.; Kuang, L.; Zhan, Q.; Wang, X.F.; Liu, S.D. Association between Diet and Lifestyle Habits and Irritable Bowel Syndrome: A Case-Control Study. Gut Liver 2015, 9, 649–656. [Google Scholar] [CrossRef]
- Ng, Q.X.; Yau, C.E.; Yaow, C.Y.L.; Chong, R.I.H.; Chong, N.Z.Y.; Teoh, S.E.; Lim, Y.L.; Soh, A.Y.S.; Ng, W.K.; Thumboo, J. What Has Longitudinal ‘Omics’ Studies Taught Us about Irritable Bowel Syndrome? A Systematic Review. Metabolites 2023, 13, 484. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Kamp, K.J.; Cain, K.C.; Utleg, A.; Burr, R.L.; Raftery, D.; Luna, R.A.; Shulman, R.J.; Heitkemper, M.M. Bile Acids and Microbiome Among Individuals with Irritable Bowel Syndrome and Healthy Volunteers. Biol. Res. Nurs. 2021, 23, 65–74. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Uranga, J.A.; Del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain-Gut Axis. Nutrients 2020, 13, 88. [Google Scholar] [CrossRef]
- Patz, M.D.; Day, H.E.; Burow, A.; Campeau, S. Modulation of the hypothalamo-pituitary-adrenocortical axis by caffeine. Psychoneuroendocrinology 2006, 31, 493–500. [Google Scholar] [CrossRef]
- Chang, L.; Sundaresh, S.; Elliott, J.; Anton, P.A.; Baldi, P.; Licudine, A.; Mayer, M.; Vuong, T.; Hirano, M.; Naliboff, B.D.; et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil. 2009, 21, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Canada. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 5 September 2023).
- Team R. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Al Saadi, T.; Idris, A.; Turk, T.; Alkhatib, M. Epidemiology and risk factors of uninvestigated dyspepsia, irritable bowel syndrome, and gastroesophageal reflux disease among students of Damascus University, Syria. J. Epidemiol. Glob. Health 2016, 6, 285–293. [Google Scholar] [CrossRef]
- Kesuma, Y.; Sekartini, R.; Timan, I.S.; Kurniawan, A.; Bardosono, S.; Firmansyah, A.; Vandenplas, Y. Irritable bowel syndrome in Indonesian adolescents. J. Pediatr. (Rio J.) 2021, 97, 197–203. [Google Scholar] [CrossRef]
- Koochakpoor, G.; Salari-Moghaddam, A.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. Association of Coffee and Caffeine Intake With Irritable Bowel Syndrome in Adults. Front. Nutr. 2021, 8, 632469. [Google Scholar] [CrossRef]
- El Sharawy, S.M.; Amer, I.F.; Elkadeem, M.Z. Irritable bowel syndrome in Egyptian medical students, prevalence and associated factors: A cross-sectional study. Pan Afr. Med. J. 2022, 41, 311. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Yuan, C.; Liu, S.; Zhang, Q.; Zhang, S.; Zhu, S. Coffee and tea intake with long-term risk of irritable bowel syndrome: A large-scale prospective cohort study. Int. J. Epidemiol. 2023, 52, 1459–1472. [Google Scholar] [CrossRef]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. Fixed-Effect vs Random-Effects Models for Meta-Analysis: 3 Points to Consider. Global Spine J. 2022, 12, 1624–1626. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Salamatullah, A.M. Relationship between the Chemical Composition and the Biological Functions of Coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef] [PubMed]
- Anty, R.; Marjoux, S.; Iannelli, A.; Patouraux, S.; Schneck, A.S.; Bonnafous, S.; Gire, C.; Amzolini, A.; Ben-Amor, I.; Saint-Paul, M.C.; et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J. Hepatol. 2012, 57, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients 2021, 13, 3042. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef]
- Bergmann, H.; Rogoll, D.; Scheppach, W.; Melcher, R.; Richling, E. The Ussing type chamber model to study the intestinal transport and modulation of specific tight-junction genes using a colonic cell line. Mol. Nutr. Food Res. 2009, 53, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef]
- Post, S.M.; de Wit, E.C.; Princen, H.M. Cafestol, the cholesterol-raising factor in boiled coffee, suppresses bile acid synthesis by downregulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase in rat hepatocytes. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3064–3070. [Google Scholar] [CrossRef]
- Xiong, F.; Zhang, L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front. Neuroendocrinol. 2013, 34, 27–46. [Google Scholar] [CrossRef]
- Su, Q.; Tun, H.M.; Liu, Q.; Yeoh, Y.K.; Mak, J.W.Y.; Chan, F.K.; Ng, S.C. Gut microbiome signatures reflect different subtypes of irritable bowel syndrome. Gut Microbes 2023, 15, 2157697. [Google Scholar] [CrossRef] [PubMed]
- Dwiyanto, J.; Hussain, M.H.; Reidpath, D.; Ong, K.S.; Qasim, A.; Lee, S.W.H.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Ethnicity influences the gut microbiota of individuals sharing a geographical location: A cross-sectional study from a middle-income country. Sci. Rep. 2021, 11, 2618. [Google Scholar] [CrossRef] [PubMed]
- Borrello, K.; Lim, U.; Park, S.Y.; Monroe, K.R.; Maskarinec, G.; Boushey, C.J.; Wilkens, L.R.; Randolph, T.W.; Le Marchand, L.; Hullar, M.A.; et al. Dietary Intake Mediates Ethnic Differences in Gut Microbial Composition. Nutrients 2022, 14, 660. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Vervoort, J.; Beekmann, K.; Baccaro, M.; Kamelia, L.; Wesseling, S.; Rietjens, I.M.C.M. Interindividual Differences in Human Intestinal Microbial Conversion of (-)-Epicatechin to Bioactive Phenolic Compounds. J. Agric. Food Chem. 2020, 68, 14168–14181. [Google Scholar] [CrossRef] [PubMed]


| Authors, Year | Region | Total Number of Subjects | Total Number of Cases | Study Population | Measure of Coffee Intake | Definition of Coffee Consumption (Lowest vs. Highest Category) | IBS Assessment Criteria | |
|---|---|---|---|---|---|---|---|---|
| Sexes Involved in Study | Age of Participants at Baseline (Years) | |||||||
| Khademolhosseini et al., 2011 [14] | Asia | 1978 | 191 | M + F | 39 | Baseline coffee intake, questionnaire | Consumer vs. abstainer | ROME II |
| Basandra et al., 2014 [15] | Asia | 200 | 33 | M + F | 20.4 | Baseline coffee intake, questionnaire | 0 cups/day vs. >2 cups/day | ROME III |
| Guo et al., 2015 [16] | Asia | 157 | 78 | M + F | 46.8/43.3 | Baseline coffee intake, questionnaire | Consumer vs. abstainer | ROME III |
| Al Saadi et al., 2016 [28] | Asia | 302 | 50 | M + F | 21.6 | Baseline coffee intake, questionnaire | 1 cup/day vs. >3 cups/day | ROME III |
| Kesuma et al., 2021 [29] | Asia | 454 | 137 | M + F | 15.8 | Baseline coffee intake, questionnaire | Consumer vs. abstainer | ROME III |
| Koochakpoor et al., 2021 [30] | Asia | 3362 | NR | M + F | 36.1 | Baseline coffee intake, questionnaire | Non-drinker vs. >3 cups/day | ROME III |
| El Sharawy et al., 2022 [31] | Africa | 182 | 50 | M + F | 23.9 | Baseline caffeine intake, questionnaire | Consumer vs. abstainer | ROME III |
| Wu et al., 2023 [32] | UK | 425,387 | 7736 | M + F | 56.22 | Baseline coffee intake, questionnaire | 0.5–1 cups/day vs. 2–3 cups/day vs. ≥4 cups/day | ICD-10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Yau, C.Y.; Loh, C.Y.L.; Lim, W.S.; Teoh, S.E.; Yau, C.E.; Ong, C.; Thumboo, J.; Namasivayam, V.S.O.; Ng, Q.X. Examining the Association between Coffee Intake and the Risk of Developing Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4745. https://doi.org/10.3390/nu15224745
Lee JY, Yau CY, Loh CYL, Lim WS, Teoh SE, Yau CE, Ong C, Thumboo J, Namasivayam VSO, Ng QX. Examining the Association between Coffee Intake and the Risk of Developing Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(22):4745. https://doi.org/10.3390/nu15224745
Chicago/Turabian StyleLee, Jasmine Yiling, Chun Yi Yau, Caitlin Yuen Ling Loh, Wei Shyann Lim, Seth En Teoh, Chun En Yau, Clarence Ong, Julian Thumboo, Vikneswaran S. O. Namasivayam, and Qin Xiang Ng. 2023. "Examining the Association between Coffee Intake and the Risk of Developing Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis" Nutrients 15, no. 22: 4745. https://doi.org/10.3390/nu15224745
APA StyleLee, J. Y., Yau, C. Y., Loh, C. Y. L., Lim, W. S., Teoh, S. E., Yau, C. E., Ong, C., Thumboo, J., Namasivayam, V. S. O., & Ng, Q. X. (2023). Examining the Association between Coffee Intake and the Risk of Developing Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrients, 15(22), 4745. https://doi.org/10.3390/nu15224745







