Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review
Abstract
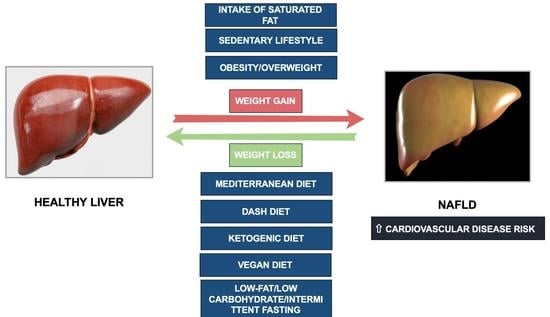
:1. Introduction
1.1. The Complex Nexus between Cardiovascular Disease and NAFLD. NAFLD Pharmacotherapy and Dietetic Approach to Managing NAFLD
1.2. Clinical Practice Guidelines for the Dietary Treatment of NAFLD
2. Nutritional Treatment in NAFLD
2.1. Mediterranean Diet and NAFLD
2.2. Low-Fat Diet and NAFLD
2.3. The Dietary Approaches to Stopping Hypertension (DASH) and NAFLD
2.4. Vegetarian Diets and NAFLD
2.5. Low/Very Low-Carbohydrate Ketogenic Diet and NAFLD
2.6. Intermittent Fasting and NAFLD
| Author [Ref.] | Study Design | Population Health Status | Sample Size | Duration | Type of Intervention/Diet Evaluated | Type of Control | Main Findings |
|---|---|---|---|---|---|---|---|
| Kontogianni et al. [33] | Observational | Recent NAFLD diagnosis | 73 | - | MedDiet | - | MedDiet negatively correlated with liver enzymes (p = 0.03), insulin resistance (p = 0.001) and severity of steatosis (p = 0.006). |
| Khalatbari-Soltani et al. [34] | Cross-sectional | Two adult populations (Fenland and CoLaus cohorts) | 13.602 | - | MedDiet | - | Greater adherence to MedDiet was associated with lower liver steatosis [0.86 (CI: 0.81–0.90)]. |
| Baratta et al. [35] | Observational | Healthy adults | 584 | - | MedDiet | - | Higher adherence to MedDiet was associated with lover IR (OR: 0.801; p = 0.018) and NAFLD (high adherence vs. low OR: 0.093; p = 0.030). |
| Kouvari et al. [36] | Observational | NAFLD | 3032 | - | MedDiet | Higher MedDiet score was inversely associated with NAFLD [0.53 CI: 0.29–0.95)]. | |
| Abenavoli et al. [37] | Randomized trial | Patients with overweight and NAFLD | 50 | 6 months | A.MedDiet B.MedDiet+ antioxidant. | Standard of care diet | MedDiet alone or with antioxidant improves insulin sensivity (p = 0.045), HOMAR-IR (p = 0.021), Tryglicerides index (p = 0.020) and fatty-liver index (p = 0.017) and anthropometric parameters (all p = 0.001). |
| Misciagna et al. [38] | Randomized controlled trial | Moderate to severe NAFLD | 98 | 6 months | Low glycemic index Mediterranean diet | Standard of care diet | Low glycemic index Mediterranean diet decreases NAFLD score [OR: 0.07 (CI: 0.02–0.12; p < 0.05)] |
| Properzi et al. [39] | Randomized controlled trial | NAFLD with cardiometabolic risk factors | 56 | 12 weeks | MedDiet | Low-fat diet | Both diets improve liver steatosis (p < 0.01) without difference betweens both groups (p = 0.32) |
| Ryan et al. [40] | Randomized controlled trial | NAFLD patients with obesity | 12 | 6 weeks | MedDiet | Low fat-high carbohydrate diet | MedDiet reduces liver steatosis (39 ± 4% vs. 7 ± 3%; p = 0.012). |
| Akhlaghi et al. [41] | Meta-analysis | NAFLD patients | 17.095 | - | MedDiet | - | A trend for the improvement of NAFLD was observed for MedDiet [0.95 (CI: 0.9–1); p = 0.05]. |
| Kawaguchi et al. [43] | Meta-analysis | NAFLD patients | 250 | - | MedDiet | Standard of care diet | MedDiet improved liver steatosis (CI: −0956 to −0.237; p = 0.001) and IR (CI: −0.713 to −0.003; p = 0.048). |
| Haigh et al. [42] | Meta-analysis | NAFLD patients | 3037 | - | MedDiet with calorie restriction | Standard of care diet | MedDiet reduced ALT (p < 0.001), AST (p = 0.004), fatty liver index (p < 0.001) and liver steatosis (p = 0.02). |
| Gepner et al. [48] | Randomized controlled trial | Patients with obesity/dyslipidemia | 278 | - | Low fat | MedDiet/Low-carbohydrate | Liver fat content reduction in all groups (- 4%compared to baseline, p < 0.001). Greater in the MedDiet/Low-carbohydrate group (p = 0.036) was observed. |
| Hekmatdoost et al. [52] | Case-control | NAFLD patients | 306 | - | DASH diet | Standard of care | Inverse relationship between the DASH diet and NAFLD risk (OR: 0.70; p < 0.05). |
| Park et al. [54] | Observational | Multiethnic Cohort | 2959 | - | DASH diet | DASH diet highest vs. lower quartile | High DASH scores are associated with a reduction in NAFLD risk (OR: 0.78; p < 0.001). |
| Watzinger et al. [55] | Cross-sectional | NAFLD patients | 136 | - | DASH diet and MedDiet | - | Diet quality scores was inversely associated with liver fat content (OR: 4.41; p = 0.04 for MedDiet score and OR: 4.41; p = 0.05 for DASH score). |
| Razavi Zade et al. [57] | Randomized controlled trial | Overweigh and obese patients with NAFLD | 60 | DASH diet | Standard of care | DASH diet reduced body max index (p = 0.06), liver enzymes (p < 0.05), triglycerides (p = 0.04), insulin resistance (p = 0.01) and inflammatory markers (p < 0.05). | |
| Chiu et al. [58] | Cross-sectional | Non-vegetarians and vegetarians | 3400 | - | Vegetarian diets | - | Vegetarian diets may be inversely associated with NAFLD (OR: 0.79; p < 0.05). |
| Jin et al. [59] | Observational | Vegetarians | 339 | - | Vegetarian diet | Vegetarian diet, was associated with lower odds of NAFLD [OR: 0.43 (CI: 0.32–0.87); p = 0.013] and cardiometabolic risk factors(Body max index, LDL cholesterol, fasting glucose and insulin resistance, all p < 0.05). | |
| Garousi et al. [60] | Randomized controlled trial | Overweight/obese adults with NAFLD | 75 | 3 months | Lacto-ovo-vegetarian diet. | Standard weight-loss diet | Lacto-ovo-vegetarian diet reduced grade of NAFLD compared to Standard weight-loss diet (67% vs. 21%; p = 0.01) |
| Ramon-Krauel et al. [62] | Randomized controlled trial | Obese children | 17 | 6 months | Low-glycemic load | Low-fat diet | Both diets improved liver steatosis (without differences between the two diets) |
| Ahn et al. [61] | Meta-analysis | Heterogeneus population with NAFLD | 370 | - | Low-carbohidrate diet | Low-fat diet | No differences between Low-carbohidrate and Low-fat diet |
| Li et al. [66] | Randomized controlled trial | Polycystic ovary syndrome | 20 | 12 weeks | Ketogenic diet | Conventional pharmacological treatment | Ketogenic diet improved menstrual cycle, body weight, blood glucose and liver function test (all p < 0.05) at 12 weeks.Ketogenic diet group reduced liver function test compared to control group (p < 0.05). |
| Cunha et al. [67] | Randomized controlled trial | Healthy participants | 39 | 2 months | Very low-calorie ketogenic diet | Low-calorie diet | Very low-calorie ketogenic diet reduced weigh (−9.7 kg vs−1.67 kg; p < 0.0001) and liver fat (4.77 vs. 0.79; p < 0.005)compared to low-calorie diet. |
| Drinda et al. [69] | Observational | T2DM and non-T2DM participants | 697 | - | Periodic fasting | - | Periodic fasting with weigh reduction rapid improved fatty liver index (Non-T2DM participants: −14.02 compared to baseline; p < 0.0001 and T2DM participants −19.15; p < 0.001 compared to baseline. Additionally, greater changes in T2DM participants p < 0.002 T2DM vs. non-T2DM). |
| Cai et al. [71] | Randomized controlled trial | NAFLD patients | 271 | 12 weeks. | Alternate-day fasting | Standard of care | Changes in fat free mass, lipids, fasting insulin, blood pressure and liver stiffness in both groups compared to baseline without differences between the two groups. |
| Holmer et al. [70] | Randomized controlled trial | NAFLD patients | 74 | 12 | Interminent calorie restriction and low-carbohydrate high-fat diet | Standard of care | Intermintent calorie restriction diet reduced hepatic fat content (−2.6%; CI: −5 to −0.2). Low-carbohidrate high fat diet reduce hepatic fat (−3.9%; CI: −6.3 to −1.4) |
| Yin et al. [73] | Meta-analysis | NAFLD patients | 417 | - | Intermittent fasting | - | Intermittent fasting improved weigh (−2.45%, CI: −3.98 to −0.91; p < 0.05) and liver enzymes (ALT: −10.54, CI: −14.01 to −7.08; p < 0.05 and ALT: −11.31, CI: −14.3 to −8.32; p < 0.05). |
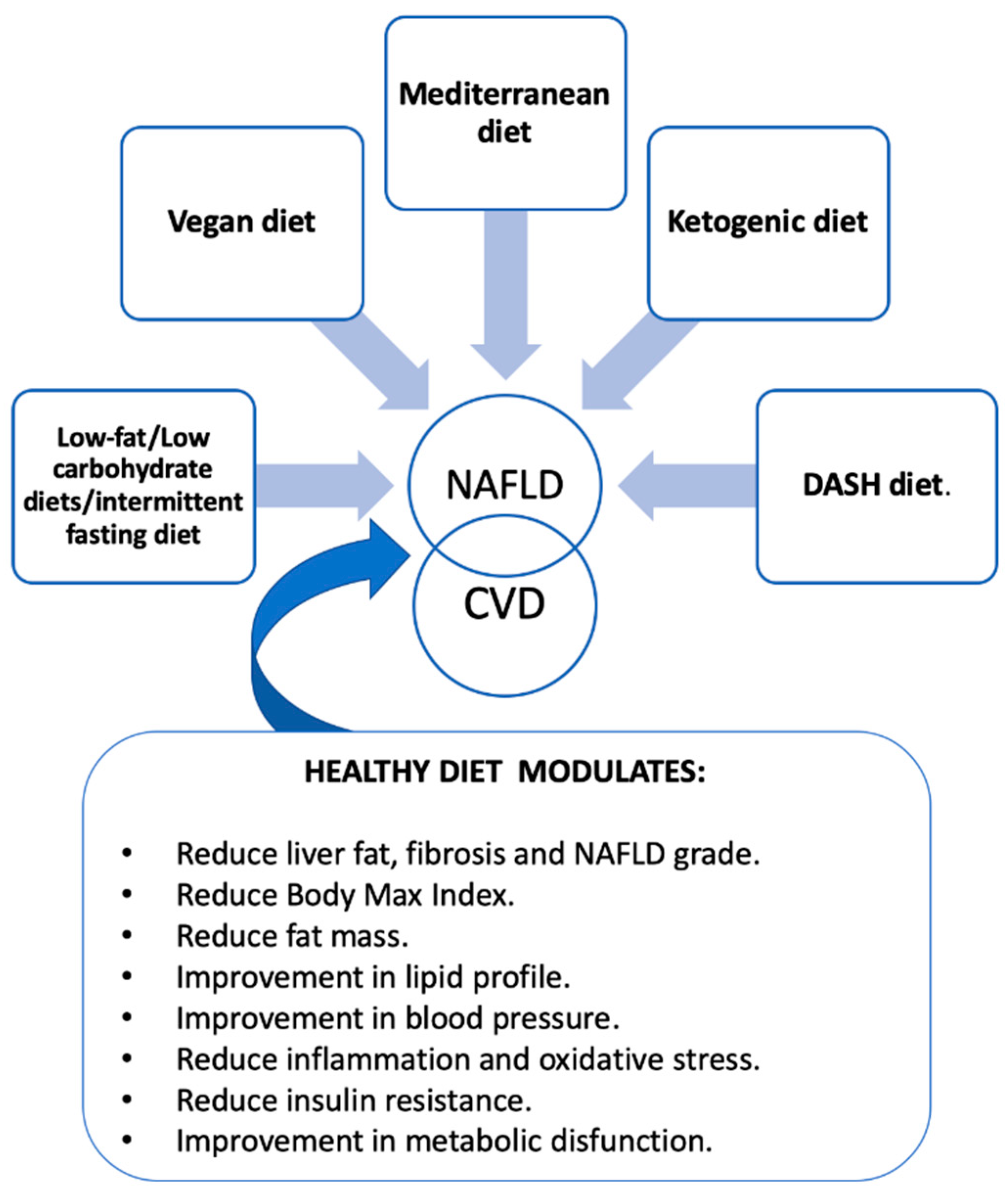
3. Factors That Modulate Dietary Patterns
4. Future Perspectives
5. Take Home Message for Healthcare Givers
- Reduce total energy intake: moderate calorie restriction (500–1000 kcal/day that can be achieved with different dietary pattern such us intermittent fasting, low carbohydrate diets, etc.) can improve NAFLD. Patients with NAFLD should aim to lose 5–10% of their body weight over a period of six months.
- Follow a Mediterranean-style diet or DASH diet: a Mediterranean diet is rich in fruits, vegetables, whole grains, legumes, nuts, fish, and olive oil, low in red and processed meat, refined carbohydrates, and saturated fat. This dietary pattern is associated with a lower risk of NAFLD and its complications.
- Limit added sugars and refined carbohydrates: high intake of added sugars and refined carbohydrates increases the risk of NAFLD. Patients should avoid or limit intake of sugar-sweetened beverages, sweets, and high-calorie snacks.
- Avoid alcohol consumption: Alcohol is a hepatotoxin and can worsen liver damage in patients with NAFLD. Patients with NAFLD should avoid alcohol or limit consumption to no more than one drink per day for women and two drinks per day for men.
6. Final Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NAFLD | non-alcoholic fatty liver disease. |
| CVD | cardiovascular disease. |
| NO | nitric oxide. |
| MUFA | monounsaturated fatty acids. |
| n-3 PUFAs | omega 3 polyunsaturated fatty acids. |
| MRI | magnetic resonance imaging. |
| T2DM | type 2 diabetes. |
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Arrese, M.; Cabrera, D.; Kalergis, A.M.; Feldstein, A.E. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 2016, 61, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
- Anstee, Q.M.; Seth, D.; Day, C.P. Genetic Factors That Affect Risk of Alcoholic and Nonalcoholic Fatty Liver Disease. Gastroenterology 2016, 150, 1728–1744.e7. [Google Scholar] [CrossRef] [PubMed]
- Severson, T.J.; Besur, S.; Bonkovsky, H.L. Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World J. Gastroenterol. 2016, 22, 6742–6756. [Google Scholar] [CrossRef]
- Machado, M.V.; Cortez-Pinto, H. Diet, Microbiota, Obesity, and NAFLD: A Dangerous Quartet. Int. J. Mol. Sci. 2016, 17, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.J.; Peloso, G.M.; Yu, H.; Butterworth, A.S.; Wang, X.; Mahajan, A.; Saleheen, D.; Emdin, C.; Alam, D.; Alves, A.C.; et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 2017, 49, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechner, K.; McKenzie, A.L.; Kränkel, N.; Von Schacky, C.; Worm, N.; Nixdorff, U.; Lechner, B.; Scherr, J.; Weingärtner, O.; Krauss, R.M. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Oseini, A.M.; Sanyal, A.J. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017, 37, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Spooner, M.H.; Jump, D.B. Omega-3 fatty acids and nonalcoholic fatty liver disease in adults and children: Where do we stand? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 103–110. [Google Scholar] [CrossRef]
- Attia, S.L.; Softic, S.; Mouzaki, M. Evolving Role for Pharmacotherapy in NAFLD/NASH. Clin. Transl. Sci. 2021, 14, 11–19. [Google Scholar] [CrossRef]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; De Vincentis, A.; Mikhailidis, D.P.; Atkin, S.L.; Mantzoros, C.S.; Jamialahmadi, T.; Sahebkar, A. Non-alcoholic fatty liver disease and steatohepatitis: State of the art on effective therapeutics based on the gold standard method for diagnosis. Mol. Metab. 2021, 50, 101049. [Google Scholar] [CrossRef]
- Kothari, S.; Dhami-Shah, H.; Shah, S.R. Antidiabetic Drugs and Statins in Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef] [PubMed]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [Green Version]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, S. Dietary approach in the treatment of nonalcoholic fatty liver disease. World J. Hepatol. 2015, 7, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Verga, S.; Tranchina, M.R.; Cottone, S.; Cerasola, G. Effects of hypocaloric very-low-carbohydrate diet vs. Mediterranean diet on endothelial function in obese women*. Eur. J. Clin. Investig. 2009, 39, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Esposito, K.; La Sala, L.; Pujadas, G.; De Nigris, V.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2014, 13, 140. [Google Scholar] [CrossRef]
- Torres-Peña, J.D.; Garcia-Rios, A.; Delgado-Casado, N.; Gomez-Luna, P.; Alcala-Diaz, J.F.; Yubero-Serrano, E.M.; Gomez-Delgado, F.; Leon-Acuña, A.; Lopez-Moreno, J.; Camargo, A.; et al. Mediterranean diet improves endothelial function in patients with diabetes and prediabetes: A report from the CORDIOPREV study. Atherosclerosis 2018, 269, 50–56. [Google Scholar] [CrossRef]
- Torres-Peña, J.D.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Mediterranean Diet and Endothelial Function: A Review of its Effects at Different Vascular Bed Levels. Nutrients 2020, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Imamura, F.; Brage, S.; De Lucia Rolfe, E.; Griffin, S.J.; Wareham, N.J.; Marques-Vidal, P.; Forouhi, N.G. The association between adherence to the Mediterranean diet and hepatic steatosis: Cross-sectional analysis of two independent studies, the UK Fenland Study and the Swiss CoLaus Study. BMC Med. 2019, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Boutari, C.; Chrysohoou, C.; Fragkopoulou, E.; Antonopoulou, S.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B.; Mantzoros, C.S. Mediterranean diet is inversely associated with steatosis and fibrosis and decreases ten-year diabetes and cardiovascular risk in NAFLD subjects: Results from the ATTICA prospective cohort study. Clin. Nutr. 2021, 40, 3314–3324. [Google Scholar] [CrossRef]
- Abenavoli, L.; Greco, M.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients 2017, 9, 870. [Google Scholar] [CrossRef]
- Misciagna, G.; del Pilar Díaz, M.; Caramia, D.V.; Bonfiglio, C.; Franco, I.; Noviello, M.R.; Chiloiro, M.; Abbrescia, D.I.; Mirizzi, A.; Tanzi, M.; et al. Effect of a low glycemic index Mediterranean diet on non-alcoholic fatty liver disease. A randomized controlled clinici trial. J. Nutr. Health Aging 2017, 21, 404–412. [Google Scholar] [CrossRef]
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Ghasemi-Nasab, M.; Riasatian, M. Mediterranean diet for patients with non-alcoholic fatty liver disease, a systematic review and meta-analysis of observational and clinical investigations. J. Diabetes Metab. Disord. 2020, 19, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Charlton, M.; Kawaguchi, A.; Yamamura, S.; Nakano, D.; Tsutsumi, T.; Zafer, M.; Torimura, T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin. Liver Dis. 2021, 41, 225–234. [Google Scholar] [CrossRef]
- Yang, Q.; Lang, X.; Li, W.; Liang, Y. The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Silverii, G.A.; Cosentino, C.; Santagiuliana, F.; Rotella, F.; Benvenuti, F.; Mannucci, E.; Cresci, B. Effectiveness of low-carbohydrate diets for long-term weight loss in obese individuals: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 1458–1468. [Google Scholar] [CrossRef]
- Bray, G.A.; Ryan, D.H. Evidence-based weight loss interventions: Individualized treatment options to maximize patient outcomes. Diabetes Obes. Metab. 2021, 23, 50–62. [Google Scholar] [CrossRef]
- Willems, A.E.M.; Sura–de Jong, M.; van Beek, A.P.; Nederhof, E.; van Dijk, G. Effects of macronutrient intake in obesity: A meta-analysis of low-carbohydrate and low-fat diets on markers of the metabolic syndrome. Nutr. Rev. 2021, 79, 429–444. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, A.; Vasquez, K.; Guishard, D.; Naji, M.; Ronning, A.; George-Alexander, G.; Vasquez, D.; Sylvester, C.; Pagano, W.; Khalida, C.; et al. Implementing DASH-aligned Congregate Meals and Self-Measured Blood Pressure in two senior centers: An open label study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1998–2009. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Gong, T.; Mu, L.; Li, J.; Yu, P.; Wang, H.; Bu, X.; Mu, L.; Mei, Y. Short-term effects of modest salt reduction combined with DASH diet on changing salt eating habits in hypertensive patients with type II diabetes. Clin. Exp. Hypertens. 2022, 44, 514–522. [Google Scholar] [CrossRef]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Hekmatdoost, A.; Shamsipour, A.; Meibodi, M.; Gheibizadeh, N.; Eslamparast, T.; Poustchi, H. Adherence to the Dietary Approaches to Stop Hypertension (DASH) and risk of Nonalcoholic Fatty Liver Disease. Int. J. Food Sci. Nutr. 2016, 67, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Lim, U.; Jacobs, S.; Monroe, K.R.; Ernst, T.; Buchthal, S.D.; Shepherd, J.A.; Wilkens, L.R.; Le Marchand, L.; Boushey, C.J. Diet Quality in Midadulthood Predicts Visceral Adiposity and Liver Fatness in Older Ages: The Multiethnic Cohort Study. Obesity 2017, 25, 1442–1450. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Noureddin, M.; Boushey, C.; Wilkens, L.; Setiawan, W. Diet Quality Association with Nonalcoholic Fatty Liver Disease by Cirrhosis Status: The Multiethnic Cohort. Curr. Dev. Nutr. 2020, 4, nzaa024. [Google Scholar] [CrossRef] [Green Version]
- Watzinger, C.; Nonnenmacher, T.; Grafetstätter, M.; Sowah, S.A.; Ulrich, C.M.; Kauczor, H.-U.; Kaaks, R.; Schübel, R.; Nattenmüller, J.; Kühn, T. Dietary Factors in Relation to Liver Fat Content: A Cross-sectional Study. Nutrients 2020, 12, 825. [Google Scholar] [CrossRef] [Green Version]
- Katsiki, N.; Stoian, A.P.; Rizzo, M. Dietary patterns in non-alcoholic fatty liver disease (NAFLD): Stay on the straight and narrow path! Clín. Investig. Arterioscler. 2022, 34, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef]
- Chiu, T.; Lin, M.-N.; Pan, W.-H.; Chen, Y.-C.; Lin, C.-L. Vegetarian diet, food substitution, and nonalcoholic fatty liver. Tzu Chi Med. J. 2018, 30, 102. [Google Scholar] [CrossRef]
- Jin, Y.; Kanaya, A.M.; Kandula, N.R.; Rodriguez, L.A.; Talegawkar, S.A. Vegetarian Diets Are Associated with Selected Cardiometabolic Risk Factors among Middle-Older Aged South Asians in the United States. J. Nutr. 2018, 148, 1954–1960. [Google Scholar] [CrossRef] [Green Version]
- Garousi, N.; Tamizifar, B.; Pourmasoumi, M.; Feizi, A.; Askari, G.; Clark, C.C.T.; Entezari, M.H. Effects of lacto-ovo-vegetarian diet vs. standard-weight-loss diet on obese and overweight adults with non-alcoholic fatty liver disease: A randomised clinical trial. Arch. Physiol. Biochem. 2021, 1–9. [Google Scholar] [CrossRef]
- Ahn, J.; Jun, D.W.; Lee, H.Y.; Moon, J.H. Critical appraisal for low-carbohydrate diet in nonalcoholic fatty liver disease: Review and meta-analyses. Clin. Nutr. 2019, 38, 2023–2030. [Google Scholar] [CrossRef]
- Ramon-Krauel, M.; Salsberg, S.L.; Ebbeling, C.B.; Voss, S.D.; Mulkern, R.V.; Apura, M.M.; Cooke, E.A.; Sarao, K.; Jonas, M.M.; Ludwig, D.S. A Low-Glycemic-Load versus Low-Fat Diet in the Treatment of Fatty Liver in Obese Children. Child. Obes. 2013, 9, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.; Kim, Y. The Effect of Low Glycemic Index and Glycemic Load Diets on Hepatic Fat Mass, Insulin Resistance, and Blood Lipid Panels in Individuals with Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2019, 17, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, M.S.; Patterson, B.W.; Klein, S. Dietary Fat and Carbohydrates Differentially Alter Insulin Sensitivity During Caloric Restriction. Gastroenterology 2009, 136, 1552–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Bai, W.-P.; Jiang, B.; Bai, L.-R.; Gu, B.; Yan, S.-X.; Li, F.-Y.; Huang, B. Ketogenic diet in women with polycystic ovary syndrome and liver dysfunction who are obese: A randomized, open-label, parallel-group, controlled pilot trial. J. Obstet. Gynaecol. Res. 2021, 47, 1145–1152. [Google Scholar] [CrossRef]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients with Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [Green Version]
- Drinda, S.; Grundler, F.; Neumann, T.; Lehmann, T.; Steckhan, N.; Michalsen, A.; Wilhelmi de Toledo, F. Effects of Periodic Fasting on Fatty Liver Index—A Prospective Observational Study. Nutrients 2019, 11, 2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmer, M.; Lindqvist, C.; Petersson, S.; Moshtaghi-Svensson, J.; Tillander, V.; Brismar, T.B.; Hagström, H.; Stål, P. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet—A randomised controlled trial. JHEP Rep. 2021, 3, 100256. [Google Scholar] [CrossRef]
- Cai, H.; Qin, Y.-L.; Shi, Z.-Y.; Chen, J.-H.; Zeng, M.-J.; Zhou, W.; Chen, R.-Q.; Chen, Z.-Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Johari, M.I.; Yusoff, K.; Haron, J.; Nadarajan, C.; Ibrahim, K.; Wong, M.S.; Abdul Hafidz, M.I.; Chua, B.; Hamid, N.; Arifin, W.N.; et al. Author Correction: A Randomised Controlled Trial on the Effectiveness and Adherence of Modified Alternate-day Calorie Restriction in Improving Activity of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 10599. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, Z.; Xiang, Y.; Peng, H.; Yang, P.; Yuan, S.; Zhang, X.; Wu, Y.; Huang, M.; Li, J. Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 709683. [Google Scholar] [CrossRef] [PubMed]
- Craigie, A.M.; Lake, A.A.; Kelly, S.A.; Adamson, A.J.; Mathers, J.C. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas 2011, 70, 266–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senee, A.; Yashwinee Bye, I.; Jeewon, R. An Analysis of the Contributors and Factors Influencing Dietary Patterns among the Elderly Population. Curr. Res. Nutr. Food Sci. J. 2022, 10, 895–903. [Google Scholar] [CrossRef]
- Kell, K.P.; Judd, S.E.; Pearson, K.E.; Shikany, J.M.; Fernández, J.R. Associations between socio-economic status and dietary patterns in US black and white adults. Br. J. Nutr. 2015, 113, 1792–1799. [Google Scholar] [CrossRef] [Green Version]
- Czarnocinska, J.; Wadolowska, L.; Lonnie, M.; Kowalkowska, J.; Jezewska-Zychowicz, M.; Babicz-Zielinska, E. Regional and socioeconomic variations in dietary patterns in a representative sample of young polish females: A cross-sectional study (GEBaHealth project). Nutr. J. 2020, 19, 26. [Google Scholar] [CrossRef]
- Dunneram, Y.; Jeewon, R. Determinants of eating habits among older adults. Progr. Nutr. 2015, 17, 274–283. [Google Scholar]
- Black, M.; Bowman, M. Nutrition and Healthy Aging. Clin. Geriatr. Med. 2020, 36, 655–669. [Google Scholar] [CrossRef]
| Identifier | Study Design | Population Health Status | Sample Size | Duration | Type of Intervention | Type of Control | Liver Endpoint |
|---|---|---|---|---|---|---|---|
| NCT05309642 | Parallel control trial | 36 BMI >25 kg/m2 and 36 BMI < 25 kg/m2 | 72 | 12 weeks | Very low calories ketogenic diet | Standard of care diet | Change on liver steatosis by MRI |
| NCT05275608 | Parallel control trial | BMI > 30–40 kg/m2 NAFLD T2DM | 20 | 90 days | Very low calories ketogenic diet | Hypocaloric Mediterranean diet | Change in liver enzymes as secondary outcome |
| NCT04440540 | Parallel control trial | Obese patients | 40 | 1 year | Lifestyle program (diet not specified) | Standard of care diet | Change of intrahepatic triglycerides content in NAFLD |
| NCT05200585 | Parallel | NAFLD | 30 | 6 months | Behavioral: Healthy Liver/Hígado Sano program | Standard of care diet | MRI liver steatosis change |
| NCT04383951 | Parallel | NAFLD | 40 | 16 weeks | Ketogenic diet | Standard of care diet | MRI liver steatosis change |
| NCT05268042 | Randomized controlled trial | Obese adolescents with NALF | 80 | 6 months | Moderately carbohydrate-restricted diet | Fat-restricted control diet | MRI liver steatosis change |
| EudraCT:2021-000152-19 | Randomized controlled trial | NAFLD and prediabetes patients | 390 | 18 months | 1.Med diet + metformin placebo pioglitazone placebo 2. Med diet + metformin + pioglitazone placebo 3. Med diet + metformin + pioglitazone | - | Evaluated witch model induce a greater regression of NAFLD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Peña, J.D.; Arenas-de Larriva, A.P.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review. Nutrients 2023, 15, 1483. https://doi.org/10.3390/nu15061483
Torres-Peña JD, Arenas-de Larriva AP, Alcala-Diaz JF, Lopez-Miranda J, Delgado-Lista J. Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review. Nutrients. 2023; 15(6):1483. https://doi.org/10.3390/nu15061483
Chicago/Turabian StyleTorres-Peña, Jose D., Antonio P. Arenas-de Larriva, Juan F. Alcala-Diaz, Jose Lopez-Miranda, and Javier Delgado-Lista. 2023. "Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review" Nutrients 15, no. 6: 1483. https://doi.org/10.3390/nu15061483
APA StyleTorres-Peña, J. D., Arenas-de Larriva, A. P., Alcala-Diaz, J. F., Lopez-Miranda, J., & Delgado-Lista, J. (2023). Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review. Nutrients, 15(6), 1483. https://doi.org/10.3390/nu15061483