Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Chemicals and Reagents
2.3. Probiotics and Postbiotics
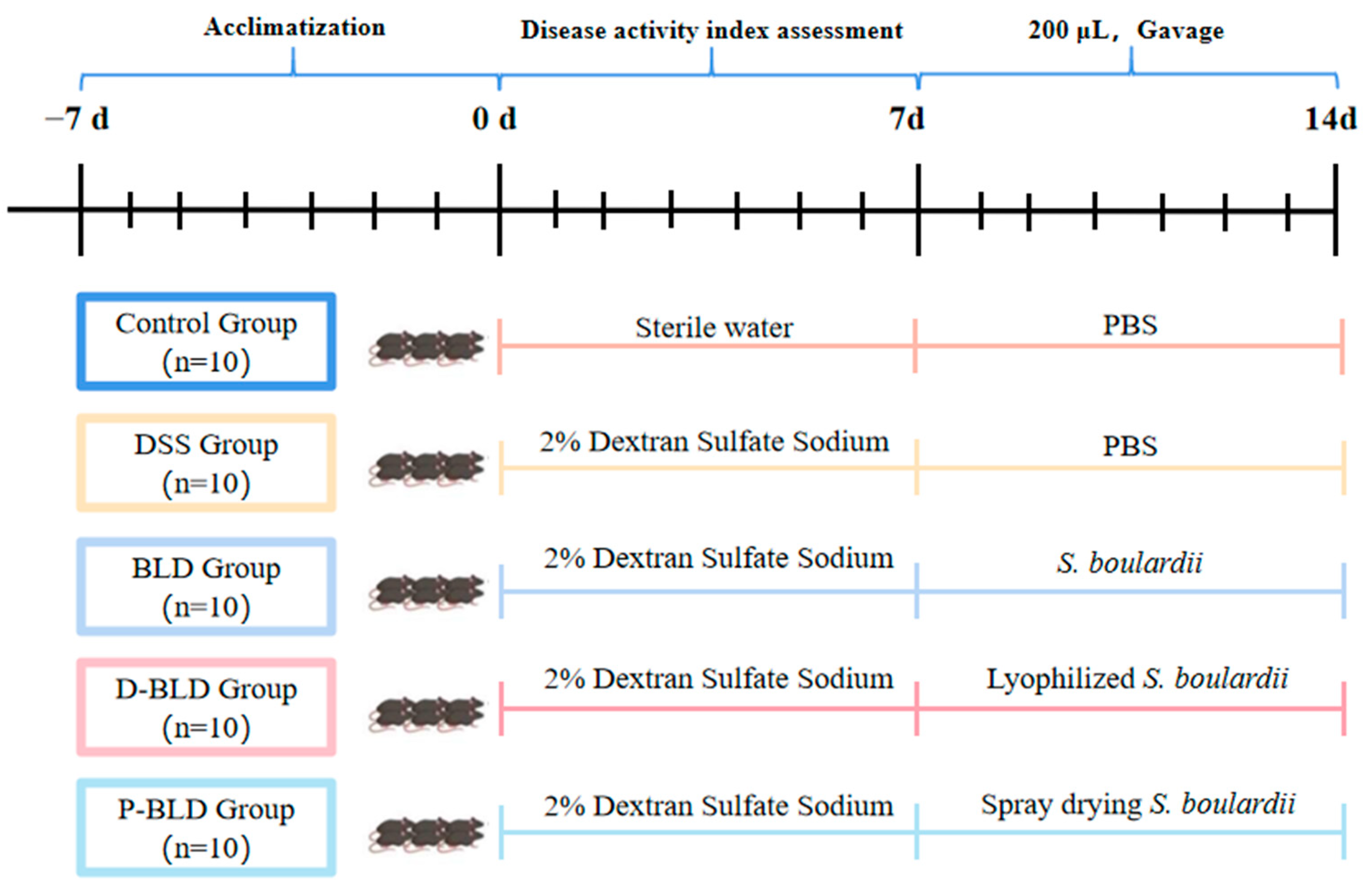
2.4. Animal Experiment
2.5. Histopathological Analysis
2.6. Measurement of Serum Inflammatory Indicators
2.7. Quantitative Real-Time Polymerase Chain Reaction Analysis for mRNA Expression
2.8. Gut Microbiota Analysis
2.9. Statistical Analysis
3. Results
3.1. Both S. boulardii and Its Postbiotics Can Alleviate the Symptoms of DSS-Induced Colitis in Mice
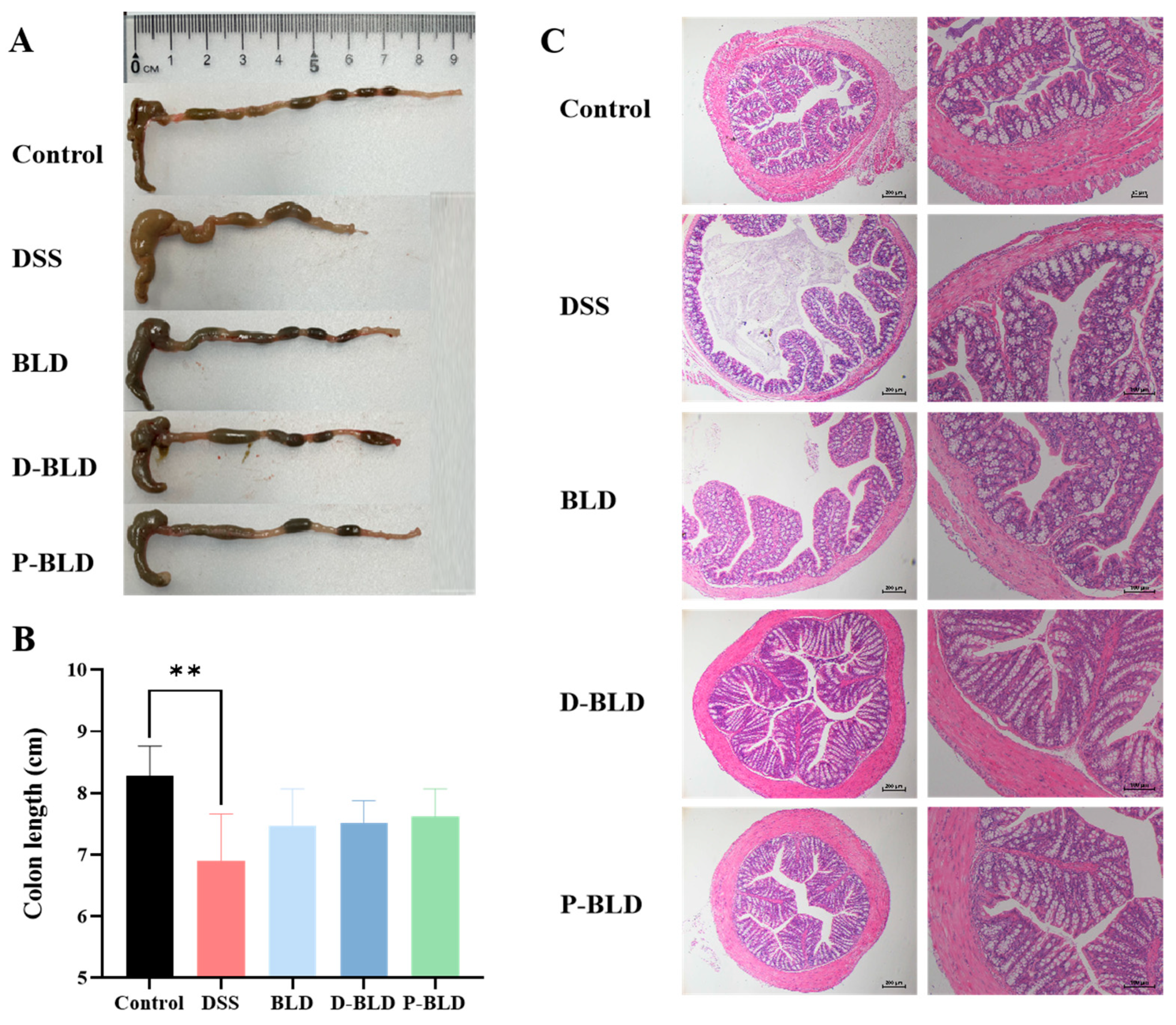
3.2. Both S. boulardii and Its Postbiotics Alleviated DSS-Induced Colonic Shortening and Pathological Changes in Mice
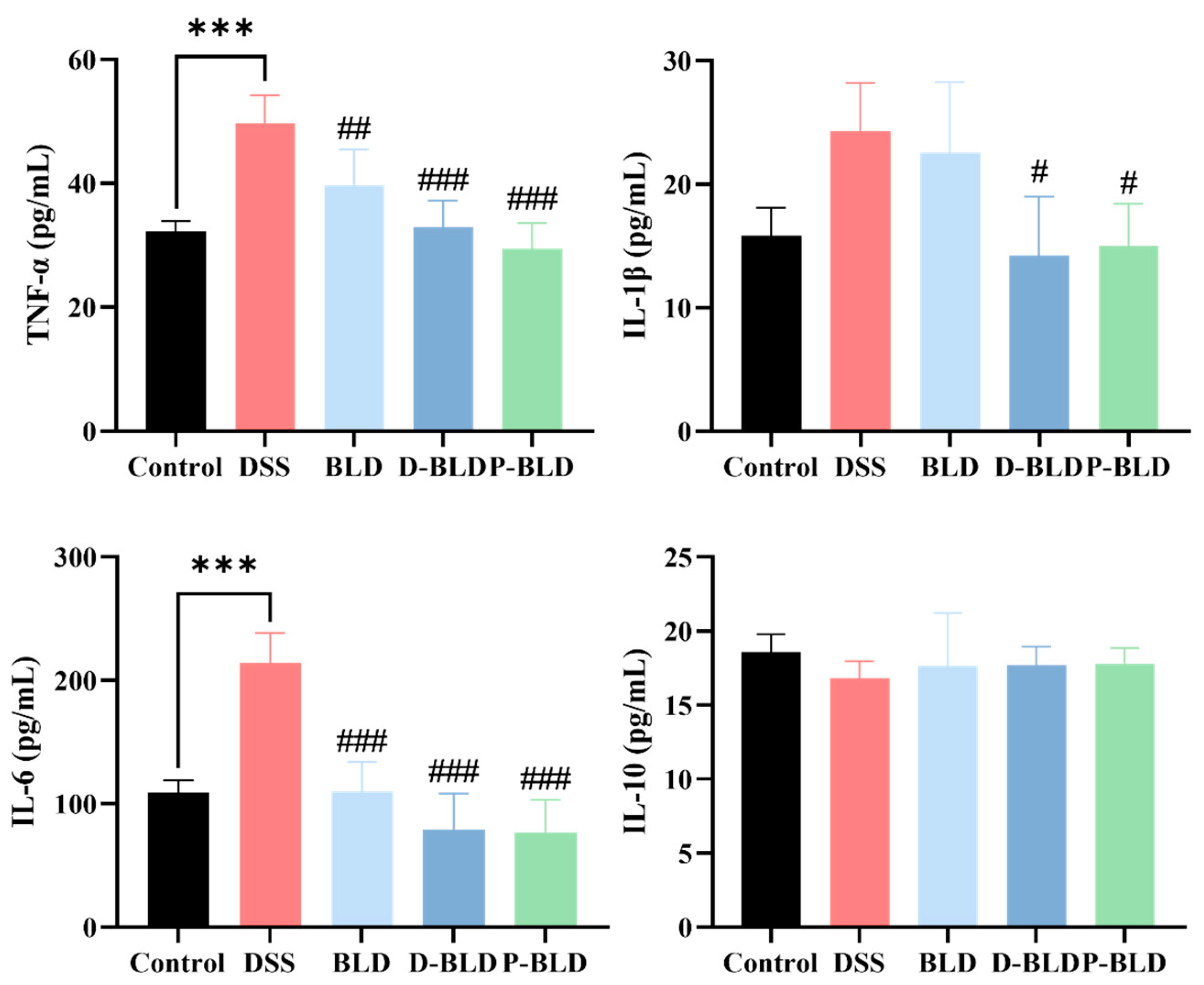
3.3. Both S. boulardii and Its Postbiotics Regulated Serum Cytokine Levels in DSS-Induced Colitis Mice
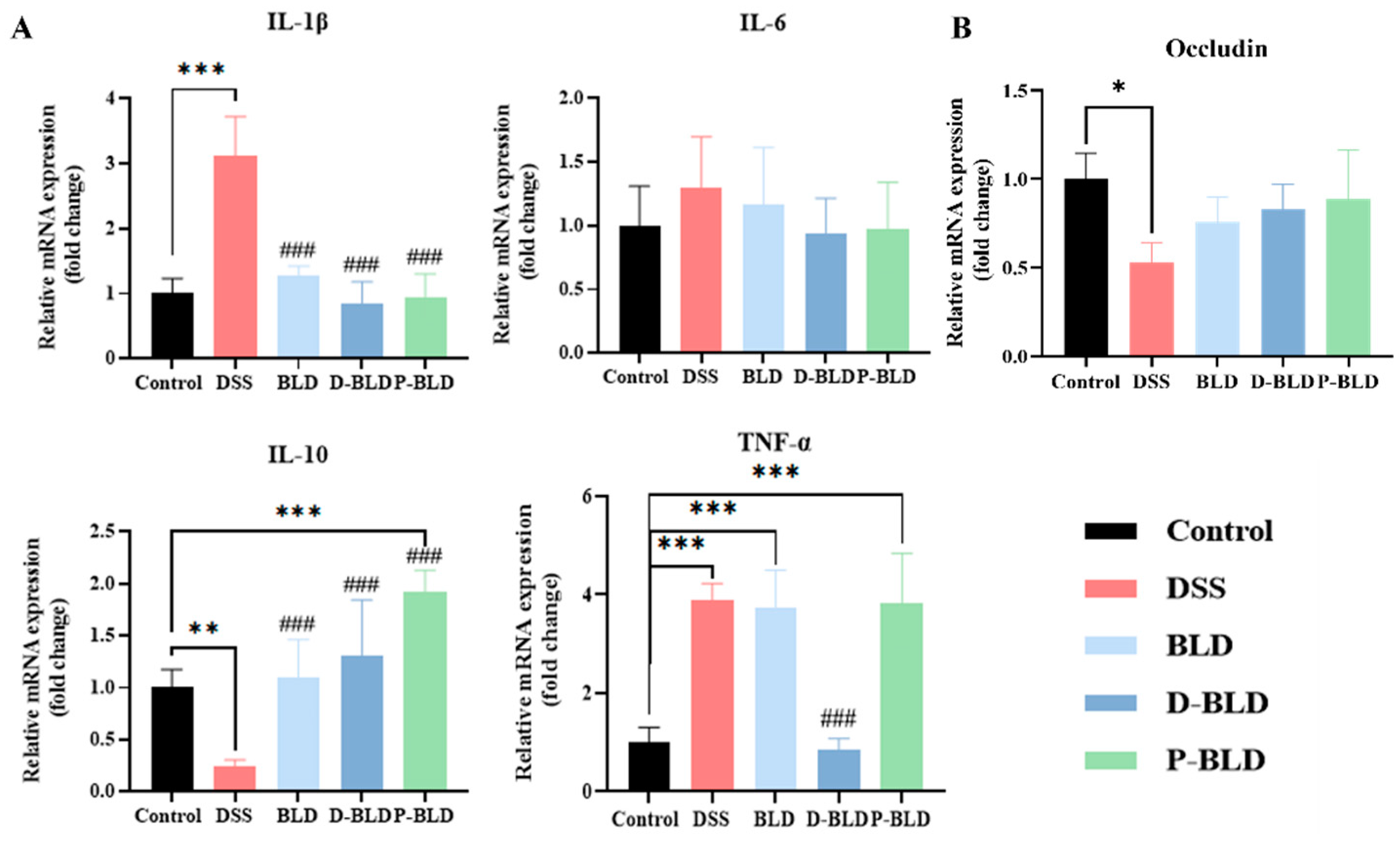
3.4. Both S. boulardii and Its Postbiotics Modulated the Expression Levels of Inflammatory Factors and Tight Junction Proteins in the Colons of Mice with DSS-Induced Colitis
3.5. Both S. boulardii and Its Postbiotics Modified Gut Microbiota in DSS-Induced Colitis Mice
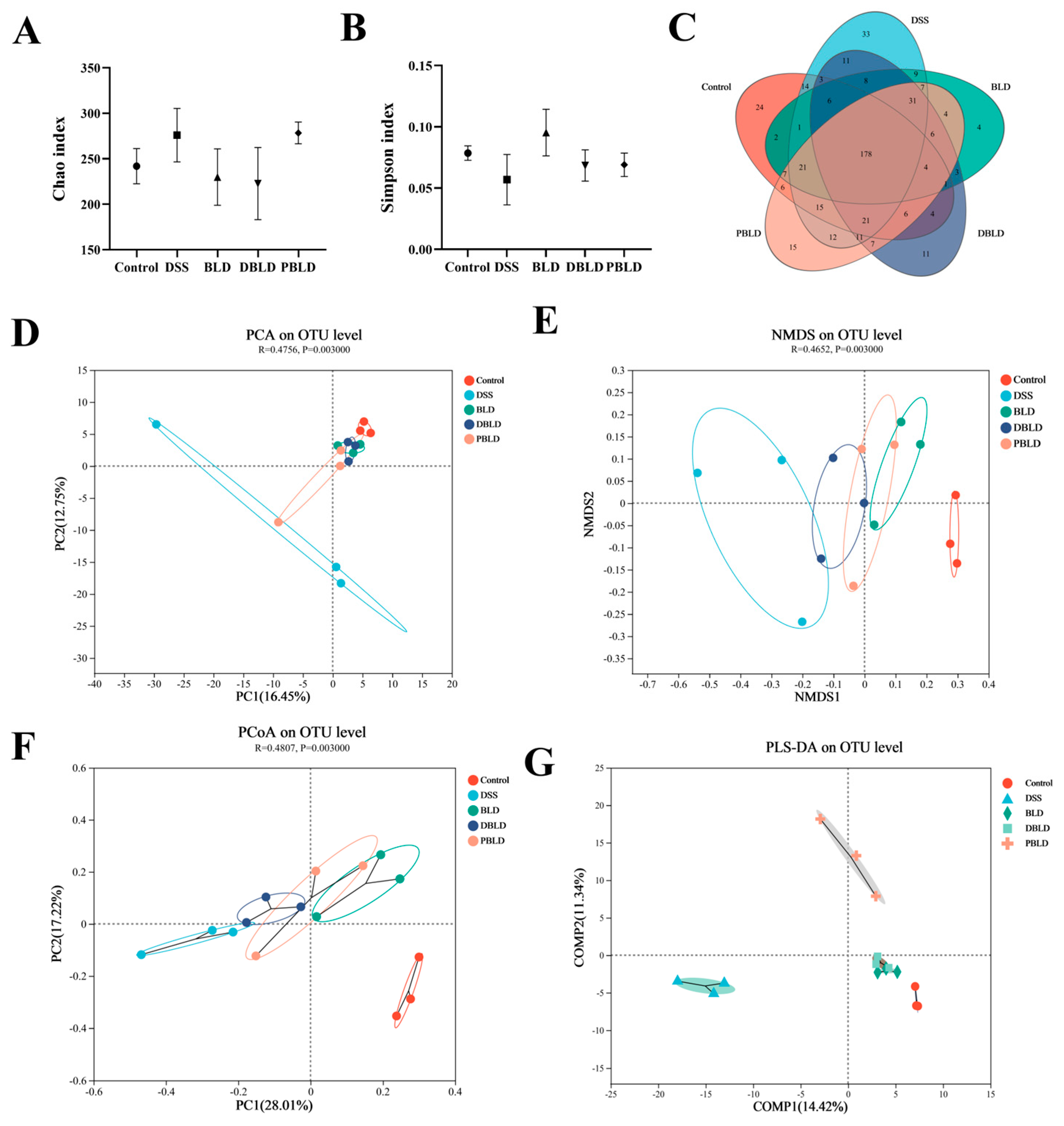
3.5.1. Gut Microbiota Overall Structure Was Altered by Both S. boulardii and Its Postbiotics
3.5.2. Gut Microbiota Composition Was Regulated by Both S. boulardii and Its Postbiotics
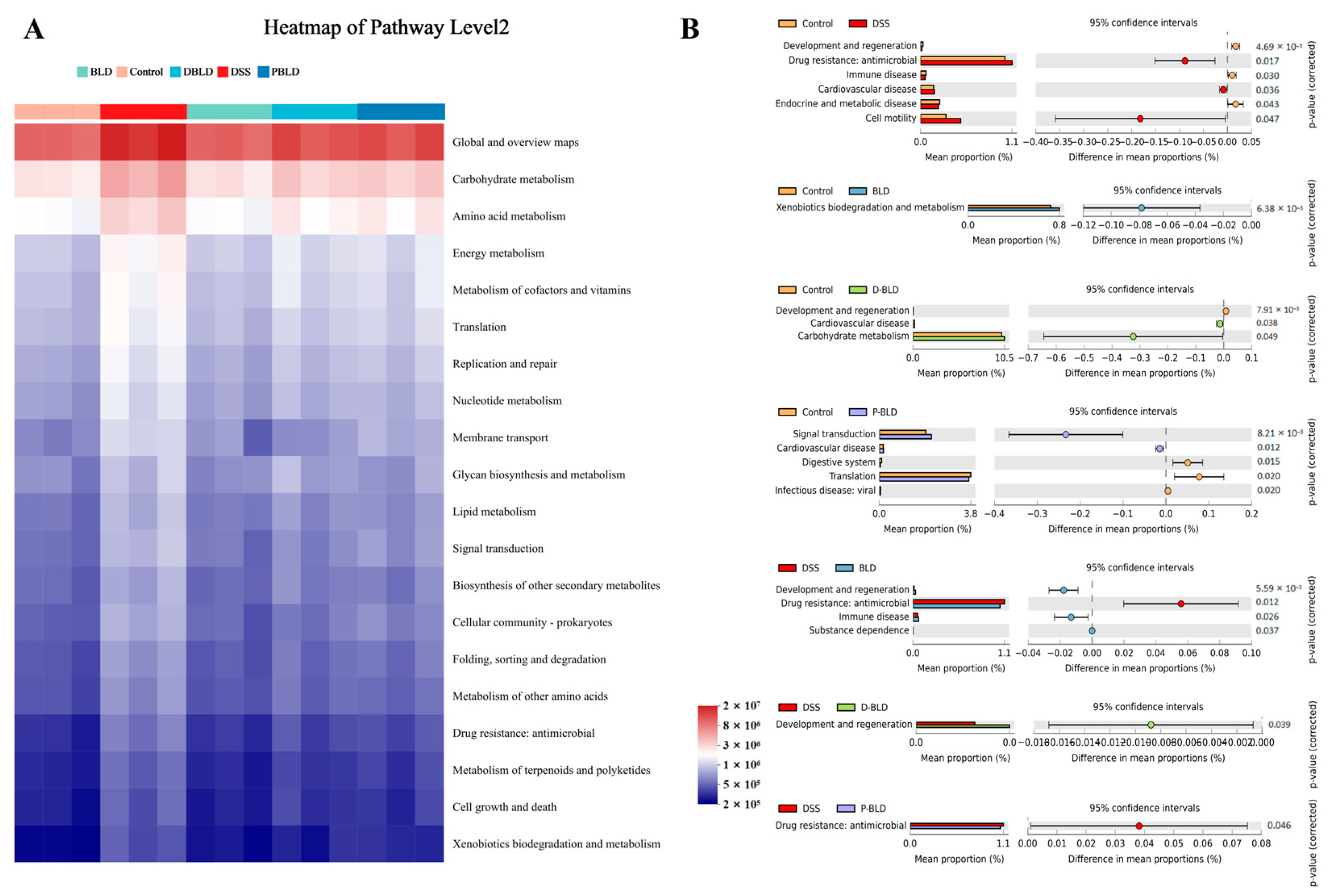
3.5.3. Functional Profile of the Gut Microbiome Was Changed by Both S. boulardii and Its Postbiotics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory Bowel Disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Wei, S.C.; Sollano, J.; Hui, Y.T.; Yu, W.; Santos Estrella, P.V.; Llamado, L.J.Q.; Koram, N. Epidemiology, burden of disease, and unmet needs in the treatment of ulcerative colitis in Asia. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 275–289. [Google Scholar] [CrossRef]
- Hvas, C.L.; Bendix, M.; Dige, A.; Dahlerup, J.F.; Agnholt, J. Current, experimental, and future treatments in inflammatory bowel disease: A clinical review. Immunopharmacol. Immunotoxicol. 2018, 40, 446–460. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, Z.; Kaur, L.; Gordon, M.; Baines, P.A.; Sinopoulou, V.; Akobeng, A.K. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 2020, CD007443. [Google Scholar] [CrossRef]
- Ansari, F.; Samakkhah, S.A.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 457–485. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Sivananthan, K.; Petersen, A.M. Review of Saccharomyces boulardii as a treatment option in IBD. Immunopharmacol. Immunotoxicol. 2018, 40, 465–475. [Google Scholar] [CrossRef]
- Martin, I.W.; Tonner, R.; Trivedi, J.; Miller, H.; Lee, R.; Liang, X.; Rotello, L.; Isenbergh, E.; Anderson, J.; Perl, T.; et al. Saccharomyces boulardii probiotic-associated fungemia: Questioning the safety of this preventive probiotic’s use. Diagn. Microbiol. Infect. Dis. 2017, 87, 286–288. [Google Scholar] [CrossRef]
- Lenka, S.; Singh, D.; Paul, S.; Gayen, A.; Chandra, M.S. boulardii Fails to Hold Its Cell Wall Integrity against Nonpathogenic E. coli: Are Probiotic Yeasts Losing the Battle? ACS Infect. Dis. 2021, 7, 733–745. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.B.; Shi, L.L.; Li, R.; Luo, Y.N.; Deng, Y.; Li, S.H.; Li, R.Z.; Liu, Z. Saccharomyces boulardii alleviates DSS-induced intestinal barrier dysfunction and inflammation in humanized mice. Food Funct. 2022, 13, 102–112. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.Z.; Sun, J.; Xu, H.Z.; Wang, M.; Zuo, X.Z.; Fu, Q.; Guo, Y.C.; Chen, Z.Y.; Zhang, P.W.; et al. Saccharomyces boulardii Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Regulating NF-κB and Nrf2 Signaling Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 1622375. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.B.; Wang, H.Y.; Ma, Y.M.; Zhao, X.H.; Zhang, X.D.; Yang, H.; Qian, J.M.; Li, J.N. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Dong, J.P.; Zheng, Y.; Wu, T.; He, Q.; Teng, G.G.; Wang, H.H. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin. Med. J. 2019, 132, 1951–1958. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.J.; Guan, L.; Zhang, Y.N.; Li, Y.; Sun, M.J. Mechanism and therapeutic effects of Saccharomyces boulardii on experimental colitis in mice. Mol. Med. Rep. 2018, 18, 5652–5662. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. J. Nutr. Biochem. 2018, 61, 129–139. [Google Scholar] [CrossRef]
- Chen, X.H.; Yang, G.X.; Song, J.H.; Xu, H.; Li, D.; Goldsmith, J.; Zeng, H.; Parsons-Wingerter, P.A.; Reinecker, H.C.; Kelly, C.P. Probiotic Yeast Inhibits VEGFR Signaling and Angiogenesis in Intestinal Inflammation. PLoS ONE 2013, 8, e64227. [Google Scholar] [CrossRef]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef]
- Basso, P.J.; Camara NO, S.; Sales-Campos, H. Microbial-Based Therapies in the Treatment of Inflammatory Bowel Disease—An Overview of Human Studies. Front. Pharmacol. 2019, 9, 1571. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, C.H.; Tang, L.J.; Yang, H. Associations between Gene Polymorphisms in Pro-Inflammatory Cytokines and the Risk of Inflammatory Bowel Disease: A Meta-Analysis. Immunol. Investig. 2021, 50, 869–883. [Google Scholar] [CrossRef]
- Funakoshi, K.; Sugimura, K.; Anezaki, K.; Bannai, H.; Ishizuka, K.; Asakura, H. Spectrum of cytokine gene expression in intestinal mucosal lesions of Crohn’s disease and ulcerative colitis. Digestion 1998, 59, 73–78. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Raizel, R.; Bonvini, A.; Tirapegui, J.; Rogero, M.M. Probiotics for inflammatory bowel diseases: A promising adjuvant treatment. Int. J. Food Sci. Nutr. 2019, 70, 20–29. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, Y.W.; Chi, S.G.; Joo, Y.S.; Kim, H.J. The Effect of Saccharomyces boulardii on Human Colon Cells and Inflammation in Rats with Trinitrobenzene Sulfonic Acid-Induced Colitis. Digest. Dis. Sci. 2009, 54, 255–263. [Google Scholar] [CrossRef]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G.H. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Ouyang, W.J.; Rutz, S.; Crellin, N.K.; Joo, Y.S.; Kim, H.J. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Sabat, R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010, 21, 315–324. [Google Scholar] [CrossRef]
- Fonseca-Camarillo, G.; Yamamoto-Furusho, J.K. Immunoregulatory Pathways Involved in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2188–2193. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Mankertz, J.; Schulzke, J.D. Altered permeability in inflammatory bowel disease: Pathophysiology and clinical implications. Curr. Opin. Gastroenterol. 2007, 23, 379–383. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wasinger, V.C.; Yau, Y.Y.; Chuang, E.; Yajnik, V.; Leong, R.W.L. Molecular Pathophysiology of Epithelial Barrier Dysfunction in Inflammatory Bowel Diseases. Proteomes 2018, 6, 17. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Kowalska-Duplaga, K.; Gosiewski, T.; Kapusta, P.; Sroka-Oleksiak, A.; Wędrychowicz, A.; Pieczarkowski, S.; Ludwig-Słomczyńska, A.H.; Wołkow, P.P.; Fyderek, K. Differences in the intestinal microbiome of healthy children and patients with newly diagnosed Crohn’s disease. Sci. Rep. 2019, 9, 18880. [Google Scholar] [CrossRef]
- Deng, L.; Wojciech, L.; Gascoigne NR, J.; Peng, G.; Tan, K.S.W. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Van Averbeke, V.; Berkell, M.; Mysara, M.; Rodriguez-Ruiz, J.P.; Xavier, B.B.; De Winter, F.H.R.; Jongers, B.; Jairam, R.K.; Hotterbeekx, A.; Goossens, H.; et al. Host Immunity Influences the Composition of Murine Gut Microbiota. Front. Immunol. 2022, 13, 828016. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Schäffler, H.; Kaschitzki, A.; Alberts, C.; Bodammer, P.; Bannert, K.; Köller, T.; Warnke, P.; Kreikemeyer, B.; Lamprecht, G. Alterations in the mucosa-associated bacterial composition in Crohn’s disease: A pilot study. Int. J. Colorectal Dis. 2016, 31, 961–971. [Google Scholar] [CrossRef]
- Nomura, K.; Ishikawa, D.; Okahara, K.; Ito, S.; Haga, K.; Takahashi, M.; Arakawa, A.; Shibuya, T.; Osada, T.; Kuwahara-Arai, K.; et al. Bacteroidetes Species Are Correlated with Disease Activity in Ulcerative Colitis. J. Clin. Med. 2021, 10, 1749. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Li, J.; Bao, Y.; Guo, Y.; Yang, W. The Dynamic Changes of Gut Microbiota in Muc2 Deficient Mice. Int. J. Mol. Sci. 2018, 19, 2809. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef]
- Li, M.X.; Li, M.Y.; Lei, J.X.; Wu, Y.Z.; Li, Z.H.; Chen, L.M.; Zhou, C.L.; Su, J.Y.; Huang, G.X.; Huang, X.Q.; et al. Huangqin decoction ameliorates DSS-induced ulcerative colitis: Role of gut microbiota and amino acid metabolism, mTOR pathway and intestinal epithelial barrier. Phytomedicine 2022, 100, 154052. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch TC, A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Sun, C.; Tang, X.; Zhang, X.; Han, D.; Liang, S.; Qu, R.; Hui, X.; Shan, Y.; Hu, L.; et al. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of Jinxiang Garlic (Allium sativum L.) Polysaccharides toward Dextran Sodium Sulfate-Induced Colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Yang, Y.; Xia, Y.; Wang, F.; Sun, Y.; Yang, Y.; Ai, L. Probiotic yeast BR14 ameliorates DSS-induced colitis by restoring the gut barrier and adjusting the intestinal microbiota. Food Funct. 2021, 12, 8386–8398. [Google Scholar] [CrossRef]
- Ren, R.; Zhao, A.Q.; Chen, L.; Wu, S.; Hung, W.-L.; Wang, B. Therapeutic effect of Lactobacillus plantarum JS19 on mice with dextran sulfate sodium induced acute and chronic ulcerative colitis. J. Sci. Food Agric. 2022; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Mar, J.S.; Nagalingam, N.A.; Song, Y.; Onizawa, M.; Lee, J.W.; Lynch, S.V. Amelioration of DSS-induced murine colitis by VSL#3 supplementation is primarily associated with changes in ileal microbiota composition. Gut Microbes 2014, 5, 494–503. [Google Scholar]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef]
- Ye, W.; Chen, Z.; He, Z.; Gong, H.; Zhang, J.; Sun, J.; Yuan, S.; Deng, J.; Liu, Y.; Zeng, A. Lactobacillus plantarum-Derived Postbiotics Ameliorate Acute Alcohol-Induced Liver Injury by Protecting Cells from Oxidative Damage, Improving Lipid Metabolism, and Regulating Intestinal Microbiota. Nutrients 2023, 15, 845. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]


| Target Gene | Nucleotide Sequence of Primer (5′ to 3′) Forward | Reverse |
|---|---|---|
| GAPDH | ATGGTGAAGGTCGGTGTGAA | TTTGCCGTGAGTGGAGTCAT |
| IL-1β | GTCGCTCAGGGTCACAAGAA | CCACACGTTGACAGCTAGGT |
| IL-6 | GGAGCCCACCAAGAACGATA | GTCACCAGCATCAGTCCCAA |
| IL-10 | AGAGAAGCATGGCCCAGAAA | ACACCTTGGTCTTGGAGCTT |
| TNF-α | AGATTCTTCCCTGAGGTGCA | ACCCCGGCCTTCCAAATAAA |
| Occludin | TTTCCTGCGGTGACTTCTCC | AAAACAGTGGTGGGGAACGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wu, J.; Jin, Y.; Huang, K.; Zhang, Y.; Liang, Z. Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients 2023, 15, 1484. https://doi.org/10.3390/nu15061484
Xu X, Wu J, Jin Y, Huang K, Zhang Y, Liang Z. Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients. 2023; 15(6):1484. https://doi.org/10.3390/nu15061484
Chicago/Turabian StyleXu, Xinge, Jingwei Wu, Yuxin Jin, Kunlun Huang, Yuanyuan Zhang, and Zhihong Liang. 2023. "Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota" Nutrients 15, no. 6: 1484. https://doi.org/10.3390/nu15061484
APA StyleXu, X., Wu, J., Jin, Y., Huang, K., Zhang, Y., & Liang, Z. (2023). Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients, 15(6), 1484. https://doi.org/10.3390/nu15061484






