The Effects of 12 Weeks of Concurrent and Combined Training on Inflammatory Markers, Muscular Performance, and Body Composition in Middle-Aged Overweight and Obese Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
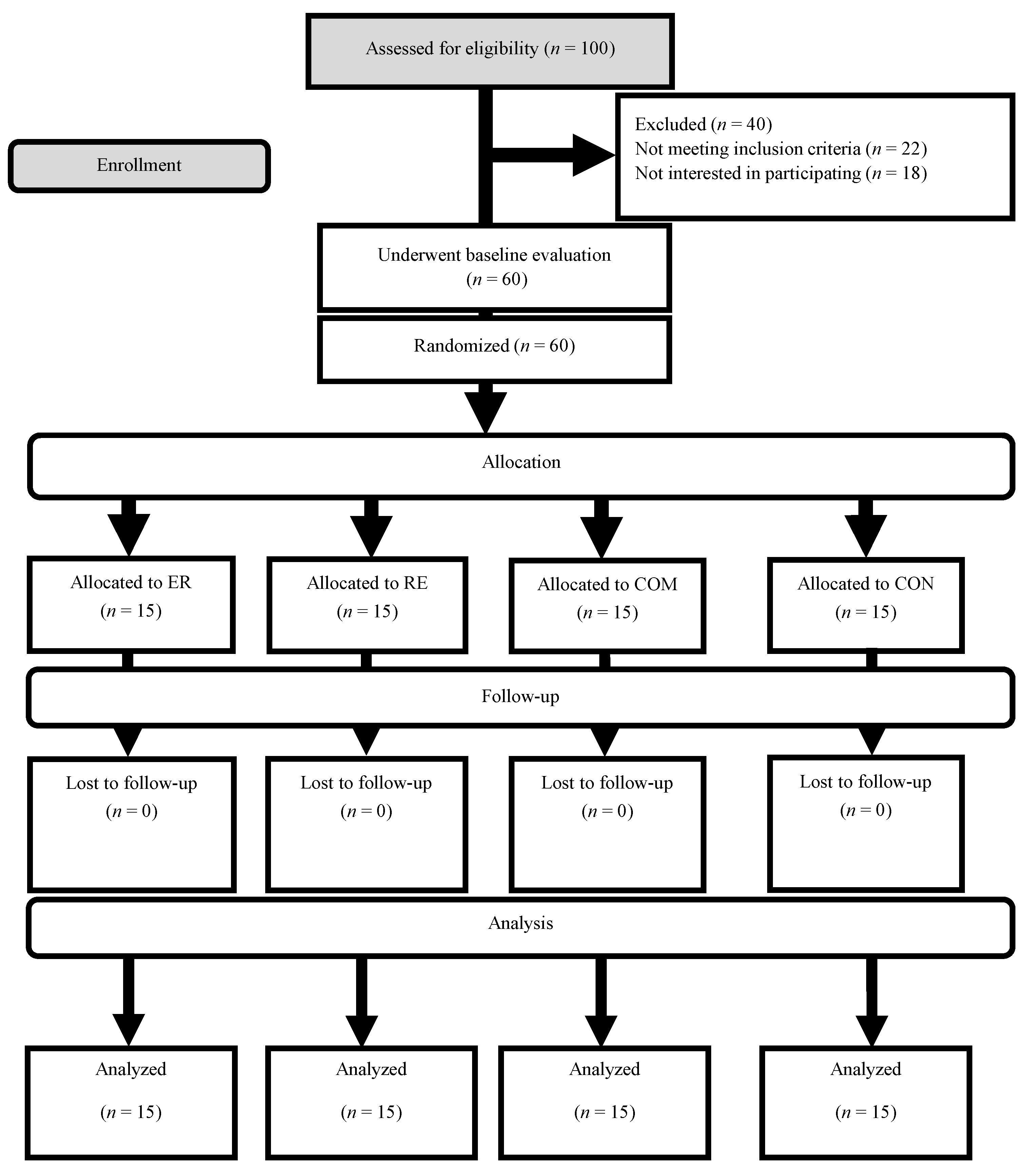
2.2. Study Design
2.3. Anthropometry and Body Composition
2.4. Blood Sampling and Analysis
2.5. Strength Testing
2.6. Power Testing
2.7. Aerobic Power
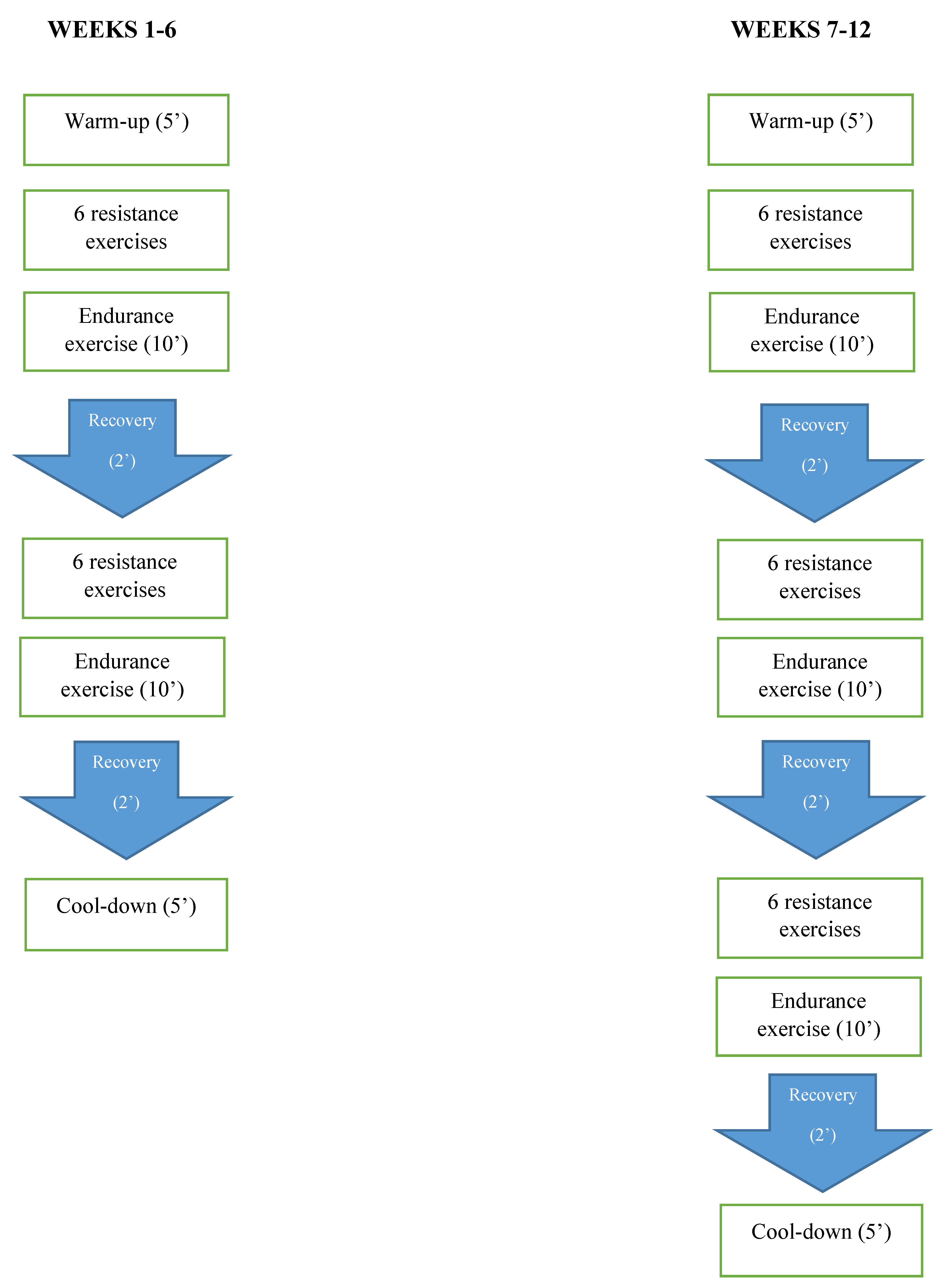
2.8. Concurrent Training
2.9. Preparatory Phase
2.10. Resistance Training Protocol
2.11. Endurance Training Protocol
2.12. Cool Down
2.13. Nutrient Intake and Dietary Analysis
2.14. Statistical Analysis
3. Results
3.1. Body Composition
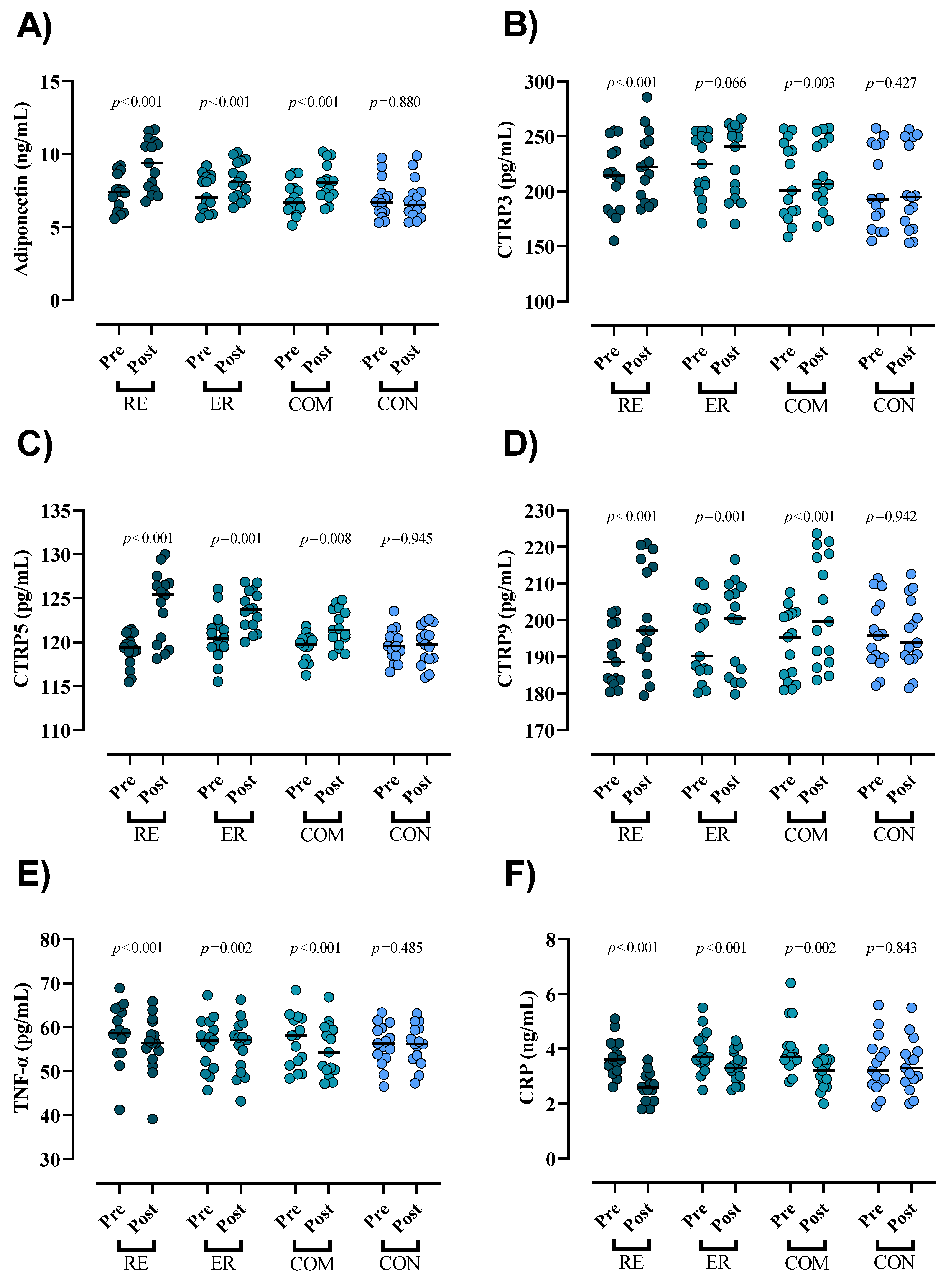
3.2. Inflammatory Markers
3.3. Muscular Performance and Dietary Intakes
3.4. Training Volume
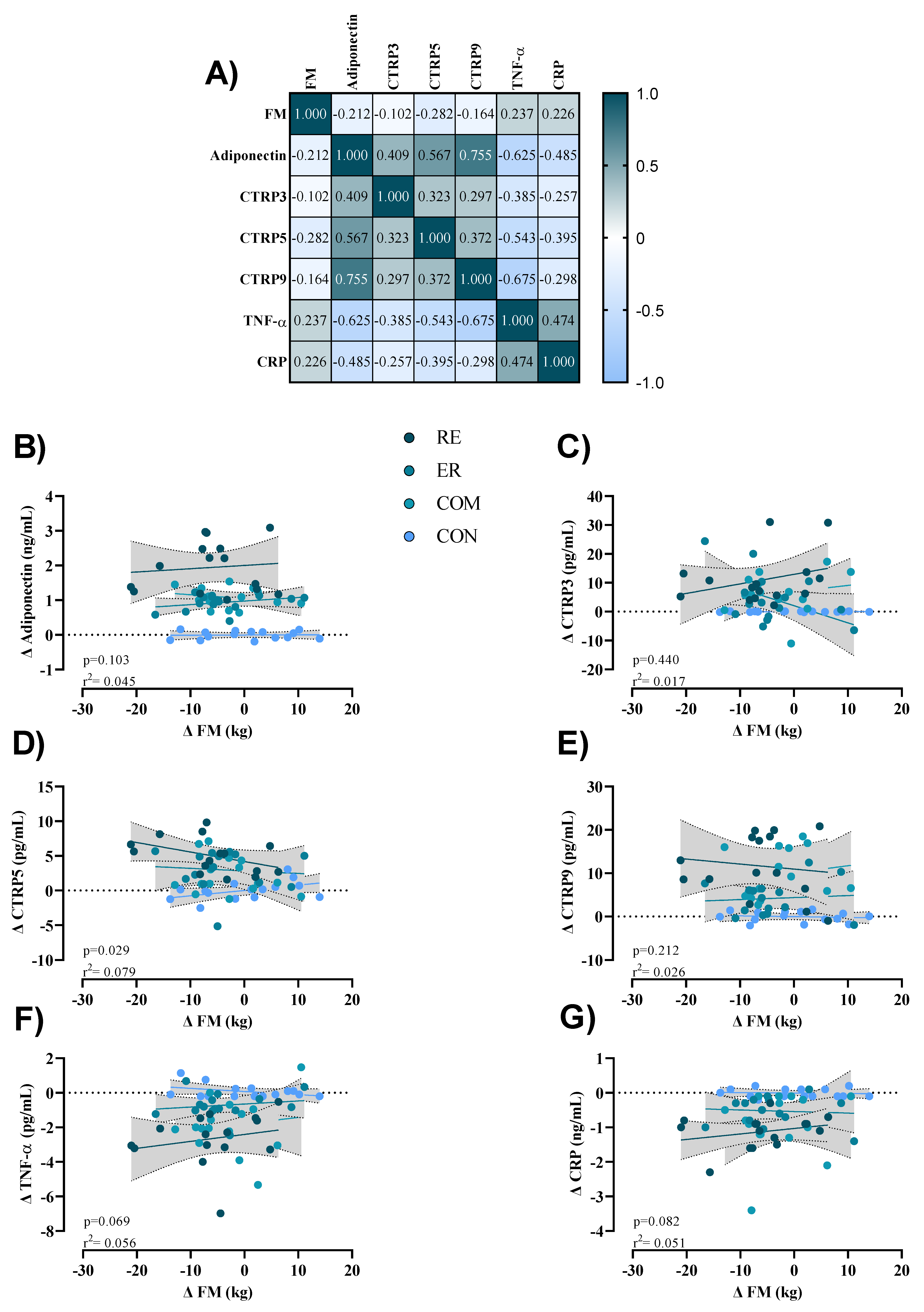
3.5. Correlations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellulu, M.S.; Patimah, I.; KhazáAi, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cosano, J.J.; Gracia-Marco, L.; Courteix, D.; Lesourd, B.; Chapier, R.; Obert, P.; Walther, G.; Vinet, A.; Thivel, D.; Muñoz-Torres, M.; et al. Cardiorespiratory Fitness and Bone Turnover Markers in Adults with Metabolic Syndrome: The Mediator Role of Inflammation. Int. J. Sport Nutr. Exerc. Metab. 2022, 33, 23–29. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Zouhal, H.; Bagheri, R.; Ashtary-Larky, D.; Wong, A.; Triki, R.; Hackney, A.C.; Laher, I.; Abderrahman, A.B. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol. Behav. 2020, 225, 113090. [Google Scholar] [CrossRef]
- Hou, H.; Ge, S.; Zhao, Z.; Wang, C.; Wang, W.; Zhao, X.; Sungur, M.A.; Alzain, M.A.; Asweto, C.O.; Zhang, J.; et al. An Updated Systematic Review and Meta-analysis of Association Between Adiponectin Gene Polymorphisms and Coronary Artery Disease. OMICS J. Integr. Biol. 2017, 21, 340–351. [Google Scholar] [CrossRef]
- Wong, G.W.; Wang, J.; Hug, C.; Tsao, T.-S.; Lodish, H.F. A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl. Acad. Sci. USA 2004, 101, 10302–10307. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Fan, W.; Sun, L. A Review of the Relationship Between CTRP Family and Coronary Artery Disease. Curr. Atheroscler. Rep. 2020, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Buechler, C. CTRP family: Linking immunity to metabolism. Trends Endocrinol. Metab. 2012, 23, 194–204. [Google Scholar] [CrossRef]
- Hofmann, C.; Chen, N.; Obermeier, F.; Paul, G.; Büchler, C.; Kopp, A.; Falk, W.; Schäffler, A. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm. Bowel Dis. 2011, 17, 2462–2471. [Google Scholar] [CrossRef]
- Schmid, A.; Kopp, A.; Hanses, F.; Karrasch, T.; Schäffler, A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem. Biophys. Res. Commun. 2014, 452, 8–13. [Google Scholar] [CrossRef]
- Shen, Y.; Li, C.; Zhang, R.Y.; Zhang, Q.; Shen, W.F.; Ding, F.H.; Lu, L. Association of increased serum CTRP5 levels with in-stent restenosis after coronary drug-eluting stent implantation: CTRP5 promoting inflammation, migration and proliferation in vascular smooth muscle cells. Int. J. Cardiol. 2017, 228, 129–136. [Google Scholar] [CrossRef]
- Wang, J.; Hang, T.; Cheng, X.M.; Li, D.M.; Zhang, Q.G.; Wang, L.J.; Peng, Y.P.; Gong, J.B. Associations of C1q/TNF-related protein-9 levels in serum and epicardial adipose tissue with coronary atherosclerosis in humans. BioMed Res. Int. 2015, 2015, 971683. [Google Scholar] [CrossRef]
- You, T.; Arsenis, N.C.; Disanzo, B.L.; LaMonte, M.J. Effects of exercise training on chronic inflammation in obesity. Sport. Med. 2013, 43, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Alipour, M.; Motevalli, M.S.; Chebbi, A.; Laher, I.; Zouhal, H. Does green tea extract enhance the anti-inflammatory effects of exercise on fat loss? Br. J. Clin. Pharmacol. 2020, 86, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Abdi, A.; Mehrabani, J.; Nordvall, M.; Wong, A.; Fallah, A.; Bagheri, R. Effects of concurrent training on irisin and fibronectin type-III domain containing 5 (FNDC5) expression in visceral adipose tissue in type-2 diabetic rats. Arch. Physiol. Biochem. 2022, 128, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, B.H.; Bagheri, R.; Ashtary-Larky, D.; Tinsley, G.M.; Eskandari, M.; Wong, A.; Kreider, R.B.; Baker, J.S. The Effects of Concurrent Training Order on Satellite Cell-Related Markers, Body Composition, Muscular and Cardiorespiratory Fitness in Older Men with Sarcopenia. J. Nutr. Health Aging 2020, 24, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Marin, P.J.; Rhea, M.R.; Wilson, S.M.; Loenneke, J.P.; Anderson, J.C. Concurrent training: A meta-analysis examining interference of aerobic and resistance exercises. J. Strength Cond. Res. 2012, 26, 2293–2307. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, J.W.; Lee, N.; Hwang, S.Y.; Cho, G.J.; Hong, H.C.; Yoo, H.J.; Hwang, T.G.; Kim, S.M.; Baik, S.H.; et al. Effects of a combined aerobic and resistance exercise program on C1q/TNF-related protein-3 (CTRP-3) and CTRP-5 levels. Diabetes Care 2013, 36, 3321–3327. [Google Scholar] [CrossRef]
- Fyfe, J.J.; Bishop, D.J.; Stepto, N.K. Interference between concurrent resistance and endurance exercise: Molecular bases and the role of individual training variables. Sport. Med. 2014, 44, 743–762. [Google Scholar] [CrossRef]
- Murach, K.A.; Bagley, J.R. Skeletal muscle hypertrophy with concurrent exercise training: Contrary evidence for an interference effect. Sport. Med. 2016, 46, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Leveritt, M.; Abernethy, P.J.; Barry, B.K.; Logan, P.A. Concurrent strength and endurance training. Sport. Med. 1999, 28, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription. Curr. Sport. Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016, 121, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Liu, J.; Guo, R.; Yan, Z.; Liu, W.; Lau, W.B.; Jiao, X.; Cao, J.; Xu, K.; et al. Implications of C1q/TNF-related protein superfamily in patients with coronary artery disease. Sci. Rep. 2020, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, C.; Zhang, Y.; Zhao, J.; Yang, M.; Tian, M.; Li, L.; Zheng, Y.; Chen, B.; Yang, G. Serum C1q/TNF-related protein-3 (CTRP3) levels are decreased in obesity and hypertension and are negatively correlated with parameters of insulin resistance. Diabetol. Metab. Syndr. 2015, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.M.; Steele, K.E.; Peterson, L.A.; Zeng, X.; Jaffe, A.E.; Schweitzer, M.A.; Magnuson, T.H.; Wong, G.W. C1q/TNF-related protein-9 (CTRP9) levels are associated with obesity and decrease following weight loss surgery. J. Clin. Endocrinol. Metab. 2016, 101, 2211–2217. [Google Scholar] [CrossRef]
- Keshavarz, S.; Kargarfard, M.; Banaei, J. The Effects of Aerobic, Resistance, And Combined Exercise on C1q/Tnf-Related Protein-5 (Ctrp-5) Levels and Glycemic Parameters in Patients with Type 2 Diabetes Mellitus. Iran. J. Diabetes Metab. 2021, 20, 179–190. [Google Scholar]
- Hasegawa, N.; Fujie, S.; Horii, N.; Uchida, M.; Kurihara, T.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aerobic exercise training-induced changes in serum C1q/TNF-related protein levels are associated with reduced arterial stiffness in middle-aged and older adults. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R94–R101. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Schäffler, A.; Fleck, M.; Schölmerich, J.; Schütz, C.; Buechler, C. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 2005, 579, 5565–5570. [Google Scholar] [CrossRef]
- Wölfing, B.; Buechler, C.; Weigert, J.; Neumeier, M.; Aslanidis, C.; Schöelmerich, J.; Schäffler, A. Effects of the new C1q/TNF-related protein (CTRP-3) “cartonectin” on the adipocytic secretion of adipokines. Obesity 2008, 16, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Choi, J.H.; Ryu, H.S.; Pak, Y.; Park, K.S.; Lee, H.K.; Lee, W. C1q Tumor Necrosis Factor α-related Protein Isoform 5 Is Increased in Mitochondrial DNA-depleted Myocytes and Activates AMP-activated Protein Kinase. J. Biol. Chem. 2009, 284, 27780–27789. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chang, S.J. Moderate exercise suppresses NF-κB signaling and activates the SIRT1-AMPK-PGC1α Axis to attenuate muscle loss in diabetic db/db mice. Front. Physiol. 2018, 9, 636. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Ge, G.; Spooner, E.; Hug, C.; Gimeno, R.; Lodish, H.F. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009, 23, 241–258. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; Li, T.; Liu, Y.; Zhu, Q.; Chen, T.; Liu, T.; Huang, C.; Zhang, J.; Zhang, Y.; et al. CTRP9 enhances carotid plaque stability by reducing pro-inflammatory cytokines in macrophages. Biochem. Biophys. Res. Commun. 2015, 458, 890–895. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, C.; Li, J.; Li, T.; Guo, H.; Liu, T.; Li, N.; Zhu, Q.; Guo, Y. Globular CTRP9 inhibits oxLDL-induced inflammatory response in RAW 264.7 macrophages via AMPK activation. Mol. Cell. Biochem. 2016, 417, 67–74. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Ashtary-Larky, D.; Bagheri, R.; Choghakhori, R.; Wong, A.; Baker, J.S.; Abbasnezhad, A. Effects of green tea supplementation on serum concentrations of adiponectin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Arch. Physiol. Biochem. 2020, 129, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef]
- Asad, M.R.; Ferdosi, M.H.; Yoosefi, Z. The effects of three training methods endurance, resistance and concurrent on adiponectin resting levels in overweighed untrained men. Procedia-Soc. Behav. Sci. 2012, 46, 440–444. [Google Scholar] [CrossRef]
- Montazeri Taleghani, H.; Soori, R.; Rezaeian, N.; Khosravi, N.J.K. Changes of plasma leptin and adiponectin levels in response to combined endurance and resistance training in sedentary postmenopausal women. Koomesh 2012, 13, 269–277. [Google Scholar]
- Hosseynzade, A.; Banitalebi, E.; Baqeri, L.; Faramarzi, M.; Mardanpour Shahkordi, Z.; Amani, S. The Effects of Two Types of Concurrent Training on Plasma Leptin and Adiponectin in Overweight Older Women. In Proceedings of the 8th International Congress on Physical Education and Sport Sciences, Tehran, Iran, 19–20 February 2015. [Google Scholar]
- Eskandari, M.; Moghadam, B.H.; Bagheri, R.; Ashtary-Larky, D.; Eskandari, E.; Nordvall, M.; Dutheil, F.; Wong, A. Effects of Interval Jump Rope Exercise Combined with Dark Chocolate Supplementation on Inflammatory Adipokine, Cytokine Concentrations, and Body Composition in Obese Adolescent Boys. Nutrients 2020, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
- Ghafourian, M.; Ashtary-Larky, D.; Chinipardaz, R.; Eskandary, N.; Mehavaran, M. Inflammatory Biomarkers’ Response to Two Different Intensities of a Single Bout Exercise Among Soccer Players. Iran. Red Crescent Med. J. 2016, 18, e21498. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Park, K.S. Responses of inflammatory cytokines following moderate intensity walking exercise in overweight or obese individuals. J. Exerc. Rehabil. 2017, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Banitalebi, E.; Baghanari, H.B. Effect of sequence order of combined training (resistance and endurance) on strength, aerobic capacity, and body composition in older women. Middle East J. Rehabil. Health 2015, 2, 2. [Google Scholar] [CrossRef]
- Baker, J.S.; Bailey, D.M.; Davies, B. The relationship between total-body mass, fat-free mass and cycle ergometry power components during 20 seconds of maximal exercise. J. Sci. Med. Sport 2001, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perroni, F.; Vetrano, M.; Rainoldi, A.; Guidetti, L.; Baldari, C. Relationship among explosive power, body fat, fat free mass and pubertal development in youth soccer players: A preliminary study. Sport Sci. Health 2014, 10, 67–73. [Google Scholar] [CrossRef]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef]
| Group | Pre (i) | Post (j) | Diff (j − i) | p-Value # | |
|---|---|---|---|---|---|
| Age (years) | R + E | 49.96 ± 3.62 | ------ | ------ | ------ |
| E + R | 51.74 ± 4.76 | ------ | ------ | ------ | |
| COM | 51.50 ± 4.34 | ------ | ------ | ------ | |
| CON | 52.63 ± 4.24 | ------ | ------ | ------ | |
| Height (cm) | R + E | 1.71 ± 0.02 | ------ | ------ | ------ |
| E + R | 1.73 ± 0.01 | ------ | ------ | ------ | |
| COM | 1.73 ± 0.02 | ------ | ------ | ------ | |
| CON | 1.72 ± 0.02 | ------ | ------ | ------ | |
| Body mass (kg) | R + E | 93.02 ± 3.65 | 87.40 ± 5.32 | −5.62 ± 4.20 d | <0.001 |
| E + R | 98.06 ± 4.32 | 93.80 ± 3.30 | −4.32 ± 3.95 | 0.001 | |
| COM | 94.56 ± 3.61 | 91.25 ± 4.80 | −3.31 ± 3.51 | 0.003 | |
| CON | 93.02 ± 3.30 | 92.19 ± 3.12 | −0.83 ± 2.66 a | 0.250 | |
| BMI (kg·m−2) | R + E | 31.68 ± 1.17 | 29.80 ± 1.40 | −1.93 ± 1.45 d | <0.001 |
| E + R | 32.90 ± 1.50 | 31.50 ± 1.41 | −1.44 ± 1.32 | 0.001 | |
| COM | 31.65± 1.43 | 30.54 ± 1.70 | −1.11 ± 1.20 | 0.003 | |
| CON | 31.30± 1.10 | 31.01 ± 1.02 | −0.30 ± 0.91 a | 0.256 | |
| Fat mass (kg) | R + E | 40.25 ± 6.91 | 34.30 ± 7.70 | −6.00 ± 8.30 | 0.014 |
| E + R | 38.73 ± 7.00 | 34.64 ± 6.20 | −4.10 ± 7.15 | 0.044 | |
| COM | 36.84 ± 7.19 | 33.93 ± 6.60 | −3.10 ± 6.30 | 0.094 | |
| CON | 38.17 ± 6.80 | 38.35 ± 5.60 | 0.17 ± 8.43 | 0.938 | |
| FFM (kg) | R + E | 52.78 ± 8.08 | 53.13 ± 10.80 | 0.35 ± 10.80 | 0.902 |
| E + R | 59.33 ± 8.27 | 59.11 ± 5.80 | −0.22 ± 7.90 | 0.914 | |
| COM | 57.71 ± 9.17 | 57.32 ± 8.25 | −0.40 ± 6.71 | 0.822 | |
| CON | 54.85 ± 8.45 | 53.90 ± 7.13 | −1.00 ± 8.64 | 0.662 | |
| WHR (m) | R + E | 0.92 ± 0.02 | 0.89 ± 0.03 | −0.03 ± 0.03 d | 0.001 |
| E + R | 0.94 ± 0.02 | 0.92 ± 0.05 | −0.02 ± 0.05 d | 0.185 | |
| COM | 0.92 ± 0.02 | 0.90 ± 0.03 | −0.03 ± 0.03 d | 0.001 | |
| CON | 0.90 ± 0.03 | 0.93± 0.02 | 0.04 ± 0.03 abc | <0.001 | |
| VO2max (mL/kg/min) | R + E | 20.38 ± 2.9 | 26.9 ± 3 | 6.5 ± 1.9 | <0.001 |
| E + R | 20.6 ± 2.9 | 29 ± 3.7 | 8.3 ± 2.9 | <0.001 | |
| COM | 20.9 ± 3.4 | 26.1 ± 4.5 | 5.2 ± 2.8 | <0.001 | |
| CON | 21.2 ± 3.1 | 20.5 ± 2.3 | −0.6 ± 2.3 | 0.323 | |
| Chest press (kg) | R + E | 28 ± 2.7 | 36.2 ± 3.2 | 8.1 ± 2.9 | <0.001 |
| E + R | 30.3 ± 2.8 | 37.2 ± 2.3 | 6.8 ± 2.4 | <0.001 | |
| COM | 30.5 ± 2.2 | 35 ± 2.3 | 4.5 ± 0.9 | <0.001 | |
| CON | 29.2 ± 3.2 | 29.7 ± 3.4 | 0.4 ± 1.5 | 0.372 | |
| Leg press (kg) | R + E | 59.2 ± 4.8 | 68.4 ± 5.3 | 8.6 ± 2.8 | <0.001 |
| E + R | 56 ± 3.1 | 63 ± 4.1 | 6.7 ± 2.9 | <0.001 | |
| COM | 57.8 ± 3.2 | 63.2 ± 3.8 | 5.2 ± 2.6 | <0.001 | |
| CON | 54.2 ± 5.3 | 53 ± 5.8 | −1.1 ± 2.6 | 0.122 | |
| Upper-body power (w) | R + E | 337.7 ± 14.8 | 362.6 ± 10.9 | 24.8 ± 12.3 | <0.001 |
| E + R | 336.8 ±13.3 | 350.2 ± 15.5 | 13.3 ± 14.9 | 0.004 | |
| COM | 339.2 ± 12.3 | 355.3 ± 11.9 | 16 ± 18.2 | 0.004 | |
| CON | 330.7 ± 11.7 | 335.6 ± 10.9 | 4.8 ± 13.5 | 0.187 | |
| Lower-body power (w) | R + E | 369.7 ± 18.5 | 410 ± 19 | 40.3 ± 20.3 | <0.001 |
| E + R | 376 ± 24.8 | 396.8 ± 22.2 | 20.8 ± 27.7 | 0.011 | |
| COM | 362.2 ± 15.2 | 376.4 ± 15.6 | 14.2 ± 13.6 | 0.001 | |
| CON | 378.8 ± 19.4 | 375.4 ± 22.4 | 3.4 ± 29.9 | 0.667 |
| Variables | Group | Pre-Training | During Training | p |
|---|---|---|---|---|
| Energy (kcal/d) | RE | 2531.2 ± 541.8 | 2414.2 ± 553.6 | 0.303 |
| ER | 2666.7 ± 671.07 | 2569.8 ± 414.7 | 0.491 | |
| COM | 2554.2 ± 712.7 | 2490.3 ± 759.2 | 0.542 | |
| CON | 2536.1 ± 330.6 | 2444.1 ± 336.6 | 0.343 | |
| Protein (g/d) | RE | 76.4 ± 17.4 | 75.1 ± 21.2 | 0.813 |
| ER | 85.1 ± 22.3 | 81.3 ± 20.6 | 0.351 | |
| COM | 78.09 ± 27.1 | 72.4 ± 22.6 | 0.182 | |
| CON | 81.8 ± 19.9 | 74.6 ± 20.4 | 0.276 | |
| Fat (g/d) | RE | 79.5 ± 24.9 | 78.7 ± 27.5 | 0.888 |
| ER | 77.4 ± 28.6 | 78.3 ± 18.4 | 0.898 | |
| COM | 82.3 ± 36.9 | 84.1 ± 38.7 | 0.704 | |
| CON | 70.1 ± 24.7 | 63.1 ± 20.6 | 0.388 | |
| CHO (g/d) | RE | 377.2 ± 89.2 | 351.1 ± 85.3 | 0.288 |
| ER | 407.2 ± 107.02 | 384.8 ± 77.8 | 0.358 | |
| COM | 375.08 ± 91.7 | 360.8 ± 109.6 | 0.565 | |
| CON | 394.4 ± 82.9 | 394.3 ± 67.4 | 0.993 |
| Groups | 1st–3rd Weeks | 4th–6th Weeks | 7th–9th Weeks | 10th–12th Weeks | ANOVA Repeated Measures | ||
|---|---|---|---|---|---|---|---|
| Main Effect | Groups Effect | Interaction Effect | |||||
| Training volume of resistance training (kg) | |||||||
| E + R | 1981.3 ± 421.5 | 2377.5 ± 505.8 a | 2971.9 ± 632.3 b | 2717.2 ± 578.1 cef | 0.001 * | 0.004 ** | 0.001 *** |
| R + E ¥ | 2280.4 ± 758.2 | 2736.5 ± 909.8 a | 3420.6 ± 1137.3 bd | 3127.4 ± 1039.8 cef | |||
| COM | 1238.8 ± 247.0 | 1486.6 ± 296.4 a | 2787.3 ± 555.9 bd | 2548.4 ± 508.2 cef | |||
| Training volume of endurance training (kJ) | |||||||
| E + R | 453.0 ± 78.2 | 659.0 ± 113.8 a | 892.4 ± 154.1 b | 1153.2 ± 199.2 cef | 0.001 * | 0.922 | 0.922 |
| R + E | 445.1 ± 92.0 | 647.4 ± 133.8 a | 876.8 ± 181.3 bd | 1133.1 ± 234.3 cef | |||
| COM | 460.1 ± 127.3 | 669.2 ± 185.1 a | 906.2 ± 250.7 bd | 1171.1 ± 324.0 cef | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri, R.; Kargarfard, M.; Jalali, K.; Ashtary-Larky, D.; Cheraghloo, N.; Ghobadi, H.; Moghadam, B.H.; Wong, A.; Nordvall, M.; Dutheil, F. The Effects of 12 Weeks of Concurrent and Combined Training on Inflammatory Markers, Muscular Performance, and Body Composition in Middle-Aged Overweight and Obese Males. Nutrients 2023, 15, 1482. https://doi.org/10.3390/nu15061482
Bagheri R, Kargarfard M, Jalali K, Ashtary-Larky D, Cheraghloo N, Ghobadi H, Moghadam BH, Wong A, Nordvall M, Dutheil F. The Effects of 12 Weeks of Concurrent and Combined Training on Inflammatory Markers, Muscular Performance, and Body Composition in Middle-Aged Overweight and Obese Males. Nutrients. 2023; 15(6):1482. https://doi.org/10.3390/nu15061482
Chicago/Turabian StyleBagheri, Reza, Mehdi Kargarfard, Khosro Jalali, Damoon Ashtary-Larky, Neda Cheraghloo, Hamid Ghobadi, Babak Hooshmand Moghadam, Alexei Wong, Michael Nordvall, and Frédéric Dutheil. 2023. "The Effects of 12 Weeks of Concurrent and Combined Training on Inflammatory Markers, Muscular Performance, and Body Composition in Middle-Aged Overweight and Obese Males" Nutrients 15, no. 6: 1482. https://doi.org/10.3390/nu15061482
APA StyleBagheri, R., Kargarfard, M., Jalali, K., Ashtary-Larky, D., Cheraghloo, N., Ghobadi, H., Moghadam, B. H., Wong, A., Nordvall, M., & Dutheil, F. (2023). The Effects of 12 Weeks of Concurrent and Combined Training on Inflammatory Markers, Muscular Performance, and Body Composition in Middle-Aged Overweight and Obese Males. Nutrients, 15(6), 1482. https://doi.org/10.3390/nu15061482








