Rationale, Design and Participants Baseline Characteristics of a Crossover Randomized Controlled Trial of the Effect of Replacing SSBs with NSBs versus Water on Glucose Tolerance, Gut Microbiome and Cardiometabolic Risk in Overweight or Obese Adult SSB Consumer: Strategies to Oppose SUGARS with Non-Nutritive Sweeteners or Water (STOP Sugars NOW) Trial and Ectopic Fat Sub-Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Inclusion and Exclusion Criteria
2.3. Randomization and Allocation Concealment
2.4. Interventions
2.5. Blinding
2.6. Trial Flow
2.7. Primary Outcomes
2.8. Secondary Outcomes
2.9. Adherence Outcomes
2.10. Exploratory Outcomes
2.11. Power Calculation
2.12. Outcome Assessment
2.13. Statistical Analysis
3. Results
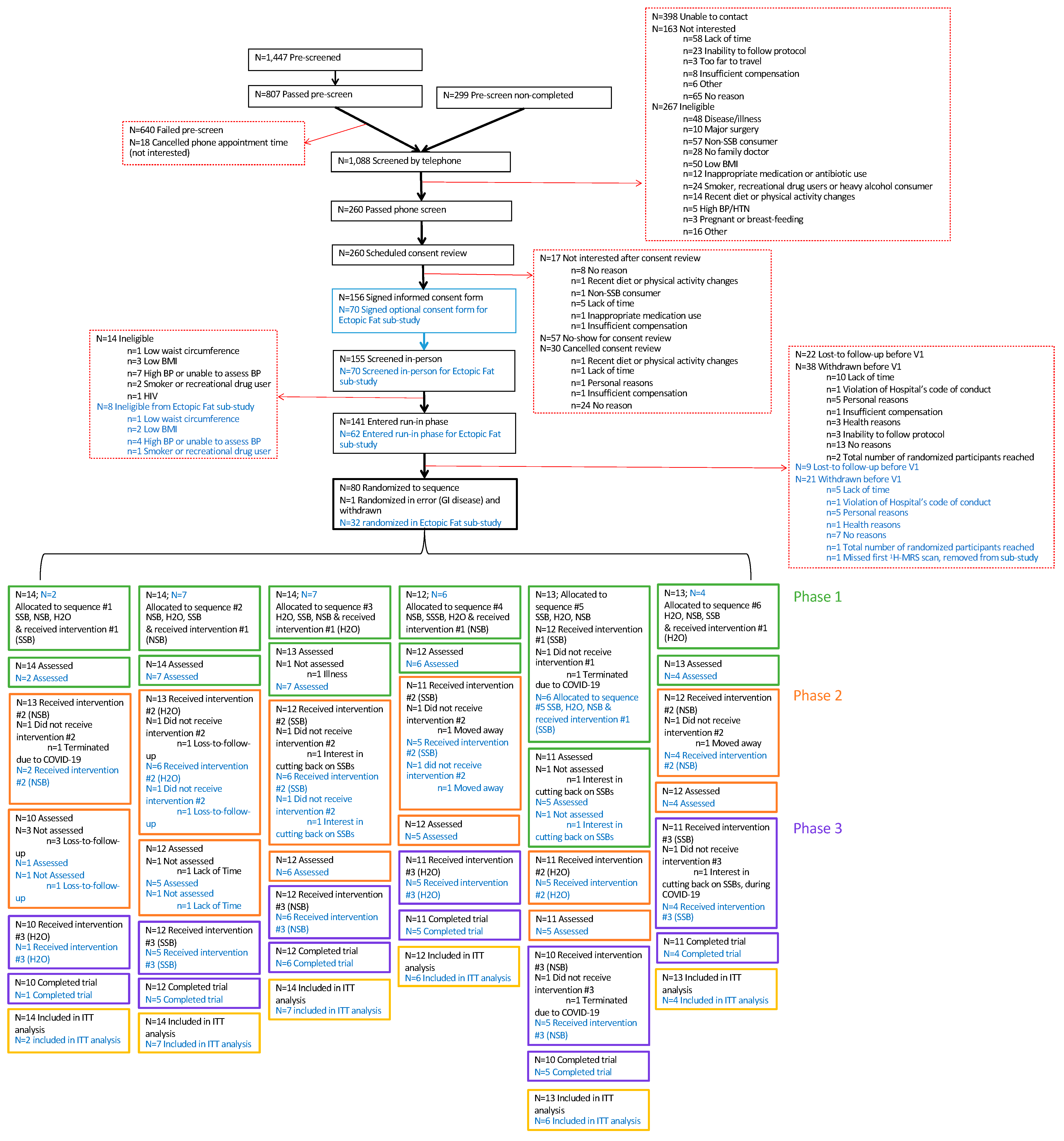
3.1. CONSORT Statement
3.2. Baseline Characteristics
4. Discussion
4.1. Strengths and Limitations
4.2. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brisbois, T.D.; Marsden, S.L.; Anderson, G.H.; Sievenpiper, J.L. Estimated intakes and sources of total and added sugars in the Canadian diet. Nutrients 2014, 6, 1899–1912. [Google Scholar] [CrossRef]
- Heart and Stroke Foundation of Canada. Sugar, Heart Disease and Stroke. Available online: https://www.heartandstroke.ca/-/media/pdf-files/canada/2017-position-statements/sugar-ps-eng.ashx (accessed on 7 September 2019).
- Diabetes Canada. Sugar & Diabetes. Available online: https://www.diabetes.ca/en-CA/advocacy---policies/our-policy-positions/sugar---diabetes (accessed on 7 September 2019).
- US Department of Health and Human Services. Cut down on Added Sugars. Available online: https://health.gov/dietaryguidelines/2015/resources/DGA_Cut-Down-On-Added-Sugars.pdf (accessed on 7 September 2019).
- Health Canada. Canada’s Dietary Guidelines for Health Professionals and Policy Makers. Available online: https://food-guide.canada.ca/static/assets/pdf/CDG-EN-2018.pdf (accessed on 7 September 2019).
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 315, h3576. [Google Scholar] [CrossRef]
- Jayalath, V.H.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.; Beyene, J.; et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef]
- Xi, B.; Huang, Y.; Reilly, K.H.; Li, S.; Zheng, R.; Barrio-Lopez, M.T.; Martinez-Gonzalez, M.A.; Zhou, D. Sugar-sweetened beverages and risk of hypertension and CVD: A dose-response meta-analysis. Br. J. Nutr. 2015, 113, 709–717. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf (accessed on 23 June 2022).
- Johnson, R.K.; Lichtenstein, A.H.; Anderson, C.A.M.; Carson, J.A.; Després, J.-P.; Hu, F.B.; Kris-Etherton, P.M.; Otten, J.J.; Towfighi, A.; Wylie-Rosett, J. Low-calorie sweetened beverages and cardiometabolic health: A science advisory from the american heart association. Circulation 2018, 138, e126–e140. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2020. Available online: https://care.diabetesjournals.org/content/diacare/suppl/2019/12/20/43.Supplement_1.DC1/Standards_of_Care_2020.pdf (accessed on 10 March 2020).
- Diabetes UK. Evidence-Based Nutrition Guidelines for the Prevention and Management Of Diabetes. Available online: https://diabetes-resources-production.s3.eu-west-1.amazonaws.com/resources-s3/2018-03/1373_Nutrition%20guidelines_0.pdf (accessed on 9 March 2020).
- Rios-Leyvraz, M.; Montez, J. Health Effects of the Use of Non-Sugar Sweeteners: A Systematic Review and Meta-Analysis; World Health Organization: Geneva, Switzerland, 2022; pp. 10–174.
- World Health Organization. Launch Event for the Public Consultation on the Draft WHO Guideline on Use of Non-Sugar Sweeteners. Available online: https://www.who.int/news-room/events/detail/2022/07/15/default-calendar/launch-event-for-the-public-consultation-on-the-draft-who-guideline-on-use-of-non-sugar-sweeteners (accessed on 18 November 2022).
- Sievenpiper, J.L.; Chan, C.B.; Dworatzek, P.D.; Freeze, C.; Williams, S.L. Diabetes Canada Clinical Practice Guidelines Expert Committee—Chapter 11: Nutrition Therapy. Can. J. Diabetes 2018, 42, S64. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. Sweeteners. Available online: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/sweeteners/eng/1387749708758/1387750396304?chap=0 (accessed on 24 July 2018).
- Kroger, M.; Meister, K.; Kava, R. Low-calorie sweeteners and other sugar substitutes: A review of the safety issues. Compr. Rev. Food Sci. Food Saf. 2006, 5, 35–47. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Roberts, A.; Nestmann, E.R. Critical review of the current literature on the safety of sucralose. Food Chem. Toxicol. 2017, 106, 324–355. [Google Scholar] [CrossRef]
- American Diabetes Association. American Heart Association/American Diabetes Association Scientific Statement:Non-nutritive Sweeteners: A Potentially Useful Option—with Caveats. Available online: http://www.diabetes.org/newsroom/press-releases/2012/ada-aha-sweetener-statement.html (accessed on 9 November 2018).
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef]
- Health Canada. 9. List of Permitted Sweeteners (Lists of Permitted Food Additives). Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/lists-permitted/9-sweeteners.html (accessed on 24 July 2018).
- Russell, C.; Baker, P.; Grimes, C.; Lindberg, R.; Lawrence, M.A. Global trends in added sugars and non-nutritive sweetener use in the packaged food supply: Drivers and implications for public health. Public Health Nutr. 2022, 1–13. [Google Scholar] [CrossRef]
- Toews, I.; Lohner, S.; de Gaudry, D.K.; Sommer, H.; Meerpohl, J.J. Association between intake of non-sugar sweeteners and health outcomes: Systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019, 364, k4718. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef]
- Malik, V.S. Non-sugar sweeteners and health. BMJ 2019, 364, k5005. [Google Scholar] [CrossRef]
- Khan, T.A.; Sievenpiper, J.L. Low-energy sweeteners and cardiometabolic health: Is there method in the madness? Am. J. Clin. Nutr. 2020, 112, 917–919. [Google Scholar] [CrossRef]
- Mela, D.J.; McLaughlin, J.; Rogers, P.J. Perspective: Standards for research and reporting on low-energy (“artificial”) sweeteners. Adv. Nutr. 2020, 11, 484–491. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S.; Bellisle, F.; Buttriss, J.; Drewnowski, A.; Fantino, M.; Gallagher, A.M.; De Graaf, K.; Goscinny, S.; Hardman, C.A. Expert consensus on low-calorie sweeteners: Facts, research gaps and suggested actions. Nutr. Res. Rev. 2020, 33, 145–154. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Khan, T.A.; Ha, V.; Viguiliouk, E.; Auyeung, R. The importance of study design in the assessment of nonnutritive sweeteners and cardiometabolic health. Can. Med. Assoc. J. 2017, 189, E1424–E1425. [Google Scholar] [CrossRef]
- Khan, T.A.; Malik, V.S.; Sievenpiper, J.L. Letter by Khan et al. regarding article, “Artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the women’s health initiative”. Stroke 2019, 50, e167–e168. [Google Scholar] [CrossRef]
- Rogers, P.; Hogenkamp, P.; De Graaf, C.; Higgs, S.; Lluch, A.; Ness, A.; Penfold, C.; Perry, R.; Putz, P.; Yeomans, M. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int. J. Obes. 2016, 40, 381–394. [Google Scholar] [CrossRef]
- McGlynn, N.D.; Khan, T.A.; Wang, L.; Zhang, R.; Chiavaroli, L.; Au-Yeung, F.; Lee, J.J.; Noronha, J.C.; Comelli, E.M.; Blanco Mejia, S.; et al. Association of Low- and No-Calorie Sweetened Beverages as a Replacement for Sugar-Sweetened Beverages with Body Weight and Cardiometabolic Risk: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e222092. [Google Scholar] [CrossRef]
- Laviada-Molina, H.; Molina-Segui, F.; Pérez-Gaxiola, G.; Cuello-García, C.; Arjona-Villicaña, R.; Espinosa-Marrón, A.; Martinez-Portilla, R.J. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: Systematic review and meta-analysis. Obes. Rev. 2020, 21, e13020. [Google Scholar] [CrossRef]
- Lee, J.; Khan, T.; Malik, V.; Hill, J.; Jeppesen, P.; Rahelic, D.; Kahleova, H.; Salas-Salvado, J.; Kendall, C.W.; Sievenpiper, J. Relation of Change or Substitution of Low Calorie Sweetened Beverages with Cardiometabolic Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Curr. Dev. Nutr. 2020, 4, 1432. [Google Scholar] [CrossRef]
- Lee, J.J.; Khan, T.A.; McGlynn, N.; Malik, V.S.; Hill, J.O.; Leiter, L.A.; Jeppesen, P.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J. Relation of Change or Substitution of Low-and No-Calorie Sweetened Beverages with Cardiometabolic Outcomes: A Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1917–1930. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Dworatzek, P.D. Food and dietary pattern-based recommendations: An emerging approach to clinical practice guidelines for nutrition therapy in diabetes. Can. J. Diabetes. 2013, 37, 51–57. [Google Scholar] [CrossRef]
- Hunter, S.R.; Reister, E.J.; Cheon, E.; Mattes, R.D. Low calorie sweeteners differ in their physiological effects in humans. Nutrients 2019, 11, 2717. [Google Scholar] [CrossRef]
- Burke, M.V.; Small, D.M. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol. Behav. 2015, 152, 381–388. [Google Scholar] [CrossRef]
- Thomson, P.; Santibanez, R.; Aguirre, C.; Galgani, J.E.; Garrido, D. Short-term impact of sucralose consumption on the metabolic response and gut microbiome of healthy adults. Br. J. Nutr. 2019, 122, 856–862. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Chang, K. Artificial Sweeteners May Disrupt Body’s Blood Sugar Controls. Available online: https://well.blogs.nytimes.com/2014/09/17/artificial-sweeteners-may-disrupt-bodys-blood-sugar-controls/ (accessed on 7 September 2019).
- Kirkey, S. Fake Sugars Linked to Obesity, Heart Disease. Available online: http://nationalpost.com/news/0717-na-sugar (accessed on 7 September 2019).
- Faherty, S. Artificial Sweeteners May Have Despicable Impacts on Gut Microbes. Available online: https://blogs.scientificamerican.com/guest-blog/artificial-sweeteners-may-have-despicable-impacts-on-gut-microbes/ (accessed on 7 September 2019).
- Sifferlin, A. Artificial Sweeteners are Linked to Weight Gain —Not Weight Loss. Available online: http://time.com/4859012/artificial-sweeteners-weight-loss/ (accessed on 7 September 2019).
- Vergano, D. Artificial Sweeteners May Trigger Blood Sugar Risks. Available online: http://news.nationalgeographic.com/news/2014/09/140917-sweeteners-artificial-blood-sugar-diabetes-health-ngfood/ (accessed on 7 September 2019).
- Leung, W. Sugar Substitutes Associated with Weight Gain and Health Problems, Study Says. Available online: https://www.theglobeandmail.com/news/national/sugar-substitutes-linked-to-weight-gain-and-health-problems-study-says/article35704562/ (accessed on 7 September 2019).
- Brait, E. More Research Needed into the Effects Sugar Substitutes Have on Health. Available online: https://www.thestar.com/life/2017/07/17/more-research-needed-into-the-effects-sugar-substitutes-have-on-health.html (accessed on 7 September 2019).
- Thompson, D. Do Artificial Sweeteners Raise Odds for Obesity? Available online: http://www.webmd.com/food-recipes/news/20170717/do-artificial-sweeteners-raise-odds-for-obesity#1 (accessed on 7 September 2019).
- Serrano, J.; Smith, K.R.; Crouch, A.L.; Sharma, V.; Yi, F.; Vargova, V.; LaMoia, T.E.; Dupont, L.M.; Serna, V.; Tang, F. High-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans and mice. Microbiome 2021, 9, 1–18. [Google Scholar] [CrossRef]
- Ahmad, S.Y.; Friel, J.; Mackay, D. The Effects of Non-Nutritive Artificial Sweeteners, Aspartame and Sucralose, on the Gut Microbiome in Healthy Adults: Secondary Outcomes of a Randomized Double-Blinded Crossover Clinical Trial. Nutrients 2020, 12, 3408. [Google Scholar] [CrossRef]
- Ahmad, S.Y.; Friel, J.K.; MacKay, D.S. The effect of the artificial sweeteners on glucose metabolism in healthy adults: A randomized, double-blinded, crossover clinical trial. Appl. Physiol. Nutr. Metab. 2020, 45, 606–612. [Google Scholar] [CrossRef]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P.; Renwick, A.G.J.N.r. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef]
- Bright, O.-J.M.; Wang, D.D.; Shams-White, M.; Bleich, S.N.; Foreyt, J.; Franz, M.; Johnson, G.; Manning, B.T.; Mattes, R.; Pi-Sunyer, X. Research priorities for studies linking intake of low-calorie sweeteners and potentially related health outcomes: Research methodology and study design. Curr. Dev. Nutr. 2017, 1, e000547. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institutes of Health Research in Partnership with Health Canada. Operating Grant: Sugar and Health ARCHIVED. Available online: https://www.researchnet-recherchenet.ca/rnr16/vwOpprtntyDtls.do?prog=2554&printfriendly=true (accessed on 9 March 2020).
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef]
- Canadian Institutes of Health Research; Natural Sciences and Engineering Research Council of Canada; Social Sciences and Humanities Research Council of Canada. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, December 2018. Available online: https://ethics.gc.ca/eng/documents/tcps2-2018-en-interactive-final.pdf (accessed on 5 October 2022).
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.; Eckel, R.; Grundy, S.; Zimmet, P.; Cleeman, J.; Donato, K.; Fruchart, J.; James, W.; Loria, C.; Smith Jr, S. A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2011; pp. 8–31.
- Lear, S.; James, P.; Ko, G.; Kumanyika, S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur. J. Clin. Nutr. 2010, 64, 42–61. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 2017, 25, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Huaman, J.-W.; Mego, M.; Manichanh, C.; Cañellas, N.; Cañueto, D.; Segurola, H.; Jansana, M.; Malagelada, C.; Accarino, A.; Vulevic, J. Effects of prebiotics vs a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology 2018, 155, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Ibrügger, S.; Gøbel, R.J.; Vestergaard, H.; Licht, T.R.; Frøkiær, H.; Linneberg, A.; Hansen, T.; Gupta, R.; Pedersen, O.; Kristensen, M. Two randomized cross-over trials assessing the impact of dietary gluten or wholegrain on the gut microbiome and host metabolic health. J. Clin. Trials. 2014, 4. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; Van Der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Leung, A.; Daskaklopoulou, S.; Dasgupta, K.; McBrien, K.; Butalia, S.; Zarnke, K.; Nerenberg, K.; Harris, K.; Nakhla, M.; Cloutier, L.; et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can. J. Cardiol. 2017, 33, 557–576. [Google Scholar] [CrossRef]
- Logue, C.; Dowey, L.R.C.; Strain, J.J.; Verhagen, H.; McClean, S.; Gallagher, A.M. Application of Liquid Chromatography-Tandem Mass Spectrometry To Determine Urinary Concentrations of Five Commonly Used Low-Calorie Sweeteners: A Novel Biomarker Approach for Assessing Recent Intakes? J. Agric. Food Chem. 2017, 65, 4516–4525. [Google Scholar] [CrossRef]
- Hales, C.; Randle, P. Effects of low-carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose-tolerance tests. Lancet 1963, 1, 790–794. [Google Scholar] [CrossRef]
- Wilkerson, H.L.; Butler, F.K.; Francis, J.O.S. The effect of prior carbohydrate intake on the oral glucose tolerance test. Diabetes 1960, 9, 386–391. [Google Scholar] [CrossRef]
- WHO Study Group on Diabetes Mellitus & World Health Organization. Diabetes Mellitus: Report of a WHO Study Group [meeting held in Geneva from 11 to 16 February 1985]; World Health Organization: Geneva, Switzerland, 1985; pp. 7–93.
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, R.S.; Giuliano, V.; Chouinard-Watkins, R.; Bazinet, R.P.J.L. Natural Abundance Carbon Isotopic Analysis Indicates the Equal Contribution of Local Synthesis and Plasma Uptake to Palmitate Levels in the Mouse Brain. Lipids 2018, 53, 481–490. [Google Scholar] [CrossRef]
- Johner, S.A.; Libuda, L.; Shi, L.; Retzlaff, A.; Joslowski, G.; Remer, T. Urinary fructose: A potential biomarker for dietary fructose intake in children. Eur. J. Clin. Nutr. 2010, 64, 1365–1370. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Salgado, A.; Carvalho, L.; Oliveira, A.; Santos, V.; Vieira, J.; Parise, E. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef]
- Retnakaran, R.; Shen, S.; Hanley, A.J.; Vuksan, V.; Hamilton, J.K.; Zinman, B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 2008, 16, 1901–1907. [Google Scholar] [CrossRef]
- Retnakaran, R.; Qi, Y.; Goran, M.; Hamilton, J. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet. Med. 2009, 26, 1198–1203. [Google Scholar] [CrossRef]
- Kahn, S.E.; Montgomery, B.; Howell, W.; Ligueros-Saylan, M.; Hsu, C.-H.; Devineni, D.; McLeod, J.F.; Horowitz, A.; Foley, J.E. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2001, 86, 5824–5829. [Google Scholar] [CrossRef]
- Phillips, D.; Clark, P.; Hales, C.; Osmond, C. Understanding oral glucose tolerance: Comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet. Med. 1994, 11, 286–292. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Wolever, T.M.; Chiasson, J.-L.; Csima, A.; Hunt, J.A.; Palmason, C.; Ross, S.A.; Ryan, E.A. Variation of postprandial plasma glucose, palatability, and symptoms associated with a standardized mixed test meal versus 75 g oral glucose. Diabetes Care 1998, 21, 336–340. [Google Scholar] [CrossRef]
- Health Canada. Draft Guidance Document on Food Health Claims Related to the Reduction in Post-Prandial Glycaemic Response. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/public-involvement-partnerships/technical-consultation-draft-guidance-document-food-health-claims-related-post-prandial-glycaemia.html (accessed on 9 March 2020).
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef]
- Braunstein, C.R.; Noronha, J.C.; Glenn, A.J.; Viguiliouk, E.; Noseworthy, R.; Khan, T.A.; Au-Yeung, F.; Blanco Mejia, S.; Wolever, T.M.; Josse, R.G. A double-blind, randomized controlled, acute feeding equivalence trial of small, catalytic doses of fructose and allulose on postprandial blood glucose metabolism in healthy participants: The Fructose and Allulose Catalytic Effects (FACE) Trial. Nutrients 2018, 10, 750. [Google Scholar] [CrossRef]
- Campos, V.; Despland, C.; Brandejsky, V.; Kreis, R.; Schneiter, P.; Chiolero, A.; Boesch, C.; Tappy, L. Sugar- and artificially sweetened beverages and intrahepatic fat: A randomized controlled trial. Obesity 2015, 23, 2335–2339. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Statist. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Food and Drug Administration. Multiple Endpoints in Clinical Trials: Guidance for Industry. Available online: https://www.fda.gov/media/162416/download (accessed on 23 January 2023).
- Tran, N.L.; Barraj, L.M.; Hearty, A.P.; Jack, M.M. Tiered intake assessment for low-and no-calorie sweeteners in beverages. Food Addit. Contam. Part A 2021, 38, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Dunford, E.K.; Taillie, L.S.; Miles, D.R.; Eyles, H.; Tolentino-Mayo, L.; Ng, S.W.J.N. Non-Nutritive Sweeteners in the Packaged Food Supply—An Assessment across 4 Countries. Nutrients 2018, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Garriguet, D. Beverage consumption of Canadian adults. Health Rep. 2008, 19, 23. [Google Scholar] [PubMed]
- GlobalData. Soft Drinks Market Analyzer Database. Available online: www.globaldata.com (accessed on 3 November 2021).
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Casals-Pascual, C.; González, A.; Vázquez-Baeza, Y.; Song, S.J.; Jiang, L.; Knight, R. Microbial diversity in clinical microbiome studies: Sample size and statistical power considerations. Gastroenterology 2020, 158, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Narayana, J.K.; Mac Aogáin, M.; Goh, W.W.B.; Xia, K.; Tsaneva-Atanasova, K.; Chotirmall, S.H. Mathematical-based microbiome analytics for clinical translation. Comput. Struct. Biotechnol. J. 2021, 19, 6272–6281. [Google Scholar] [CrossRef] [PubMed]
- Diana Sherifali, R.; Robyn, L. Diabetes Canada clinical practice guidelines expert committee. Can. J. Diabet. 2018, 42, S6–S9. [Google Scholar]
- Sedgwick, P.; Greenwood, N. Understanding the Hawthorne effect. BMJ 2015, 351, h4672. [Google Scholar] [CrossRef]
- Statistics Canada. Health Fact Sheets. Overweight and Obese Adults. 2018. Available online: https://www150.statcan.gc.ca/n1/pub/82-625-x/2019001/article/00005-eng.htm (accessed on 14 January 2023).
- Jones, A.C.; Kirkpatrick, S.I.; Hammond, D. Beverage consumption and energy intake among Canadians: Analyses of 2004 and 2015 national dietary intake data. Nutr. J. 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Nikpartow, N.; Danyliw, A.D.; Whiting, S.J.; Lim, H.J.; Vatanparast, H.J.P.h.n. Beverage consumption patterns of Canadian adults aged 19 to 65 years. Public Health Nutr. 2012, 15, 2175–2184. [Google Scholar] [CrossRef]
- Brancati, F.L.; Whelton, P.K.; Kuller, L.H.; Klag, M.J. Diabetes mellitus, race, and socioeconomic status a population-based study. Ann. Epidemiol. 1996, 6, 67–73. [Google Scholar] [CrossRef]
- Canadian Institute for Health Information. Diabetes Care Gaps and Disparities in Canada. Available online: https://publications.gc.ca/collections/collection_2012/icis-cihi/H117-5-7-2009-eng.pdf (accessed on 11 October 2021).
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children. Available online: http://apps.who.int/iris/bitstream/handle/10665/149782/9789241549028_eng.pdf;jsessionid=F9FAD19E165BB45830BA1A484FC6FD93?sequence=1 (accessed on 9 November 2018).
- Health Canada. Summary of Guiding Principles and Recommendations. Available online: https://www.foodguideconsultation.ca/guiding-principles-summary (accessed on 25 August 2017).
- Diabetes Canada. Diabetes Canada’s Position on Sugars. Available online: http://www.diabetes.ca/about-cda/public-policy-position-statements/sugars (accessed on 25 August 2017).
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015—2020 Dietary Guidelines for Americans. 8th Edition. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 20 January 2022).
 = Randomization event: AHRC-REDCap program; Blocked (Latin squares) randomization; Allocation concealment; 1H-MRS = proton magnetic resonance spectroscopy;
= Randomization event: AHRC-REDCap program; Blocked (Latin squares) randomization; Allocation concealment; 1H-MRS = proton magnetic resonance spectroscopy;  = Water;
= Water;  = sugar-sweetened beverage (SSB);
= sugar-sweetened beverage (SSB);  = non-nutritive sweetened beverage (NSB); N = number; OGTT = 2-h 75 g oral glucose tolerance test.
= non-nutritive sweetened beverage (NSB); N = number; OGTT = 2-h 75 g oral glucose tolerance test.
 = Randomization event: AHRC-REDCap program; Blocked (Latin squares) randomization; Allocation concealment; 1H-MRS = proton magnetic resonance spectroscopy;
= Randomization event: AHRC-REDCap program; Blocked (Latin squares) randomization; Allocation concealment; 1H-MRS = proton magnetic resonance spectroscopy;  = Water;
= Water;  = sugar-sweetened beverage (SSB);
= sugar-sweetened beverage (SSB);  = non-nutritive sweetened beverage (NSB); N = number; OGTT = 2-h 75 g oral glucose tolerance test.
= non-nutritive sweetened beverage (NSB); N = number; OGTT = 2-h 75 g oral glucose tolerance test.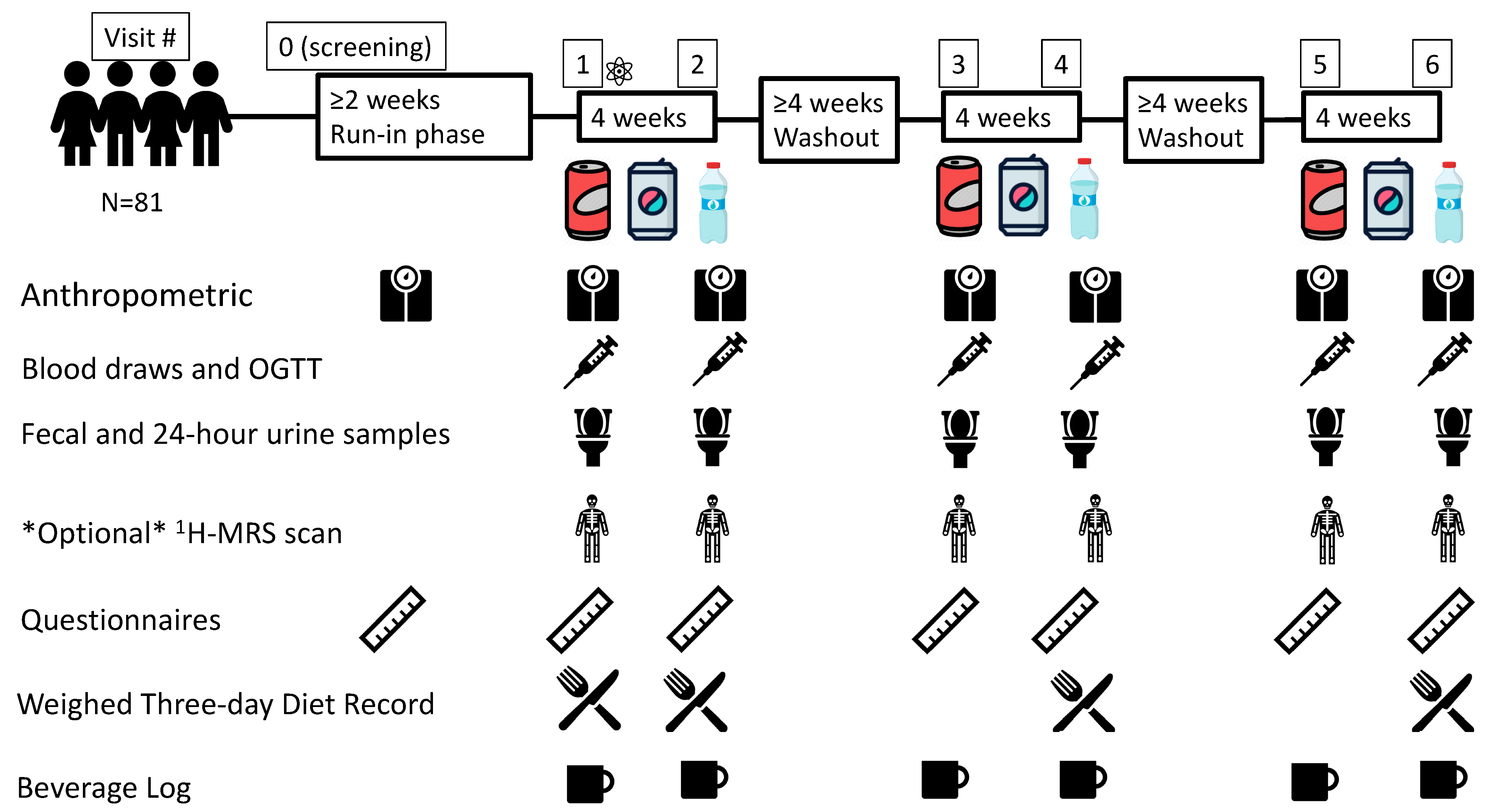
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age between 18 and 75 years | Age below 18 years and greater than 75 years |
| Regularly consumes SSBs (≥1355 mL serving per day) 2 | BMI < 23 kg/m2 for Asian individuals and <25 kg/m2 for other individuals 1 |
| BMI ≥ 23 kg/m2 for Asian individuals and ≥25 kg/m2 for other individuals 1 | Waist circumference <94 cm in men, <80 cm in women in Europid, Sub-Saharan African, Eastern Mediterranean, and Middle Eastern individuals; <90 cm in men and <80 cm in women for South Asian, Chinese, Japanese, and South and Central American individuals 1 |
| Waist circumference >94 cm in men, >80 cm in women in Europid, Sub-Saharan African, Eastern Mediterranean, and Middle Eastern individuals; >90 cm in men and >80 cm in women for South Asian, Chinese, Japanese, and South and Central American individuals [61,62,63,64] 1 | Not regularly drinking SSBs (<1355 mL can serving per day) 2 |
| Otherwise healthy | Pregnancy or breast-feeding females, or females planning on becoming pregnant throughout the study duration |
| Has a primary care physician | Regular medication use that have a clinically relevant effect on the primary outcomes (exceptions include birth control and PRN meds such as Advil, Tylenol, etc.) |
| Antibiotic use in the last 3 months [65,66,67,68,69] | |
| Use of CAM deemed inappropriate by investigators | |
| Diabetes | |
| Self-reported hypertension or systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 100 mmHg [70] when measured at screening visit 1 | |
| Polycystic ovarian syndrome | |
| Cardiovascular disease | |
| Gastrointestinal disease | |
| Previous bariatric surgery | |
| Liver disease | |
| Hyper-or hypothyroidism | |
| Kidney disease | |
| Chronic infection | |
| Lung disease | |
| Cancer/malignancy | |
| Schizophrenia spectrum and other psychotic disorders, bipolar and related disorders, and dissociative disorders | |
| Major surgery in the last 6 months | |
| Other major illness or health-related incidence within the last 6 months | |
| Regular cigarettes smoker | |
| Regular recreational drug users | |
| Heavy alcohol users (>3 drinks/day) | |
| Does not have a primary care physician | |
| Participation in any trials within the last 6 months or planning on participating in other trials for the duration of this study | |
| Plans to make dietary or physical activity changes throughout study duration | |
| Any condition or circumstance which would prevent you from having an 1H-MRS scan (e.g., having prostheses or metal implants, tattoos, or claustrophobia) (this is only applicable for the 32 participants in the Ectopic Fat sub-study) |
| Intervention Phase 1 | Intervention Phase 2 | Intervention Phase 3 | |
|---|---|---|---|
| Sequence Group 1 | A | B | C |
| Sequence Group 2 | B | C | A |
| Sequence Group 3 | C | A | B |
| Sequence Group 4 | B | A | C |
| Sequence Group 5 | A | C | B |
| Sequence Group 6 | C | B | A |
| Group | |||
|---|---|---|---|
| SSB Arm (42 g Sugars, 140 kcal) | NSB Arm (0 g Sugars, 0 kcal) | NNS Sweetener | Water Arm (0 g Sugars, 0 kcal) |
| Coca-Cola | Diet Coke | Aspartame and acesulfame–potassium | Still |
| Coke Zero | Aspartame and acesulfame–potassium | ||
| Pepsi | Diet Pepsi | Aspartame and acesulfame–potassium | Carbonated |
| Canada Dry Ginger Ale | Diet Canada Dry Ginger Ale | Aspartame and acesulfame–potassium | |
| Sprite | Sprite Zero | Aspartame and acesulfame–potassium | |
| 7UP | Diet 7UP | Aspartame and acesulfame–potassium | |
| Orange Crush | Diet Orange Crush | Sucralose | |
| Outcome | Mean Change ± SD | Correlation | N | N (Corrected for 20% Attrition) | Alpha | Power (1-Beta) (%) |
|---|---|---|---|---|---|---|
| Primary Outcome Family | ||||||
| Weighed UniFrac Distance | 0.04 ± 0.07 [43] | 0.70 | 60 | 75 | Largest p-value at alpha 0.0375 *; If failed test second p-value at alpha 0.025 | 98 |
| Glucose iAUC (mmol/L/min) | 44.81 ± 113.00 (20%) [88,89] | 0.70 | 60 | 75 | 89 | |
| Secondary Outcome Family | ||||||
| Waist Circumference (cm) | 1.00 ± 7.17 [35,90] | 0.70 | 60 | 75 | 0.05 | 35 |
| Body Weight (kg) | 1.00 ± 10.10 [35,90] | 0.70 | 60 | 75 | 0.05 | 20 |
| Fasting Plasma Glucose (mmol/L) | 1.0 ± 2.20 [91] | 0.70 | 60 | 75 | 0.05 | 99 |
| 2-Hour Plasma Glucose (mmol/L) | 1.40 ± 1.40 [91] | 0.70 | 60 | 75 | 0.05 | 99 |
| Matsuda ISIOGTT | 0.35 ± 1.26 [91] | 0.70 | 60 | 75 | 0.05 | 81 |
| Ectopic Fat Sub-Study | ||||||
| IHCL (1H-MRS) (%) | 5.00 ± 10.00 [92] | 0.67 | 25 | 32 | 0.05 | 80 |
| Variable | Main Trial (N = 80) | Ectopic Fat Sub-Study (N = 32) |
|---|---|---|
| Anthropometry | Mean ± SD | Mean ± SD |
| Age (years) | 42.34 ± 12.99 | 42.16 ± 12.91 |
| Females, n (%) | 41 (51) | 16 (50) |
| Height (cm) | 167.19 ± 10.66 | 167.90 ± 9.23 |
| Weight (kg) | 93.99 ± 18.89 | 95.25 ± 19.68 |
| BMI (kg/m2) | 33.71 ± 6.75 | 33.70 ± 6.03 |
| Waist circumference (cm) | 108.69 ± 13.50 | 110.31 ± 13.67 |
| Systolic blood pressure (mmHg) | 116.37 ± 12.49 | 76.68 ± 9.12 |
| Diastolic blood pressure (mmHg) | 76.24 ± 9.03 | 72.34 ± 9.76 |
| FPG (mmol/L) | 5.57 ± 1.19 | 5.77 ± 1.75 |
| 2 h-PG (mmol/L) | 7.26 ± 3.11 | 7.99 ± 4.05 |
| IHCL (%) | NA | 9.7 ± 9.2 |
| Self-reported ethnicity | n (%) | n (%) |
| Aboriginal | 2 (3) | 1 (3) |
| European | 36 (45) | 18 (56) |
| African/Caribbean | 5 (6) | 2 (6) |
| Latin American | 6 (8) | 3 (9) |
| Indian | 5 (6) | 2 (6) |
| East Asian | 6 (8) | 1 (3) |
| Southeast Asian | 6 (8) | 2 (6) |
| Mixed ethnicity | 14 (18) | 3 (9) |
| Highest level of education | n (%) | n (%) |
| Grade 9 | 1 (1) | 0 (0) |
| High School Diploma or High School Equivalent | 18 (23) | 10 (31) |
| College Certificate or Diploma | 18 (23) | 9 (28) |
| Undergraduate Degree | 27 (34) | 8 (25) |
| Graduate Degree (including post-graduate) | 16 (20) | 5 (16) |
| Work status | n (%) | n (%) |
| Full-time (≥32 h/week) | 40 (50) | 14 (44) |
| Part-time (≤32 h/week) | 13 (16) | 5 (16) |
| Casual employee | 6 (8) | 6 (19) |
| Stay at home parent | 6 (8) | 2 (6) |
| Full-time student | 3 (4) | 1 (3) |
| Disability | 3 (4) | 1 (3) |
| Multiple work status | 6 (8) | 2 (6) |
| Other | 3 (4) | 1 (3) |
| Alcohol intake | n (%) | n (%) |
| None | 21 (26) | 6 (19) |
| 1–2 times per year | 9 (11) | 4 (13) |
| Every 2–3 months | 16 (20) | 9 (28) |
| 1–2 times per month | 16 (20) | 3 (9) |
| 1–2 times per week | 15 (19) | 8 (25) |
| Daily | 3 (4) | 2 (6) |
| Regular medication use | n (%) | n (%) |
| Participants taking medications | 23 (29) | 12 (38) |
| Aspirin | 1 (4) | 1 (8) |
| Paracetamol | 1 (4) | 0 (0) |
| Combined inhaled corticosteroids and short-acting bronchodilators | 1 (4) | 0 (0) |
| Oral contraceptive | 2 (9) | 1 (8) |
| Statins | 1 (4) | 0 (0) |
| Antihistamine | 2 (9) | 0 (0) |
| Anxiolytic/anticonvulsant | 1 (4) | 0 (0) |
| Migraine relief | 1 (4) | 1 (8) |
| PReP | 1 (4) | 1 (8) |
| Short-acting bronchodilators | 1 (4) | 0 (0) |
| Stimulant | 1 (4) | 1 (8) |
| Topical corticosteroid | 1 (4) | 1 (8) |
| Mixed | 9 (39) | 6 (50) |
| ACEi + statin | 1 (11) | 1 (17) |
| Stimulant + diuretic + ACEi + antidepressant + antipsychotic + anxiolytic/anticonvulsant + medical marijuana + combined inhaled corticosteroids and long-acting bronchodilators + antacid | 1 (11) | 0 (0) |
| Short-acting bronchodilator + combined inhaled corticosteroids and long-acting bronchodilators + inhaled corticosteroid | 1 (11) | 1 (17) |
| Short-acting bronchodilators + antihistamine | 1 (11) | 1 (17) |
| Aspirin + Feminizing hormone therapy + oral disinfectant and antiseptic + topical antibiotics + diuretic | 1 (11) | 1 (17) |
| Combined ARB and diuretic +statin | 1 (11) | 0 (0) |
| ARB + Ursodiol | 1 (11) | 1 (17) |
| Statin + Aspirin | 1 (11) | 0 (0) |
| Antihistamine + ibuprofen | 1 (11) | 1 (17) |
| Supplement use | n (%) | n (%) |
| Participants taking supplements | 23 (29) | 8 (25) |
| Recreational marijuana | 2 (9) | 1 (13) |
| Multivitamin | 6 (26) | 3 (38) |
| Vitamin B | 1 (4) | 1 (13) |
| Vitamin C | 2 (9) | 0 (0) |
| Calcium | 1 (4) | 0 (0) |
| Iron | 1 (4) | 0 (0) |
| Fiber | 1 (4) | 0 (0) |
| Glucosamine | 1 (4) | |
| Mixed | 8 (35) | 3 (38) |
| Multivitamin + fiber | 1 (13) | 1 (33) |
| Omega-3 + super cod liver oil + vitamin D + combination calcium and magnesium | 1 (13) | 0 (0) |
| Vitamin B + multivitamin | 1 (13) | 0 (0) |
| Omega-3 + turmeric | 1 (13) | 0 (0) |
| Zinc + vitamin B + vitamin C + fiber | 1 (13) | 1 (33) |
| Combination calcium and vitamin D + vitamin A + vitamin B + vitamin C + vitamin D + vitamin E + combination vitamin K and vitamin D + combination omega-3 and omega-6 + magnesium | 1 (13) | 0 (0) |
| Glucosamine + vitamin D + coEQ + combination omega-3 and vitamin E + ginkgo biloba + turmeric + multivitamin | 1 (13) | 0 (0) |
| Vitamin D + vitamin E + vitamin C | 1 (13) | 1 (33) |
| Baseline background NNS intake | n (%) | n (%) |
| From beverages | 21 (26) | 10 (31) |
| From foods | 4 (5) | 0 (0) |
| As added sweeteners | 5 (6) | 1 (3) |
| Multiple sources | 6 (8) | 1 (3) |
| None | 44 (55) | 20 (63) |
| Baseline SSB intake | n (%) | n (%) |
| Cola | ||
| Coca-Cola | 35 (44) | 17 (53) |
| Pepsi | 11 (14) | 4 (13) |
| Non-cola | ||
| Canada Dry Ginger Ale | 21 (26) | 6 (19) |
| Sprite | 7 (9) | 4 (13) |
| 7-UP | 3 (4) | 0 (0) |
| Orange Crush | 3 (4) | 1 (3) |
| Baseline mean SSB intake/day | Mean servings/day * (range) | Mean servings/day * (range) |
| Overall | 1.84 (1–5) | 2.02 (1–5) |
| Cola | ||
| Coca-Cola | 1.93 (1–5) | 2.00 (1–5) |
| Pepsi | 2.27 (1–4) | 3.00 (2–4) |
| Non-cola | ||
| Canada Dry Ginger Ale | 1.53 (1–5) | 1.94 (1–5) |
| Sprite | 1.86 (1–4) | 1.50 (1–2) |
| 7-UP | 2.00 | 0.00 |
| Orange Crush | 1.00 | 1.00 |
| NSB equivalents (NNS blends) | n (%) | n (%) |
| Cola | ||
| Coke Zero (Asp and Ace-K) | 18 (23) | 9 (28) |
| Diet Coke (Asp and Ace-K) | 15 (19) | 7 (22) |
| Diet Pepsi (Asp and Ace-K) | 11 (14) | 4 (13) |
| Diet Coke or Coke Zero (Asp and Ace-K) | 2 (3) | 1 (3) |
| Non-cola | ||
| Canada Dry Diet Ginger Ale (Asp and Ace-K) | 20 (25) | 5 (16) |
| Sprite Zero (Asp and Ace-K) | 7 (9) | 4 (13) |
| Diet Orange Crush (Sucralose) | 4 (5) | 2 (6) |
| Diet 7-UP (Asp and Ace-K) | 3 (4) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub-Charette, S.; McGlynn, N.D.; Lee, D.; Khan, T.A.; Blanco Mejia, S.; Chiavaroli, L.; Kavanagh, M.E.; Seider, M.; Taibi, A.; Chen, C.T.; et al. Rationale, Design and Participants Baseline Characteristics of a Crossover Randomized Controlled Trial of the Effect of Replacing SSBs with NSBs versus Water on Glucose Tolerance, Gut Microbiome and Cardiometabolic Risk in Overweight or Obese Adult SSB Consumer: Strategies to Oppose SUGARS with Non-Nutritive Sweeteners or Water (STOP Sugars NOW) Trial and Ectopic Fat Sub-Study. Nutrients 2023, 15, 1238. https://doi.org/10.3390/nu15051238
Ayoub-Charette S, McGlynn ND, Lee D, Khan TA, Blanco Mejia S, Chiavaroli L, Kavanagh ME, Seider M, Taibi A, Chen CT, et al. Rationale, Design and Participants Baseline Characteristics of a Crossover Randomized Controlled Trial of the Effect of Replacing SSBs with NSBs versus Water on Glucose Tolerance, Gut Microbiome and Cardiometabolic Risk in Overweight or Obese Adult SSB Consumer: Strategies to Oppose SUGARS with Non-Nutritive Sweeteners or Water (STOP Sugars NOW) Trial and Ectopic Fat Sub-Study. Nutrients. 2023; 15(5):1238. https://doi.org/10.3390/nu15051238
Chicago/Turabian StyleAyoub-Charette, Sabrina, Néma D. McGlynn, Danielle Lee, Tauseef Ahmad Khan, Sonia Blanco Mejia, Laura Chiavaroli, Meaghan E. Kavanagh, Maxine Seider, Amel Taibi, Chuck T. Chen, and et al. 2023. "Rationale, Design and Participants Baseline Characteristics of a Crossover Randomized Controlled Trial of the Effect of Replacing SSBs with NSBs versus Water on Glucose Tolerance, Gut Microbiome and Cardiometabolic Risk in Overweight or Obese Adult SSB Consumer: Strategies to Oppose SUGARS with Non-Nutritive Sweeteners or Water (STOP Sugars NOW) Trial and Ectopic Fat Sub-Study" Nutrients 15, no. 5: 1238. https://doi.org/10.3390/nu15051238
APA StyleAyoub-Charette, S., McGlynn, N. D., Lee, D., Khan, T. A., Blanco Mejia, S., Chiavaroli, L., Kavanagh, M. E., Seider, M., Taibi, A., Chen, C. T., Ahmed, A., Asbury, R., Erlich, M., Chen, Y.-T., Malik, V. S., Bazinet, R. P., Ramdath, D. D., Logue, C., Hanley, A. J., ... Sievenpiper, J. L. (2023). Rationale, Design and Participants Baseline Characteristics of a Crossover Randomized Controlled Trial of the Effect of Replacing SSBs with NSBs versus Water on Glucose Tolerance, Gut Microbiome and Cardiometabolic Risk in Overweight or Obese Adult SSB Consumer: Strategies to Oppose SUGARS with Non-Nutritive Sweeteners or Water (STOP Sugars NOW) Trial and Ectopic Fat Sub-Study. Nutrients, 15(5), 1238. https://doi.org/10.3390/nu15051238








