Effectiveness of Apigenin, Resveratrol, and Curcumin as Adjuvant Nutraceuticals for Calvarial Bone Defect Healing: An In Vitro and Histological Study on Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Dental Pulp Stem Cells Cultures
2.2. In Vitro Study Design
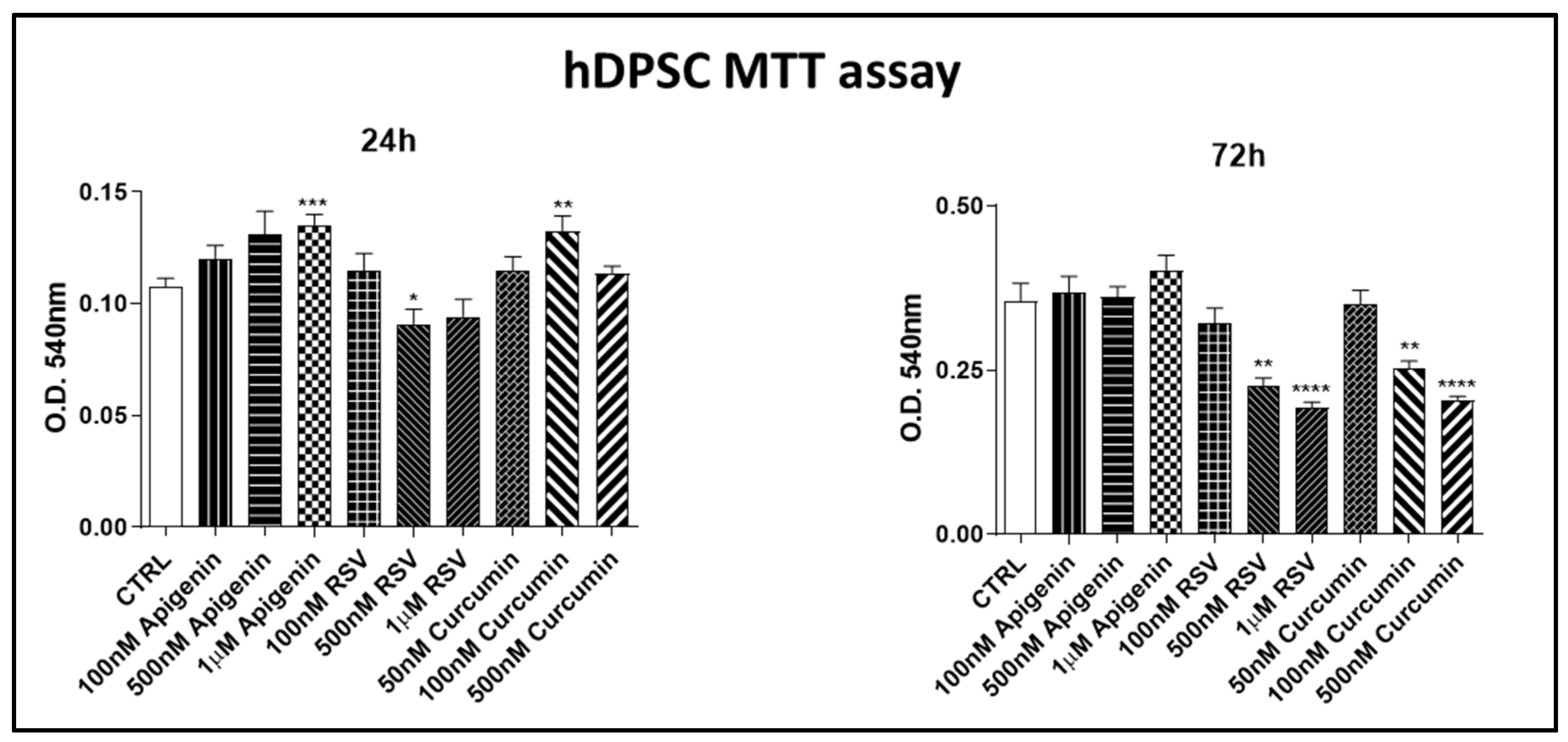
2.3. Cell Proliferation Assay
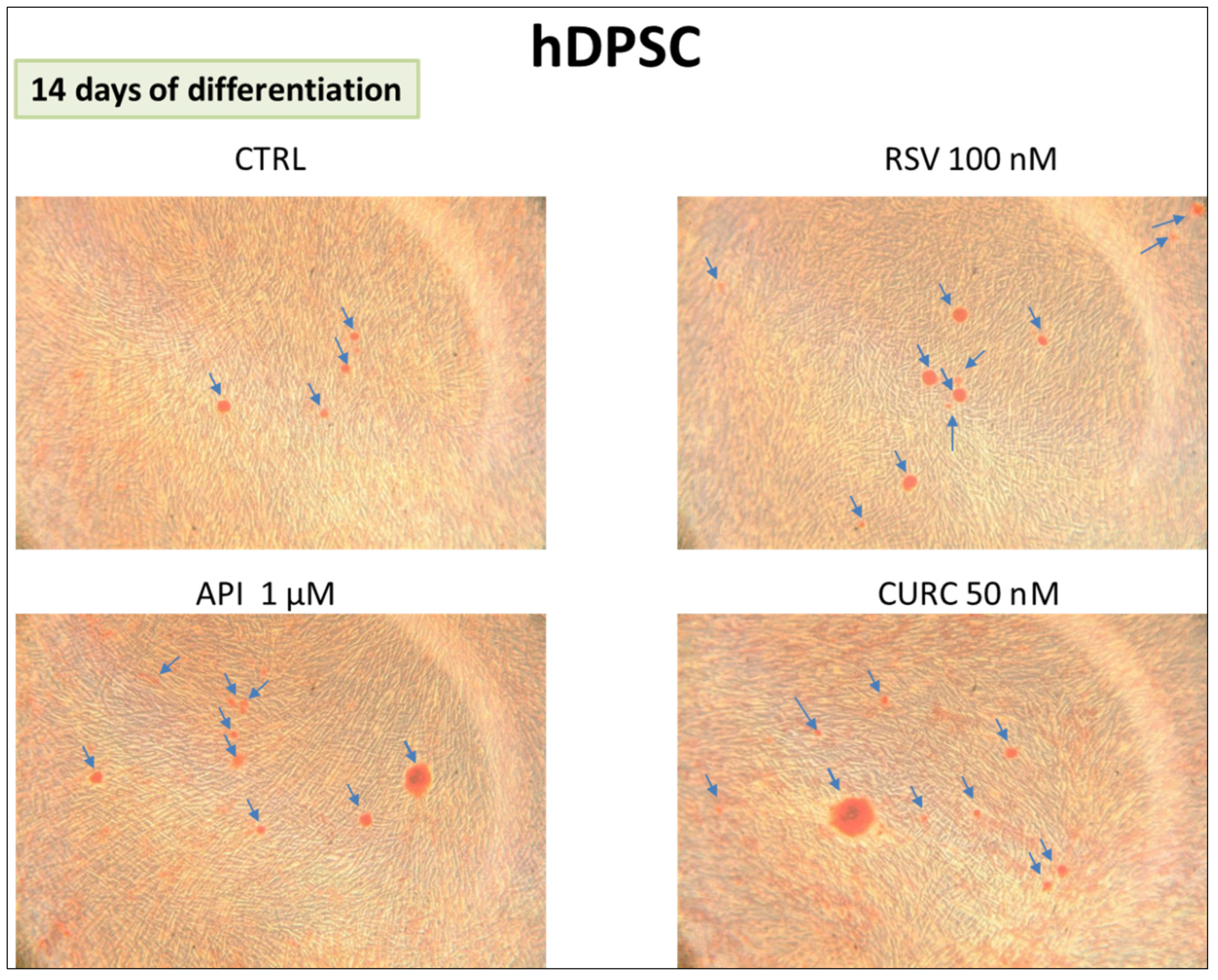
2.4. Alizarin Red Staining
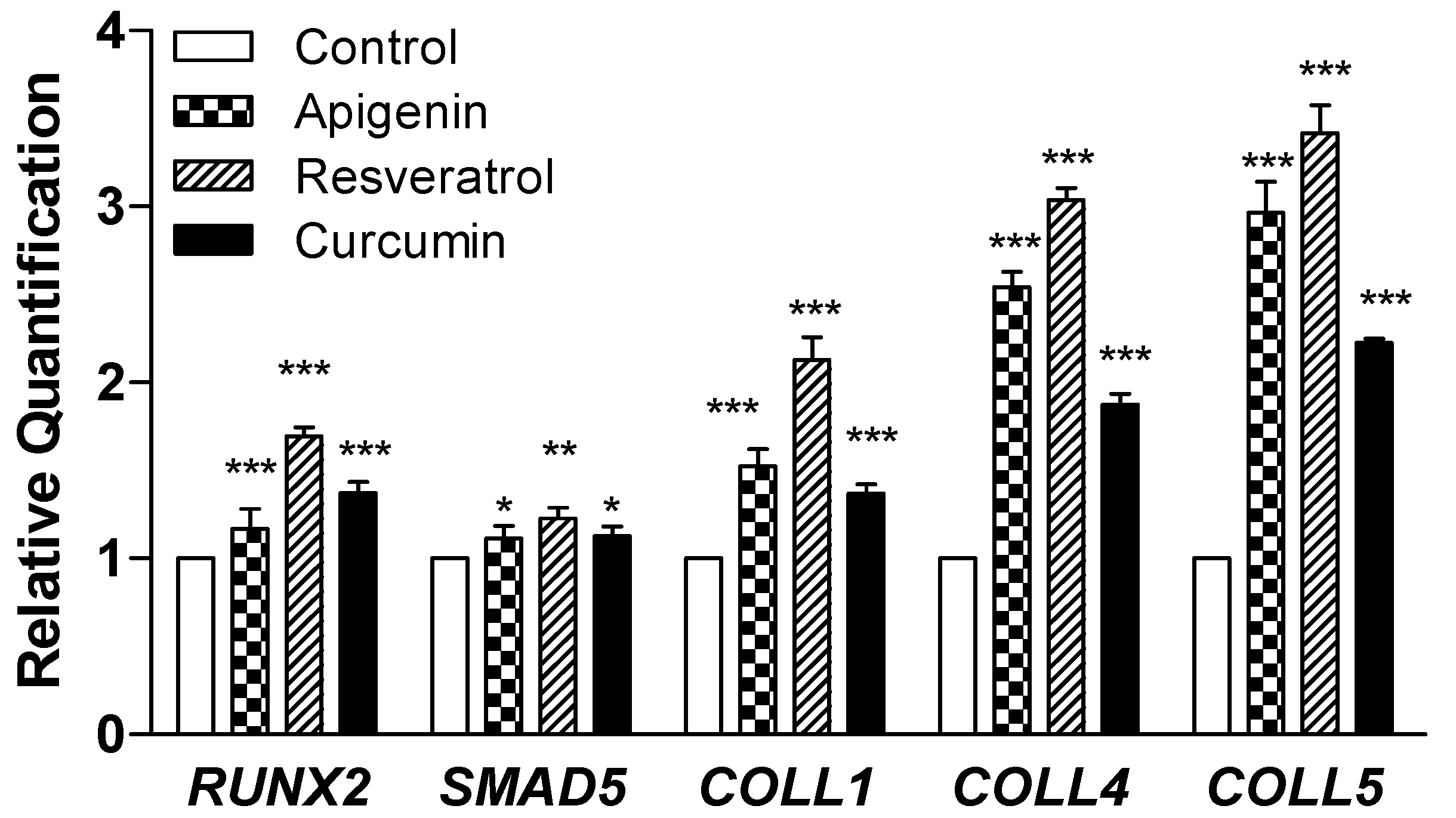
2.5. Quantitative Real-Time PCR for Gene Expression Analysis
2.6. In Vivo Animal Study
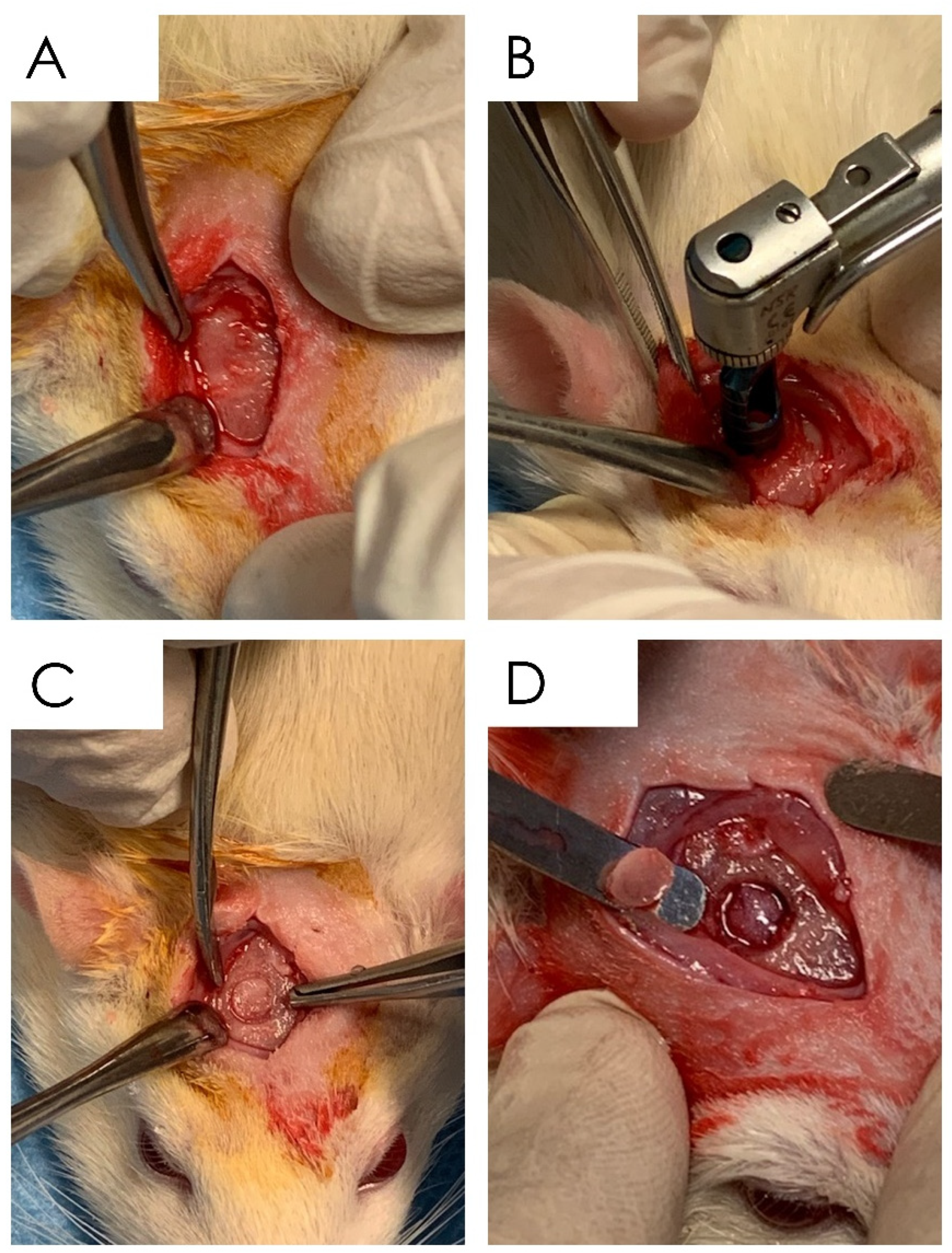
2.6.1. Surgical Procedure
- (1)
- Group A: Ctr—empty bone defect;
- (2)
- Group B: Resveratrol (resveratrol 98%, No. 3183, Galeno SRL, Comeana, Italy) 1 mL (10 mg/kg)—empty bone defect/administration of resveratrol by gastric gavage in a single dose daily [40];
- (3)
- Group C: Curcumin (curcumin 95%, No. 4507, Galeno SRL, Comeana, Italy) 1 mL (10 mg/kg)—empty bone defect/administration of curcumin by gastric gavage in a single dose daily [41];
- (4)
2.6.2. Specimen Processing
2.7. Statistical Analysis
3. Results
3.1. In Vitro Procedure
3.1.1. Cell Proliferation Assay
3.1.2. Alizarin Red Assay
3.1.3. Gene Expression
3.1.4. In Vivo Procedure
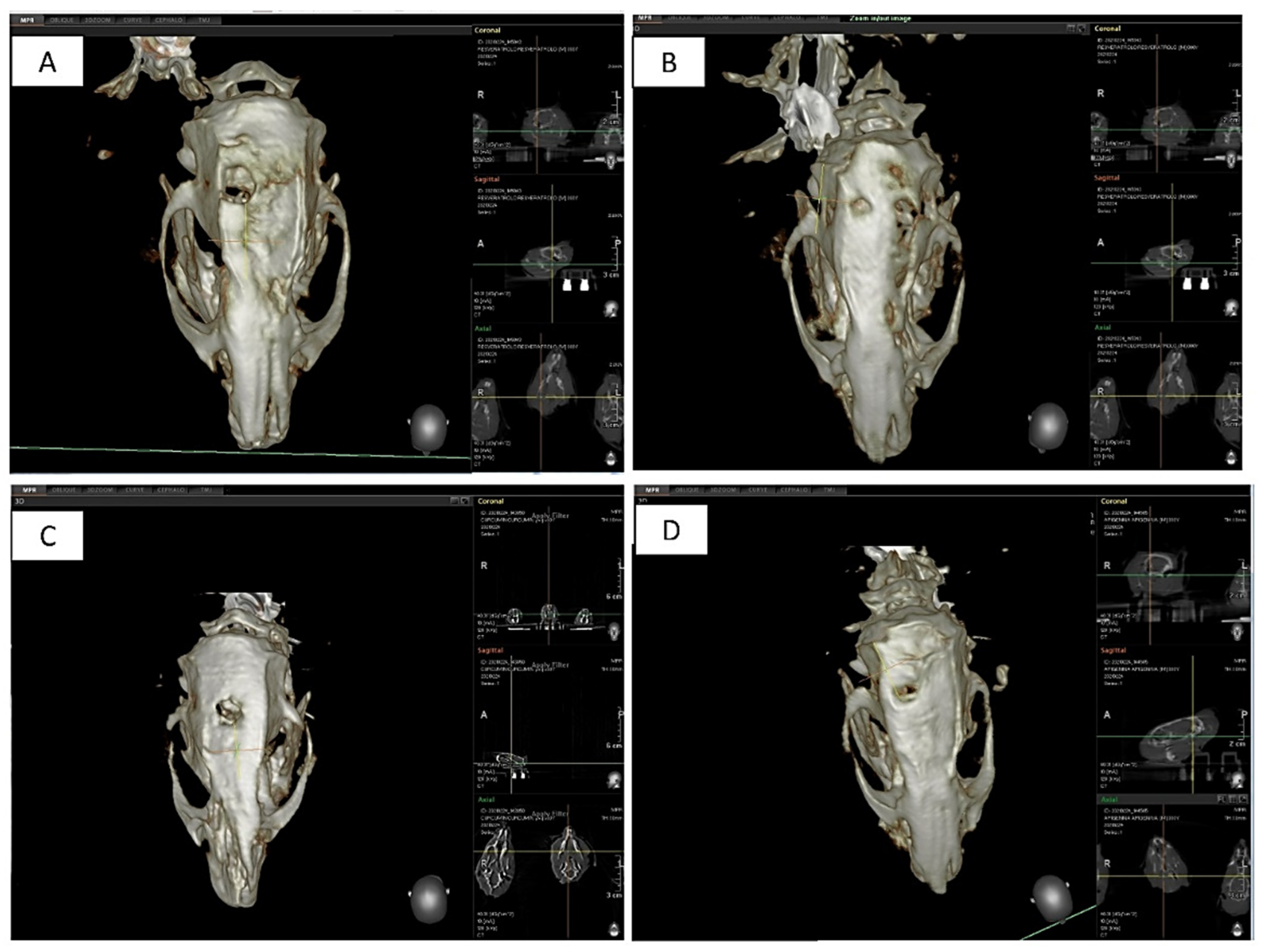
3.1.5. Tomography Assessment
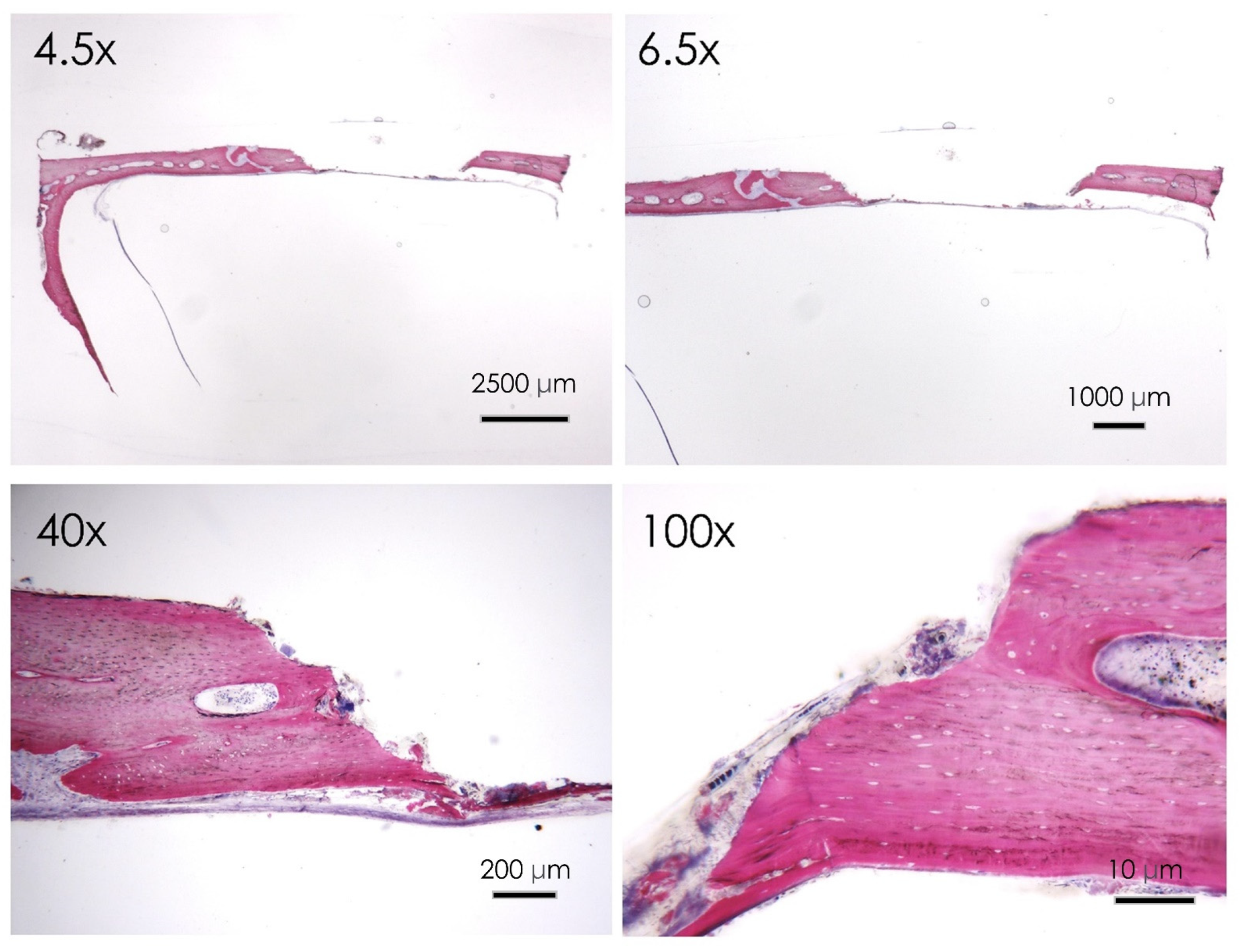
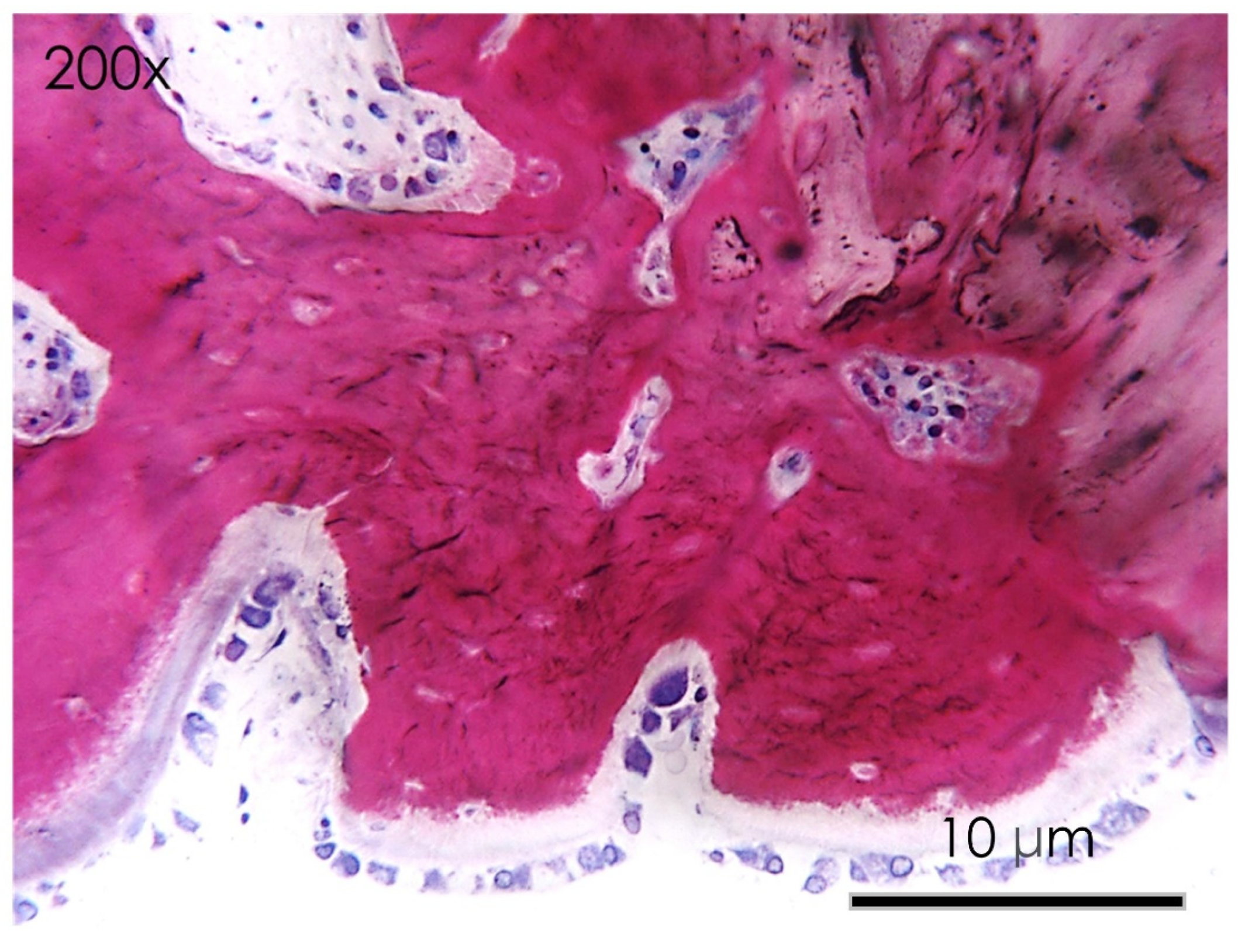
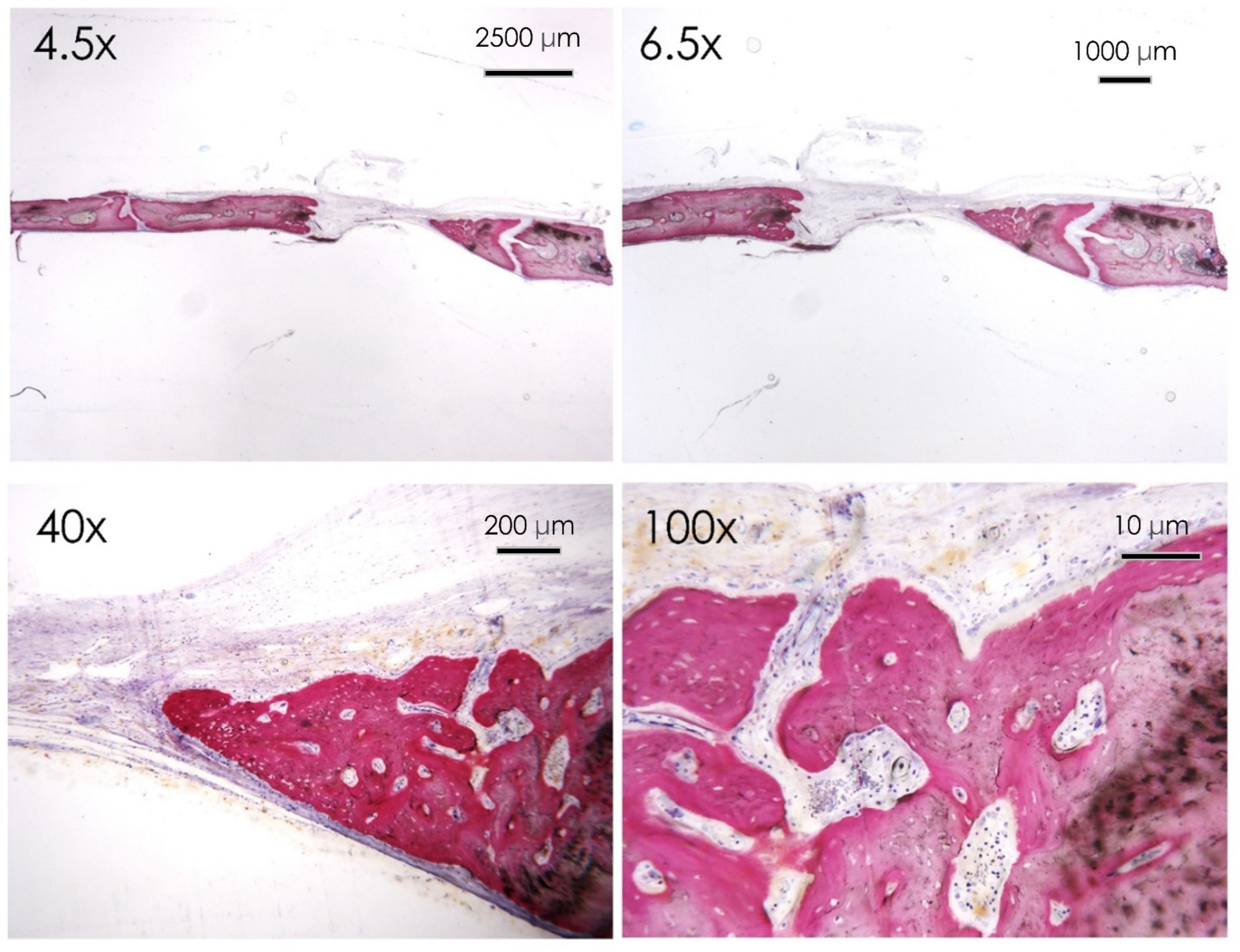
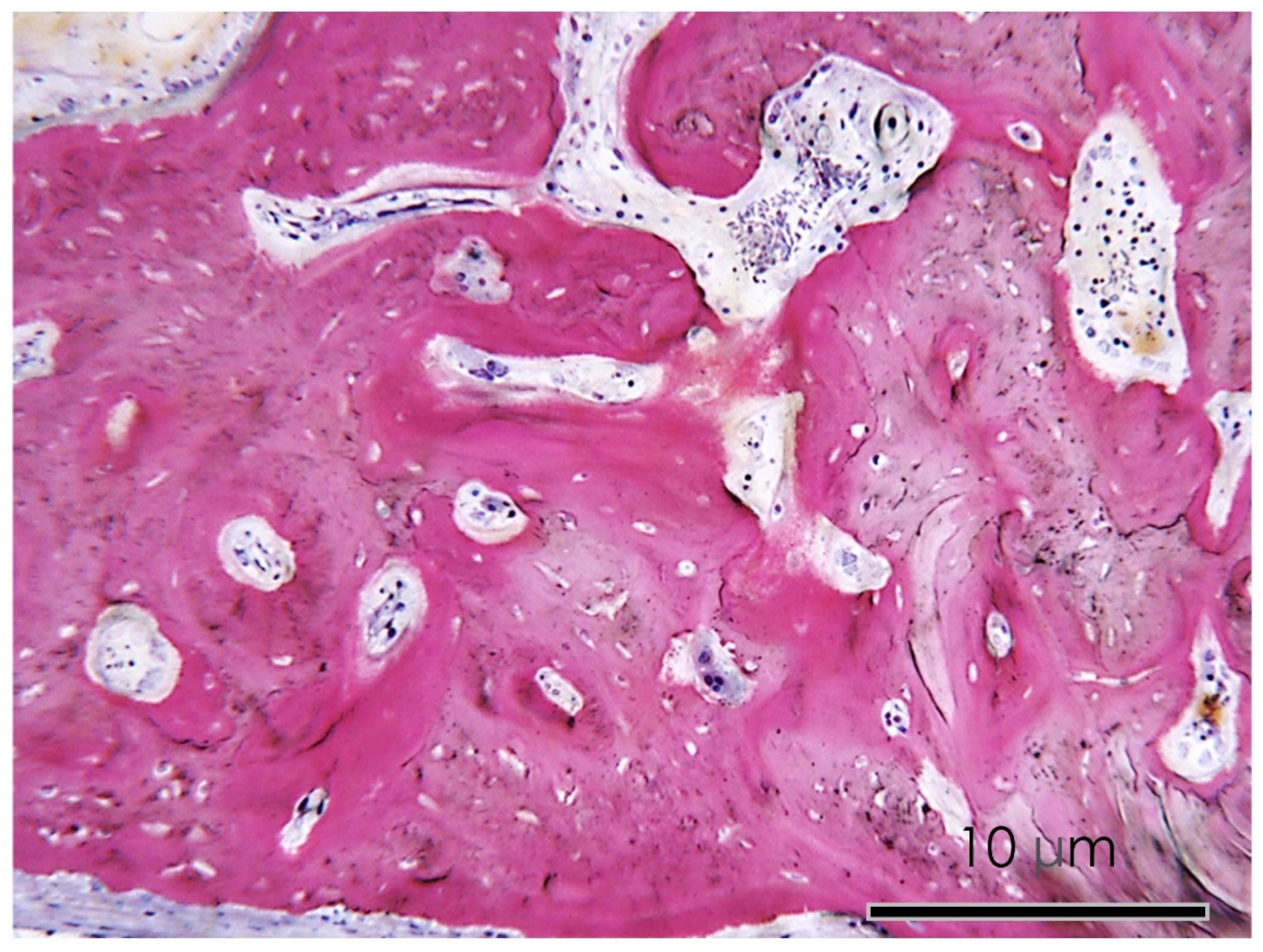
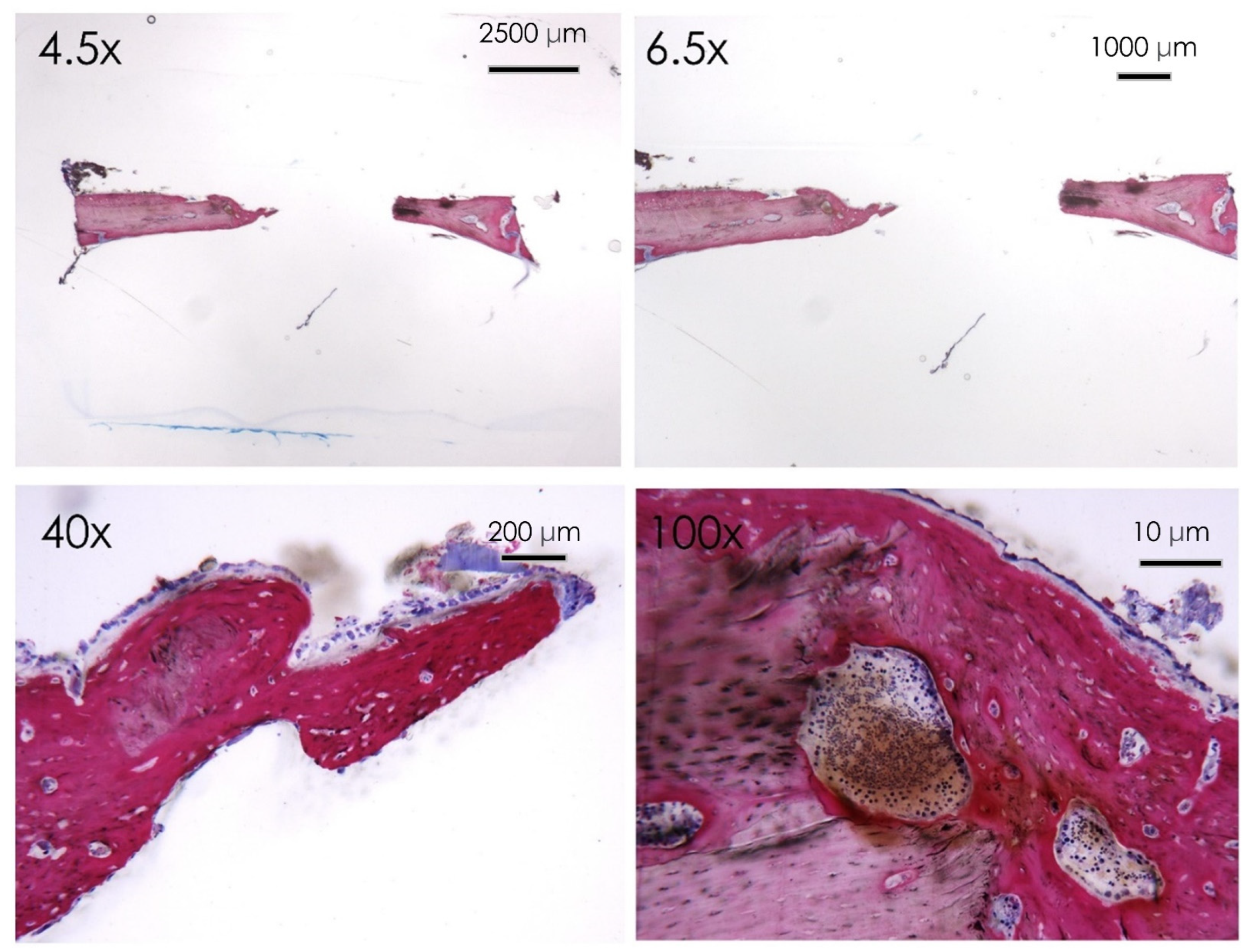
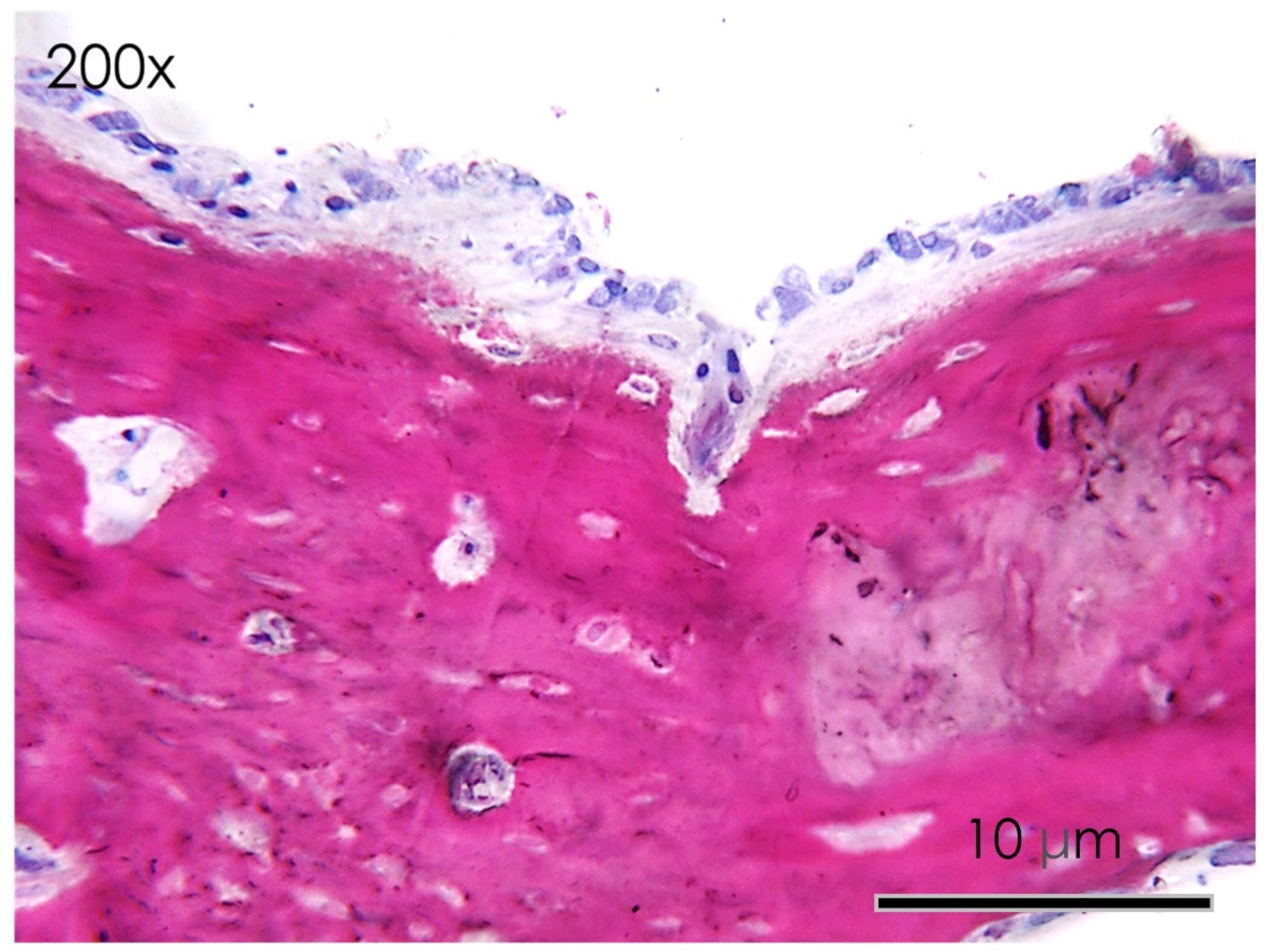
3.1.6. Histological Assay
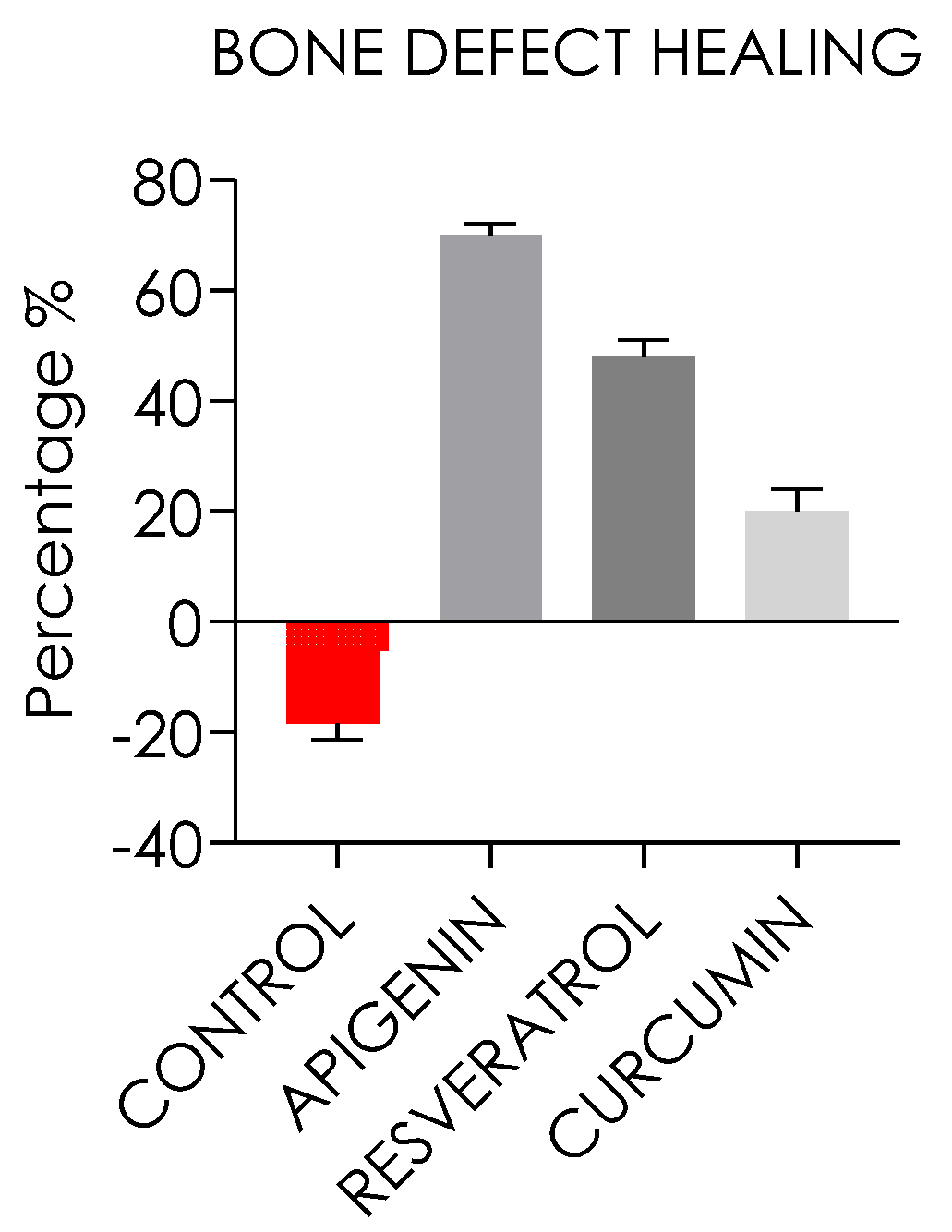
3.1.7. Bone Defect Repair
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gazdag, A.R.; Lane, J.M.; Glaser, D.; Forster, R.A. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J. Am. Acad. Orthop. Surg. 1995, 3, 1–8. [Google Scholar] [CrossRef]
- Nkenke, E.; Neukam, F.W. Autogenous Bone Harvesting and Grafting in Advanced Jaw Resorption: Morbidity, Resorption and Implant Survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. S2), S203–S217. [Google Scholar]
- Scarano, A.; Crincoli, V.; Di Benedetto, A.; Cozzolino, V.; Lorusso, F.; Podaliri Vulpiani, M.; Grano, M.; Kalemaj, Z.; Mori, G.; Grassi, F.R. Bone Regeneration Induced by Bone Porcine Block with Bone Marrow Stromal Stem Cells in a Minipig Model of Mandibular “Critical Size” Defect. Stem Cells Int. 2017, 2017, 9082869. [Google Scholar] [CrossRef]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef]
- Cooper, G.M.; Mooney, M.P.; Gosain, A.K.; Campbell, P.G.; Losee, J.E.; Huard, J. Testing the Critical Size in Calvarial Bone Defects: Revisiting the Concept of a Critical-Size Defect. Plast. Reconstr. Surg. 2010, 125, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Gupta, S.C.; Karelia, D.; Gilhooley, P.J.; Shakibaei, M.; Aggarwal, B.B. Dietary Nutraceuticals as Backbone for Bone Health. Biotechnol. Adv. 2018, 36, 1633–1648. [Google Scholar] [CrossRef]
- Tumedei, M.; Mancinelli, R.; Di Filippo, E.S.; Marrone, M.; Iezzi, G.; Piattelli, A.; Fulle, S. Osteogenic Potential of Human Dental Pulp Stem Cells Co-Cultured with Equine Bone Substitute Combined with Melatonin. Int. J. Periodontics Restor. Dent. 2022, 42. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and Angiogenesis: The Potential for Engineering Bone. Eur. Cell. Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharm. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the Debate for a Regulatory Framework: Nutraceutical Regulatory Framework. Br. J. Clin. Pharm. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining “Nutraceuticals”: Neither Nutritious nor Pharmaceutical. Br. J. Clin. Pharm. 2017, 83, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals in Hypercholesterolaemia: An Overview. Br. J. Pharm. 2017, 174, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Feldt-Rasmussen, U.; Bonofiglio, D.; Asamoah, E. Nutraceutical Supplements in the Thyroid Setting: Health Benefits beyond Basic Nutrition. Nutrients 2019, 11, 2214. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-W.; Chen, K.-M.; Hassanshahi, M.; Tang, Q.; Howe, P.R.; Xian, C.J. Childhood Cancer Chemotherapy-Induced Bone Damage: Pathobiology and Protective Effects of Resveratrol and Other Nutraceuticals. Ann. N. Y. Acad. Sci. 2017, 1403, 109–117. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dånmark, S.; Edlund, U.; Finne-Wistrand, A.; He, X.; Norgård, M.; Blomén, E.; Hultenby, K.; Andersson, G.; Lindgren, U. Resveratrol-Conjugated Poly-ε-Caprolactone Facilitates in Vitro Mineralization and in Vivo Bone Regeneration. Acta Biomater. 2011, 7, 751–758. [Google Scholar] [CrossRef]
- Ornstrup, M.J.; Harsløf, T.; Kjær, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol Increases Bone Mineral Density and Bone Alkaline Phosphatase in Obese Men: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef]
- Xi, H.; Gao, Y.; Yang, F.; Li, W.; Ma, H.; Chen, K. Effect of resveratrol on peak bone mass in growing rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 2017, 46, 578–584. [Google Scholar]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of Resveratrol on Bone Health in Type 2 Diabetic Patients. A Double-Blind Randomized-Controlled Trial. Nutr. Diabetes 2018, 8, 51. [Google Scholar] [CrossRef]
- Pino, D.S.; Casarin, R.C.; Pimentel, S.P.; Cirano, F.R.; Corrêa, M.G.; Ribeiro, F.V. Effect of Resveratrol on Critical-Sized Calvarial Defects of Diabetic Rats: Histometric and Gene Expression Analysis. J. Oral Maxillofac. Surg. 2017, 75, 2561.e1–2561.e10. [Google Scholar] [CrossRef]
- Jung, W.-W. Protective Effect of Apigenin against Oxidative Stress-Induced Damage in Osteoblastic Cells. Int. J. Mol. Med. 2014, 33, 1327–1334. [Google Scholar] [CrossRef]
- Goto, T.; Hagiwara, K.; Shirai, N.; Yoshida, K.; Hagiwara, H. Apigenin Inhibits Osteoblastogenesis and Osteoclastogenesis and Prevents Bone Loss in Ovariectomized Mice. Cytotechnology 2015, 67, 357–365. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, C.; Zha, X.; Xu, Z.; Li, L.; Liu, Y.; Xu, L.; Cui, L.; Xu, D.; Zhu, B. Apigenin Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells through JNK and P38 MAPK Pathways. Mol. Cell. Biochem. 2015, 407, 41–50. [Google Scholar] [CrossRef]
- D’Amico, E.; Pierfelice, T.V.; Iezzi, G.; Di Pietro, N.; Lepore, S.; Lorusso, F.; Scarano, A.; Pandolfi, A.; Piattelli, A.; Petrini, M. Apigenin Promotes Proliferation and Mineralization of Human Osteoblasts and Up-Regulates Osteogenic Markers. Appl. Sci. 2022, 12, 8510. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Z.; Cui, Y.; Shao, Y.; Tao, Y.; Mei, L. Apigenin from Daily Vegetable Celery Can Accelerate Bone Defects Healing. J. Funct. Foods 2019, 54, 412–421. [Google Scholar] [CrossRef]
- Tong, J.; Shen, Y.; Zhang, Z.; Hu, Y.; Zhang, X.; Han, L. Apigenin Inhibits Epithelial-Mesenchymal Transition of Human Colon Cancer Cells through NF-ΚB/Snail Signaling Pathway. Biosci. Rep. 2019, 39, BSR20190452. [Google Scholar] [CrossRef] [PubMed]
- Ganai, S.A. Plant-Derived Flavone Apigenin: The Small-Molecule with Promising Activity against Therapeutically Resistant Prostate Cancer. Biomed. Pharm. 2017, 85, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rohanizadeh, R.; Deng, Y.; Verron, E. Therapeutic Actions of Curcumin in Bone Disorders. BoneKEy Rep. 2016, 5, 793. [Google Scholar] [CrossRef]
- Chang, H.-I.; Su, Y.-H.; Lin, Y.-J.; Chen, P.-J.; Shi, C.-S.; Chen, C.-N.; Yeh, C.-C. Evaluation of the Protective Effects of Curcuminoid (Curcumin and Bisdemethoxycurcumin)-Loaded Liposomes against Bone Turnover in a Cell-Based Model of Osteoarthritis. DDDT 2015, 9, 2285. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Kawata, Y.; Amano, S.; Hanazawa, S. Stimulatory Effect of Curcumin on Osteoclast Apoptosis. Biochem. Pharmacol. 2000, 59, 1577–1581. [Google Scholar] [CrossRef]
- Kunihiro, A.G.; Luis, P.B.; Brickey, J.A.; Frye, J.B.; Chow, H.-H.S.; Schneider, C.; Funk, J.L. Beta-Glucuronidase Catalyzes Deconjugation and Activation of Curcumin-Glucuronide in Bone. J. Nat. Prod. 2019, 82, 500–509. [Google Scholar] [CrossRef]
- Hussan, F.; Ibraheem, N.G.; Kamarudin, T.A.; Shuid, A.N.; Soelaiman, I.N.; Othman, F. Curcumin Protects against Ovariectomy-Induced Bone Changes in Rat Model. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Thiagarajan, R.; Rastrelli, L.; Daglia, M.; Sobarzo-Sánchez, E.; Alinezhad, H.; Nabavi, S.M. Curcumin: A Natural Product for Diabetes and Its Complications. Curr. Top. Med. Chem. 2015, 15, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, G.; Tokuda, H.; Yamamoto, N.; Matsushima-Nishiwaki, R.; Mizutani, J.; Kozawa, O.; Otsuka, T. Resveratrol Amplifies BMP-4-Stimulated Osteoprotegerin Synthesis via P38 MAP Kinase in Osteoblasts. Mol. Med. Rep. 2015, 12, 3849–3854. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-C.; Hou, S.-M.; Chen, R.-J.; Peng, H.-W.; Hsieh, C.-F.; Kuo, M.-L.; Yen, M.-L. Resveratrol Promotes Osteogenesis of Human Mesenchymal Stem Cells by Upregulating RUNX2 Gene Expression via the SIRT1/FOXO3A Axis. J. Bone Min. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; Illescas-Montes, R.; Ramos-Torrecillas, J.; de Luna-Bertos, E.; Ruiz, C.; García-Martínez, O. Bone Protective Effect of Extra-Virgin Olive Oil Phenolic Compounds by Modulating Osteoblast Gene Expression. Nutrients 2019, 11, 1722. [Google Scholar] [CrossRef]
- Pan, F.-F.; Shao, J.; Shi, C.-J.; Li, Z.-P.; Fu, W.-M.; Zhang, J.-F. Apigenin Promotes Osteogenic Differentiation of Mesenchymal Stem Cells and Accelerates Bone Fracture Healing via Activating Wnt/β-Catenin Signaling. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E760–E771. [Google Scholar] [CrossRef]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural Compounds Targeting Major Cell Signaling Pathways: A Novel Paradigm for Osteosarcoma Therapy. J. Hematol. Oncol. 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.Z.; Casarin, R.C.V.; Cirano, F.R.; Tenenbaum, H.T.; Casati, M.Z. Systemic Treatment with Resveratrol and/or Curcumin Reduces the Progression of Experimental Periodontitis in Rats. J. Periodontal Res. 2017, 52, 201–209. [Google Scholar] [CrossRef]
- Folwarczna, J.; Zych, M.; Trzeciak, H.I. Effects of Curcumin on the Skeletal System in Rats. Pharmacol. Rep. 2010, 62, 900–909. [Google Scholar] [CrossRef]
- Vieira, G.; Cavalli, J.; Gonçalves, E.C.D.; Braga, S.F.P.; Ferreira, R.S.; Santos, A.R.S.; Cola, M.; Raposo, N.R.B.; Capasso, R.; Dutra, R.C. Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor. Biomolecules 2020, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Ha, S.K.; Kang, T.H.; Oh, M.S.; Cho, M.H.; Lee, S.Y.; Park, J.-H.; Kim, S.Y. Protective Effect of Apigenin on Ovariectomy-Induced Bone Loss in Rats. Life Sci. 2008, 82, 1217–1223. [Google Scholar] [CrossRef]
- Yi, L.-T.; Li, J.-M.; Li, Y.-C.; Pan, Y.; Xu, Q.; Kong, L.-D. Antidepressant-like Behavioral and Neurochemical Effects of the Citrus-Associated Chemical Apigenin. Life Sci. 2008, 82, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Alkhutari, A.S.; Abotaleb, B.; Altairi, N.H.; Del Fabbro, M. Do Osteoconductive Bone Substitutes Result in Similar Bone Regeneration for Maxillary Sinus Augmentation When Compared to Osteogenic and Osteoinductive Bone Grafts? A Systematic Review and Frequentist Network Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A Narrative Review of the Histological and Histomorphometrical Evaluation of the Peri-Implant Bone in Loaded and Unloaded Dental Implants. A 30-Year Experience (1988–2018). Int. J. Environ. Res. Public Health 2020, 17, 2088. [Google Scholar] [CrossRef]
- Isacco, C.G.; Nguyen, K.C.D.; Ballini, A.; Paduanelli, G.; Pham, V.H.; Aityan, S.K.; Schiffman, M.; Tran, T.C.; Huynh, T.D.; Filgueira, L.; et al. Innovative Scaffold Solution for Bone Regeneration Made of Beta-Tricalcium Phosphate Granules, Autologous Fibrin Fold, and Peripheral Blood Stem Cells. In Regenerative Medicine and Plastic Surgery; Duscher, D., Shiffman, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 167–179. ISBN 978-3-030-19961-6. [Google Scholar]
- Gao, P.; Zhang, H.; Liu, Y.; Fan, B.; Li, X.; Xiao, X.; Lan, P.; Li, M.; Geng, L.; Liu, D.; et al. Beta-Tricalcium Phosphate Granules Improve Osteogenesis in Vitro and Establish Innovative Osteo-Regenerators for Bone Tissue Engineering in Vivo. Sci. Rep. 2016, 6, 23367. [Google Scholar] [CrossRef]
- Yoon, M.-S.; Lee, J.S.; Choi, B.-M.; Jeong, Y.-I.; Lee, C.-M.; Park, J.-H.; Moon, Y.; Sung, S.-C.; Lee, S.K.; Chang, Y.H. Apigenin Inhibits Immunostimulatory Function of Dendritic Cells: Implication of Immunotherapeutic Adjuvant. Mol. Pharmacol. 2006, 70, 1033–1044. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, Y.; Xiao, B.; Ren, G.-F. Effects of Resveratrol on Bone Mineral Density in Ovarectomized Rats. Int. J. Biomed. Sci. IJBS 2005, 1, 76. [Google Scholar]
- Cirano, F.R.; Pimentel, S.P.; Casati, M.Z.; Corrêa, M.G.; Pino, D.S.; Messora, M.R.; Silva, P.H.F.; Ribeiro, F.V. Effect of Curcumin on Bone Tissue in the Diabetic Rat: Repair of Peri-Implant and Critical-Sized Defects. Int. J. Oral Maxillofac. Surg. 2018, 47, 1495–1503. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Le Maitre, C.L.; Cross, N.A.; Jordan-Mahy, N. The Effect of Apigenin and Chemotherapy Combination Treatments on Apoptosis-Related Genes and Proteins in Acute Leukaemia Cell Lines. Sci. Rep. 2022, 12, 8858. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Choi, Y.M.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin Enhances Skeletal Muscle Hypertrophy and Myoblast Differentiation by Regulating Prmt. Oncotarget 2017, 8, 78300. [Google Scholar] [CrossRef] [PubMed]
- Iaculli, F.; Di Filippo, E.S.; Piattelli, A.; Mancinelli, R.; Fulle, S. Dental Pulp Stem Cells Grown on Dental Implant Titanium Surfaces: An in Vitro Evaluation of Differentiation and MicroRNAs Expression. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-B.; Yang, J.-J.; Choi, K.-H.; Bae, J.; Lee, J.-Y.; Kim, S.-E.; Shin, S.-W. Effect of RhBMP-2 Immobilized Anorganic Bovine Bone Matrix on Bone Regeneration. Int. J. Mol. Sci. 2015, 16, 16034–16052. [Google Scholar] [CrossRef] [PubMed]
- Polak, S.J.; Levengood, S.K.L.; Wheeler, M.B.; Maki, A.J.; Clark, S.G.; Johnson, A.J.W. Analysis of the Roles of Microporosity and BMP-2 on Multiple Measures of Bone Regeneration and Healing in Calcium Phosphate Scaffolds. Acta Biomater. 2011, 7, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.M.; Lee, S.-Y.; Long, F. Bmp Induces Osteoblast Differentiation through Both Smad4 and MTORC1 Signaling. Mol. Cell. Biol. 2017, 37, e00253-16. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, A.; Tanaka, T.; Chazono, M.; Komaki, H.; Kitasato, S.; Inagaki, N.; Akiyama, S.; Marumo, K. Effects of Micro-Porosity and Local BMP-2 Administration on Bioresorption of β-TCP and New Bone Formation. Biomater. Res. 2019, 23, 12. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Q.; Shu, Y.; Zeng, Z.; Huang, B.; Feng, Y.; Zhang, B.; Wang, X.; Lei, Y.; Ye, Z.; et al. Transcriptomic Landscape Regulated by the 14 Types of Bone Morphogenetic Proteins (BMPs) in Lineage Commitment and Differentiation of Mesenchymal Stem Cells (MSCs). Genes Dis. 2019, 6, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Innamorati, G.; Valenti, M.T. Transcription Factor Runx2 and Its Application to Bone Tissue Engineering. Stem Cell Rev. Rep. 2012, 8, 891–897. [Google Scholar] [CrossRef]
- Liu, T.M.; Lee, E.H. Transcriptional Regulatory Cascades in Runx2-Dependent Bone Development. Tissue Eng. B Rev. 2013, 19, 254–263. [Google Scholar] [CrossRef]
- Pereira, R.D.S.; Menezes, J.D.; Bonardi, J.P.; Griza, G.L.; Okamoto, R.; Hochuli-Vieira, E. Histomorphometric and Immunohistochemical Assessment of RUNX2 and VEGF of BiogranTM and Autogenous Bone Graft in Human Maxillary Sinus Bone Augmentation: A Prospective and Randomized Study. Clin. Implant Dent. Relat. Res. 2017, 19, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-W.; Wang, T.-H.; Yan, P.-P.; Chu, L.-W.; Yu, J.; Gao, Z.-D.; Li, Y.-Z.; Guo, B.-L. Curcumin Improves Bone Microarchitecture and Enhances Mineral Density in APP/PS1 Transgenic Mice. Phytomedicine 2011, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Attari, F.; Zahmatkesh, M.; Aligholi, H.; Mehr, S.E.; Sharifzadeh, M.; Gorji, A.; Mokhtari, T.; Khaksarian, M.; Hassanzadeh, G. Curcumin as a Double-Edged Sword for Stem Cells: Dose, Time and Cell Type-Specific Responses to Curcumin. DARU J. Pharm. Sci. 2015, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-S.; Lai, C.-H.; Shen, C.-Y.; Pai, P.-Y.; Chen, R.-J.; PadmaViswanadha, V.; Yang, C.-K.; Chen, M.-C.; Lin, Y.-M.; Huang, C.-Y. Orally Administered Resveratrol Enhances the Therapeutic Effect of Autologous Transplanted Adipose-Derived Stem Cells on Rats with Diabetic Hepatopathy. Biotech. Histochem. 2020, 95, 37–45. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, Y.-J.; Jang, J.-H.; Song, H. Positive Modulation of Osteogenesis- and Osteoclastogenesis-Related Gene Expression with Strontium-Containing Microstructured Ti Implants in Rabbit Cancellous Bone. J. Biomed. Mater. Res. A 2013, 101, 298–306. [Google Scholar] [CrossRef]

| Rat Grimace Scale (Mean, SD) | Day 1 | Day 3 | Day 7 | Day 14 | Day 30 |
|---|---|---|---|---|---|
| Control Group | 1.7 ± 0.4 | 1.1 ± 0.5 | 0.6 ± 0.4 | 0.3 ± 0.4 | 0.2 ± 0.4 |
| Resveratrol Group | 1.7 ± 0.5 | 1.2 ± 0.5 | 0.6 ± 0.5 | 0.2 ± 0.4 | 0.2 ± 0.4 |
| Apigenin Group | 1.8 ± 0.4 | 1.1 ± 0.6 | 0.6 ± 0.4 | 0.3 ± 0.5 | 0.2 ± 0.5 |
| Curcumin Group | 1.7 ± 0.4 | 1.2 ± 0.5 | 0.6 ± 0.4 | 0.2 ± 0.5 | 0.2 ± 0.4 |
| p value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, F.; Scarano, A.; Fulle, S.; Valbonetti, L.; Mancinelli, R.; Di Filippo, E.S. Effectiveness of Apigenin, Resveratrol, and Curcumin as Adjuvant Nutraceuticals for Calvarial Bone Defect Healing: An In Vitro and Histological Study on Rats. Nutrients 2023, 15, 1235. https://doi.org/10.3390/nu15051235
Lorusso F, Scarano A, Fulle S, Valbonetti L, Mancinelli R, Di Filippo ES. Effectiveness of Apigenin, Resveratrol, and Curcumin as Adjuvant Nutraceuticals for Calvarial Bone Defect Healing: An In Vitro and Histological Study on Rats. Nutrients. 2023; 15(5):1235. https://doi.org/10.3390/nu15051235
Chicago/Turabian StyleLorusso, Felice, Antonio Scarano, Stefania Fulle, Luca Valbonetti, Rosa Mancinelli, and Ester Sara Di Filippo. 2023. "Effectiveness of Apigenin, Resveratrol, and Curcumin as Adjuvant Nutraceuticals for Calvarial Bone Defect Healing: An In Vitro and Histological Study on Rats" Nutrients 15, no. 5: 1235. https://doi.org/10.3390/nu15051235
APA StyleLorusso, F., Scarano, A., Fulle, S., Valbonetti, L., Mancinelli, R., & Di Filippo, E. S. (2023). Effectiveness of Apigenin, Resveratrol, and Curcumin as Adjuvant Nutraceuticals for Calvarial Bone Defect Healing: An In Vitro and Histological Study on Rats. Nutrients, 15(5), 1235. https://doi.org/10.3390/nu15051235










