Comprehensive Investigation on Associations between Dietary Intake and Blood Levels of Fatty Acids and Colorectal Cancer Risk
Abstract
:1. Introduction
2. Methods
2.1. Literature Search and Selection
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analysis
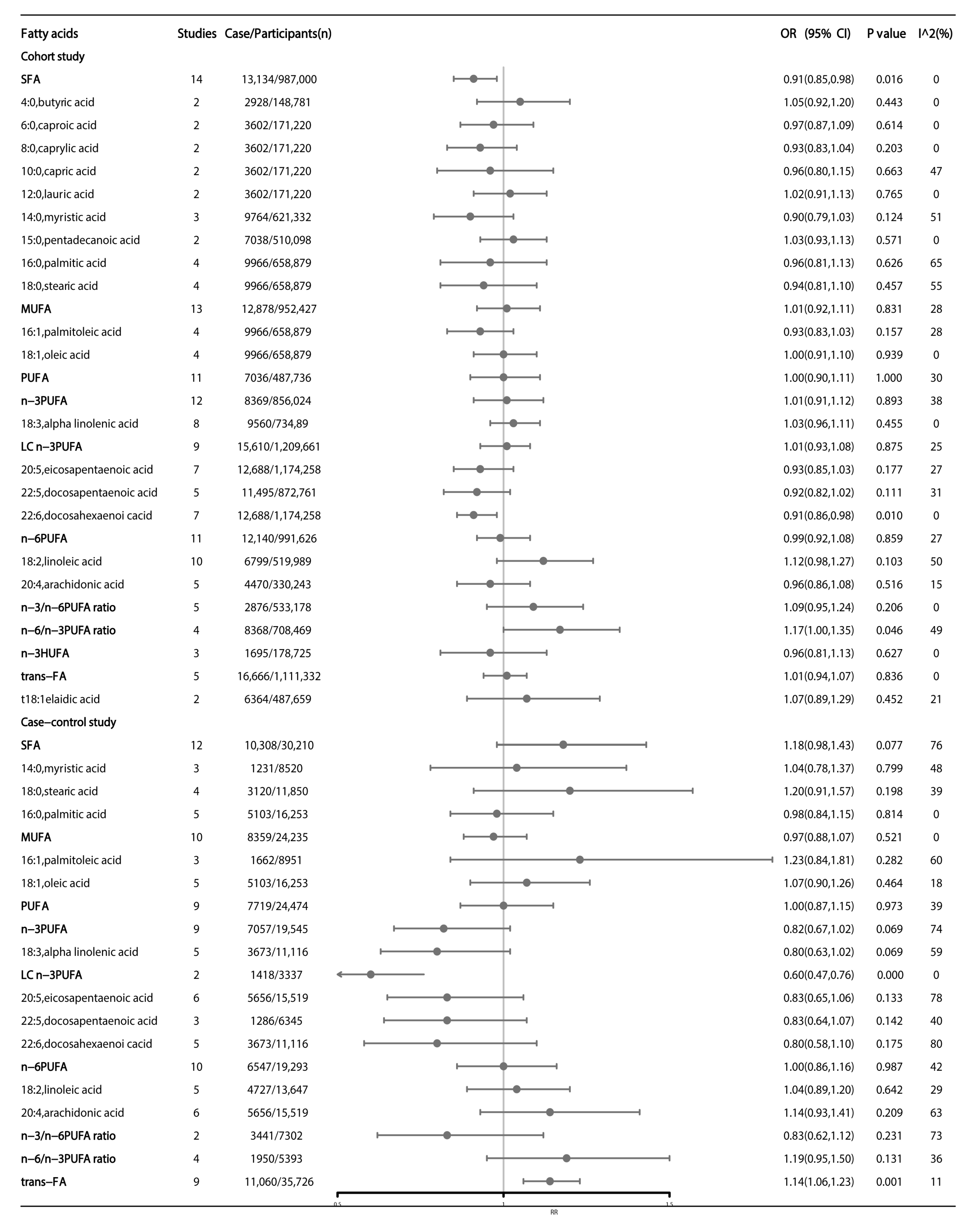
3. Results
3.1. Meta-Analyses of Observational Studies
3.2. Association between Dietary Fatty Acids Intake and Colorectal Cancer
3.2.1. Main Analysis
3.2.2. Subgroup Analysis
3.2.3. Association between Blood Fatty Acids and Colorectal Cancer
3.2.4. Mendelian Randomization Studies
4. Discussion
4.1. N-3 Polyunsaturated Fatty Acids (n-3 PUFA)
4.2. N-6 Polyunsaturated Fatty Acids (n-6 PUFA)
4.3. Saturated Fatty Acids (SFAs)
4.4. Trans Fatty Acids (Trans-FA)
4.5. Conflict and Harmony of Dietary and Blood FAs in Their Associations with CRC
4.6. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The association of diet, gut microbiota and colorectal cancer: What we eat may imply what we get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Baena, R.; Salinas, P. Diet and colorectal cancer. Maturitas 2015, 80, 258–264. [Google Scholar] [CrossRef]
- Nguyen, S.; Li, H.; Yu, D.; Cai, H.; Gao, J.; Gao, Y.; Luu, H.N.; Tran, H.; Xiang, Y.B.; Zheng, W.; et al. Dietary fatty acids and colorectal cancer risk in men: A report from the Shanghai Men’s Health Study and a meta-analysis. Int. J. Cancer 2021, 148, 77–89. [Google Scholar] [CrossRef]
- Aglago, E.K.; Murphy, N.; Huybrechts, I.; Nicolas, G.; Casagrande, C.; Fedirko, V.; Weiderpass, E.; Rothwell, J.A.; Dahm, C.C.; Olsen, A.; et al. Dietary intake and plasma phospholipid concentrations of saturated, monounsaturated and trans fatty acids and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Int. J. Cancer 2021, 149, 856–882. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Fuchs, C.S.; Ogino, S.; Hu, F.B.; Mozaffarian, D.; Ma, J.; Willett, W.C.; Giovannucci, E.L.; Wu, K. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int. J. Cancer 2014, 135, 2413–2423. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Intake or Blood Levels of n-3 Polyunsaturated Fatty Acids and Risk of Colorectal Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2020, 29, 288–299. [Google Scholar] [CrossRef]
- Hodge, A.M.; Williamson, E.J.; Bassett, J.K.; MacInnis, R.J.; Giles, G.G.; English, D.R. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int. J. Cancer 2015, 137, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.H.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am. J. Clin. Nutr. 2000, 71, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Nutritional epidemiology issues in chronic disease at the turn of the century. Epidemiol. Rev. 2000, 22, 82–86. [Google Scholar] [CrossRef]
- Kim, M.; Park, K. Dietary Fat Intake and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2018, 10, 1963. [Google Scholar] [CrossRef]
- Geelen, A.; Schouten, J.M.; Kamphuis, C.; Stam, B.E.; Burema, J.; Renkema, J.M.; Bakker, E.J.; van’t Veer, P.; Kampman, E. Fish consumption, n-3 fatty acids, and colorectal cancer: A meta-analysis of prospective cohort studies. Am. J. Epidemiol. 2007, 166, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Zhou, J.D.; Dong, J.Y.; Ding, W.Q.; Wu, J.C. Dietary intake of n-3 fatty acids and colorectal cancer risk: A meta-analysis of data from 489 000 individuals. Br. J. Nutr. 2012, 108, 1550–1556. [Google Scholar] [CrossRef]
- Chen, G.C.; Qin, L.Q.; Lu, D.B.; Han, T.M.; Zheng, Y.; Xu, G.Z.; Wang, X.H. N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: Meta-analysis of prospective studies. Cancer Causes Control 2015, 26, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Hou, J.; Sun, J.; Guo, N.; Wang, Z. Dietary Intake of N-3 and N-6 Polyunsaturated Fatty Acids and Risk of Cancer: Meta-Analysis of Data from 32 Studies. Nutr. Cancer 2021, 73, 901–913. [Google Scholar] [CrossRef]
- Yang, B.; Wang, F.L.; Ren, X.L.; Li, D. Biospecimen long-chain N-3 PUFA and risk of colorectal cancer: A meta-analysis of data from 60,627 individuals. PLoS ONE 2014, 9, e110574. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. N-6 Polyunsaturated Fatty Acids and Risk of Cancer: Accumulating Evidence from Prospective Studies. Nutrients 2020, 12, 2523. [Google Scholar] [CrossRef]
- Michels, N.; Specht, I.O.; Heitmann, B.L.; Chajes, V.; Huybrechts, I. Dietary trans-fatty acid intake in relation to cancer risk: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Deng, T.; Chen, Y.; Zhao, Z.; Wen, Y.; Chen, Y.; Li, X.; Zeng, G. Association between vasectomy and risk of testicular cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0194606. [Google Scholar] [CrossRef] [PubMed]
- Markozannes, G.; Kanellopoulou, A.; Dimopoulou, O.; Kosmidis, D.; Zhang, X.; Wang, L.; Theodoratou, E.; Gill, D.; Burgess, S.; Tsilidis, K.K. Systematic review of Mendelian randomization studies on risk of cancer. BMC Med. 2022, 20, 41. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- van Enst, W.A.; Ochodo, E.; Scholten, R.J.; Hooft, L.; Leeflang, M.M. Investigation of publication bias in meta-analyses of diagnostic test accuracy: A meta-epidemiological study. BMC Med. Res. Methodol. 2014, 14, 70. [Google Scholar] [CrossRef]
- Siegmann, E.M.; Muller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Gromer, T.W. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.C.; Fournier, A.; et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated With Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. Hepatol. 2020, 18, 654–666.e656. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Serini, S.; Chen, Y.Q.; Su, H.M.; Lim, K.; Cittadini, A.; Calviello, G. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. Biomed. Res. Int. 2015, 2015, 143109. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260.e1216. [Google Scholar] [CrossRef]
- Hall, M.N.; Chavarro, J.E.; Lee, I.M.; Willett, W.C.; Ma, J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1136–1143. [Google Scholar] [CrossRef]
- Daniel, C.R.; McCullough, M.L.; Patel, R.C.; Jacobs, E.J.; Flanders, W.D.; Thun, M.J.; Calle, E.E. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 516–525. [Google Scholar] [CrossRef]
- Howe, P.; Meyer, B.; Record, S.; Baghurst, K. Dietary intake of long-chain omega-3 polyunsaturated fatty acids: Contribution of meat sources. Nutrition 2006, 22, 47–53. [Google Scholar] [CrossRef]
- Stephenson, J.A.; Al-Taan, O.; Arshad, A.; West, A.L.; Calder, P.C.; Morgan, B.; Metcalfe, M.S.; Dennison, A.R. Unsaturated fatty acids differ between hepatic colorectal metastases and liver tissue without tumour in humans: Results from a randomised controlled trial of intravenous eicosapentaenoic and docosahexaenoic acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 405–410. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Reddy, B.S.; Mangat, S.; Sheinfil, A.; Weisburger, J.H.; Wynder, E.L. Effect of type and amount of dietary fat and 1,2-dimethylhydrazine on biliary bile acids, fecal bile acids, and neutral sterols in rats. Cancer Res. 1977, 37, 2132–2137. [Google Scholar]
- Romagnolo, D.F.; Donovan, M.G.; Doetschman, T.C.; Selmin, O.I. n-6 Linoleic Acid Induces Epigenetics Alterations Associated with Colonic Inflammation and Cancer. Nutrients 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albani, V.; Celis-Morales, C.; Marsaux, C.F.; Forster, H.; O’Donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Navas-Carretero, S.; San-Cristobal, R.; et al. Exploring the association of dairy product intake with the fatty acids C15:0 and C17:0 measured from dried blood spots in a multipopulation cohort: Findings from the Food4Me study. Mol. Nutr. Food Res. 2016, 60, 834–845. [Google Scholar] [CrossRef]
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Unger, A.L.; Torres-Gonzalez, M.; Kraft, J. Dairy Fat Consumption and the Risk of Metabolic Syndrome: An Examination of the Saturated Fatty Acids in Dairy. Nutrients 2019, 11, 2200. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef]
- Vinikoor, L.C.; Millikan, R.C.; Satia, J.A.; Schroeder, J.C.; Martin, C.F.; Ibrahim, J.G.; Sandler, R.S. trans-Fatty acid consumption and its association with distal colorectal cancer in the North Carolina Colon Cancer Study II. Cancer Causes Control 2010, 21, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Laake, I.; Carlsen, M.H.; Pedersen, J.I.; Weiderpass, E.; Selmer, R.; Kirkhus, B.; Thune, I.; Veierod, M.B. Intake of trans fatty acids from partially hydrogenated vegetable and fish oils and ruminant fat in relation to cancer risk. Int. J. Cancer 2013, 132, 1389–1403. [Google Scholar] [CrossRef]
- Limburg, P.J.; Liu-Mares, W.; Vierkant, R.A.; Wang, A.H.; Harnack, L.; Flood, A.P.; Sellers, T.A.; Cerhan, J.R. Prospective evaluation of trans-fatty acid intake and colorectal cancer risk in the Iowa Women’s Health Study. Int. J. Cancer 2008, 123, 2717–2719. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, S.M.; Cook, N.R.; Lee, I.M.; Buring, J.E. Dietary fat and fatty acids and risk of colorectal cancer in women. Am. J. Epidemiol. 2004, 160, 1011–1022. [Google Scholar] [CrossRef]
- Baer, D.J.; Judd, J.T.; Clevidence, B.A.; Tracy, R.P. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: A randomized crossover study. Am. J. Clin. Nutr. 2004, 79, 969–973. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- May-Wilson, S.; Sud, A.; Law, P.J.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hanninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Pro-inflammatory fatty acid profile and colorectal cancer risk: A Mendelian randomisation analysis. Eur. J. Cancer 2017, 84, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Isom, C.A.; Shrubsole, M.J.; Cai, Q.; Smalley, W.E.; Ness, R.M.; Zheng, W.; Murff, H.J. Arachidonic acid and colorectal adenoma risk: A Mendelian randomization study. Clin. Epidemiol. 2019, 11, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.K.; Prynne, C.J.; Roberts, C.; Olson, A.; Nicholson, S.K.; Whitton, C.; Teucher, B.; Bates, B.; Henderson, H.; Pigott, S.; et al. National Diet and Nutrition Survey: Fat and fatty acid intake from the first year of the rolling programme and comparison with previous surveys. Br. J. Nutr. 2012, 107, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Woutersen, R.A.; Appel, M.J.; van Garderen-Hoetmer, A.; Wijnands, M.V. Dietary fat and carcinogenesis. Mutat. Res. 1999, 443, 111–127. [Google Scholar] [CrossRef]
- de Antueno, R.J.; Knickle, L.C.; Smith, H.; Elliot, M.L.; Allen, S.J.; Nwaka, S.; Winther, M.D. Activity of human Delta5 and Delta6 desaturases on multiple n-3 and n-6 polyunsaturated fatty acids. FEBS Lett. 2001, 509, 77–80. [Google Scholar] [CrossRef]
- Hansen-Petrik, M.B.; McEntee, M.F.; Johnson, B.T.; Obukowicz, M.G.; Masferrer, J.; Zweifel, B.; Chiu, C.H.; Whelan, J. Selective inhibition of Delta-6 desaturase impedes intestinal tumorigenesis. Cancer Lett. 2002, 175, 157–163. [Google Scholar] [CrossRef]
- Rifkin, S.B.; Shrubsole, M.J.; Cai, Q.; Smalley, W.E.; Ness, R.M.; Swift, L.L.; Zheng, W.; Murff, H.J. PUFA levels in erythrocyte membrane phospholipids are differentially associated with colorectal adenoma risk. Br. J. Nutr. 2017, 117, 1615–1622. [Google Scholar] [CrossRef] [Green Version]



| Fatty Acid | Colon Cancer | Rectum Cancer | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Cases/Total Participants | RR (95% CI) | p Value | I2 (%) | No of Studies | Cases/Total Participants | RR (95% CI) | p Value | I2 (%) | |
| n-3 PUFA | 7 | 4268/310,898 | 0.83 (0.67, 1.02) | 0.077 | 59 | 7 | 1786/314,886 | 1.02 (0.80, 1.29) | 0.873 | 38 |
| ALA | 6 | 2716/315,918 | 0.96 (0.83, 1.11) | 0.597 | 0 | 6 | 2461/396,911 | 1.02 (0.83, 1.27) | 0.824 | 46 |
| EPA | 5 | 7591/734,064 | 0.93 (0.82, 1.06) | 0.264 | 24 | 4 | 2873/729,661 | 0.87 (0.72, 1.04) | 0.133 | 12 |
| DHA | 5 | 6148/789,647 | 0.92 (0.81, 1.04) | 0.174 | 15 | 5 | 3215/789,647 | 0.87 (0.76, 0.99) | 0.041 | 0 |
| DPA | 4 | 5686/672,953 | 0.87 (0.76, 0.99) | 0.040 | 16 | 4 | 2966/672,953 | 0.90 (0.78, 1.03) | 0.132 | 0 |
| n-6 PUFA | 6 | 2253/272,456 | 0.96 (0.83, 1.13) | 0.645 | 0 | 8 | 2976/397,186 | 1.03 (0.82, 1.28) | 0.824 | 43 |
| LA | 7 | 3950/281,344 | 1.15 (1.02, 1.29) | 0.023 | 0 | 6 | 1170/248,618 | 1.19 (0.89, 1.61) | 0.246 | 55 |
| AA | 4 | 2918/185,865 | 0.98 (0.84, 1.14) | 0.799 | 0 | 3 | 607/181,462 | 0.81 (0.63, 1.04) | 0.100 | 0 |
| Total PUFA | 8 | 4660/141,264 | 1.07 (0.97, 1.19) | 0.165 | 10 | 4 | 1060/70,044 | 1.02 (0.86, 1.22) | 0.811 | 23 |
| Total MUFA | 11 | 5407/239,203 | 0.97 (0.87, 1.07) | 0.517 | 12 | 7 | 1728/80,303 | 1.01 (0.88, 1.16) | 0.892 | 0 |
| Oleic acid | 3 | 2951/70,043 | 0.88 (0.76, 1.03) | 0.105 | 4 | - | - | - | - | - |
| Total SFA | 10 | 6371/233,272 | 0.99 (0.89, 1.10) | 0.844 | 7 | 6 | 1605/72,358 | 1.01 (0.87, 1.17) | 0.876 | 3 |
| Palmitic acid | 3 | 2951/68,029 | 0.91 (0.77, 1.07) | 0.242 | 0 | - | - | - | - | - |
| Trans FA | 3 | 3087/9692 | 1.14 (1.02, 1.27) | 0.019 | 10 | - | - | - | - | - |
| Ratio n-6/n-3 | - | - | - | - | - | 2 | 2226/549,403 | 1.25 (1.05, 1.48) | 0.013 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Li, D.; Wang, L.; Zhang, H.; Jiang, F.; Zhang, R.; Xu, L.; Yang, N.; Dai, S.; Xu, X.; et al. Comprehensive Investigation on Associations between Dietary Intake and Blood Levels of Fatty Acids and Colorectal Cancer Risk. Nutrients 2023, 15, 730. https://doi.org/10.3390/nu15030730
Lu Y, Li D, Wang L, Zhang H, Jiang F, Zhang R, Xu L, Yang N, Dai S, Xu X, et al. Comprehensive Investigation on Associations between Dietary Intake and Blood Levels of Fatty Acids and Colorectal Cancer Risk. Nutrients. 2023; 15(3):730. https://doi.org/10.3390/nu15030730
Chicago/Turabian StyleLu, Ying, Doudou Li, Lijuan Wang, Han Zhang, Fangyuan Jiang, Rongqi Zhang, Liying Xu, Nan Yang, Shuhui Dai, Xiaolin Xu, and et al. 2023. "Comprehensive Investigation on Associations between Dietary Intake and Blood Levels of Fatty Acids and Colorectal Cancer Risk" Nutrients 15, no. 3: 730. https://doi.org/10.3390/nu15030730






