Extra Virgin Olive Oil and Cardiovascular Protection in Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Characterization of the Organic EVOO
2.1.1. Olea Extract
2.1.2. HPLC-DAD-MS Analysis of Olea Extract
2.2. In Vitro Study
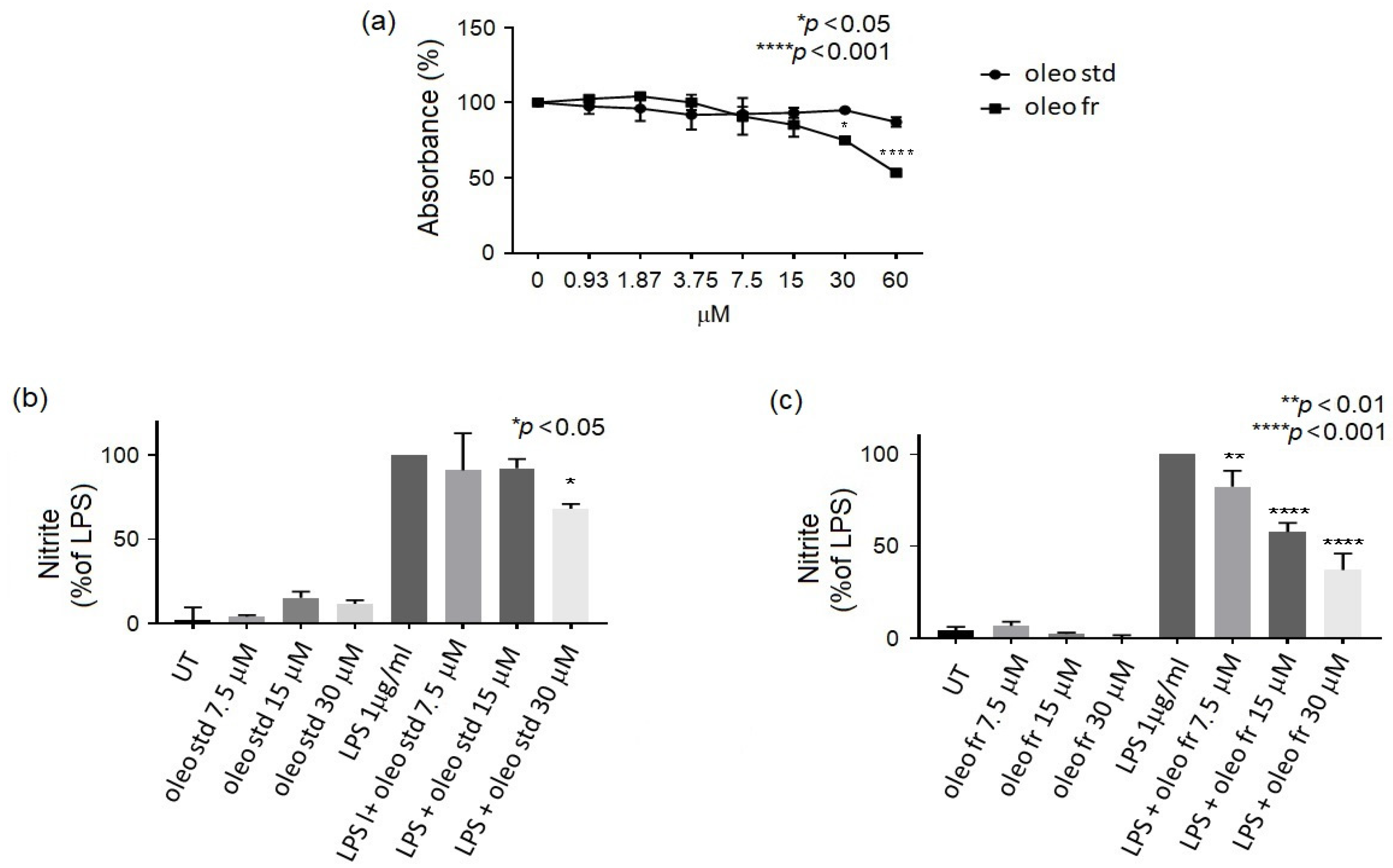
2.2.1. MTT Assay
2.2.2. Nitrite Assay
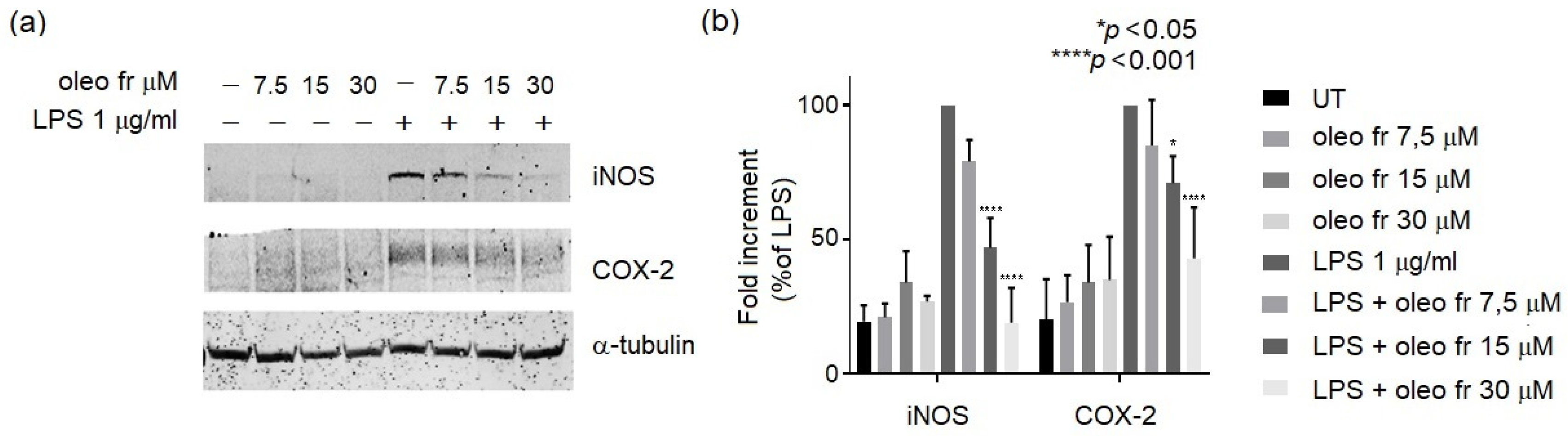
2.2.3. Western Blotting Analysis
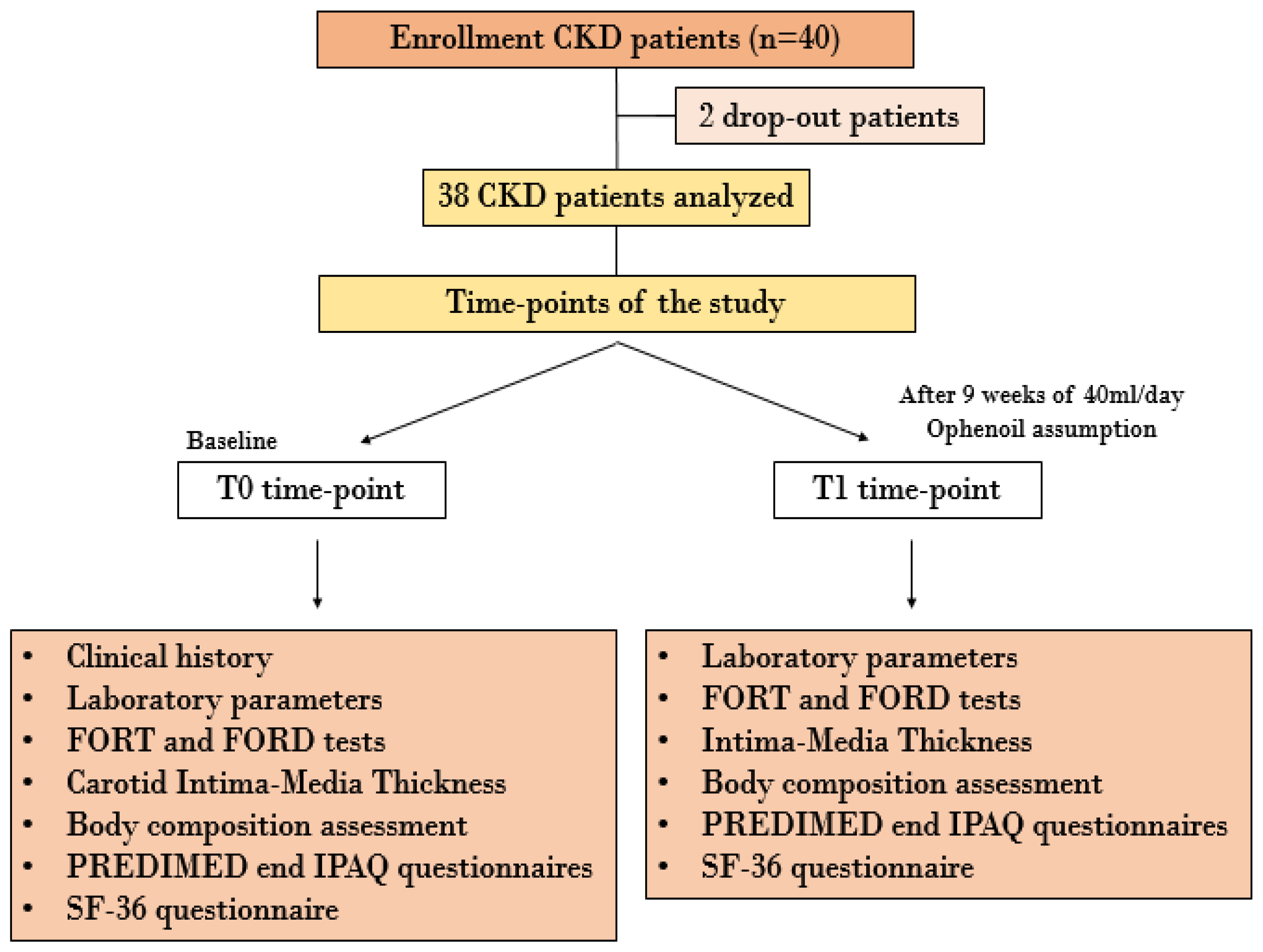
2.3. In Vivo Study
2.3.1. Sample Size
- The null hypothesis was rejected if the mean of the population from which the sample was extracted differed from the sample mean by an amount, expressed in absolute value, equal to or greater than 46.2% of the standard deviation;
- For the hypotheses’ (null and alternative) verification, the one-tailed Gauss z test with α = 0.05 (first type error) and β = 0.10 (second type error) was adopted in order to give a test power equal to 90%.
2.3.2. Enrolled Patients
2.3.3. Blood and Urinary Analysis
2.3.4. Atherogenic Indices
2.3.5. Body Composition Assessment
2.3.6. Doppler Ultrasound of Epiaortic Vessels
2.3.7. Questionnaires
2.3.8. Statistical Analysis
3. Results
3.1. Qualitative and Quantitative Characterization of the Organic EVOO
Oleocanthal Fraction Characterization
3.2. In Vitro Study
3.3. In Vivo Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ADMA | Asymmetric Dimethylarginine |
| AGEs | Advanced Glycation End Products |
| BCM | Body Cellular Mass |
| BCMI | Body Cellular Mass Index |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| BMR | Basal Metabolic Rate |
| CAPS | Carotid Atherosclerosis Progression Study |
| CIMT | Carotid Intima-Media Thickness |
| CKD | Chronic Kidney Disease |
| COX | Cyclooxygenase Enzymes |
| CRP | C-Reactive Protein |
| CV | Cardiovascular |
| DDAH | Enzyme Dimethylarginine Di-Methylaminohydrolase |
| ECW | Extra Cell Water |
| ED | Endothelial Dysfunction |
| EFSA | European Food Safety Authority |
| EPIC | European Prospective Investigation |
| ESR | Erythrocyte Sedimentation Rate |
| EVOO | Extra Virgin Olive Oil |
| FFM | Fat Free Mass |
| FM | Fat Mass |
| FORD | Free Oxygen Radical Defense |
| FORT | Free Oxygen Radical Test |
| SF-36 | Short Form 36 Health Survey |
| GFR | Glomerular Filtration Rate |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HMG-CoA | Hydroxy-methyl-glutaryl-coenzyme A |
| HPLC-DAD-MS | High-Performance Liquid Chromatography Coupled with Diode Array Detector-Mass Spectrometry |
| HT | Hydroxytyrosol |
| ICW | Intra Cell Water |
| IL | Interleukin |
| IPAQ | International Physical Activity Questionnaires |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| LMR | Lymphocyte-To-Monocyte Ratio |
| LPS | Lipopolysaccharide |
| MPCs | Minor Phenolic Compounds |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MUFAs | Monounsaturated Fatty Acids |
| NF-kB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLR | Neutrophil-To-Lymphocyte Ratio |
| NO | Nitric Oxide |
| Oleo fr | Oleocanthal fraction |
| Oleo std | Oleocanthal standard |
| OS | Oxidative Stress |
| PLR | Platelet-To-Lymphocyte Ratio |
| PREDIMED | Prevención Con Dieta Mediterránea |
| PTV | Policlinico Tor Vergata |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| TBW | Total Body Water |
| TC | Total Cholesterol |
| TNF | Tumor Necrosis Factor |
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [Green Version]
- Weiner, D.E.; Tighiouart, H.; Elsayed, E.F.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. The Framingham predictive instrument in chronic kidney disease. J. Am. Coll. Cardiol. 2007, 50, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Reichel, H.; Zee, J.; Tu, C.; Young, E.; Pisoni, R.L.; Stengel, B.; Duttlinger, J.; Lonnemann, G.; Robinson, B.M.; Pecoits-Filho, R.; et al. Chronic kidney disease progression and mortality risk profiles in Germany: Results from the Chronic Kidney Disease Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2020, 35, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noce, A.; Canale, M.P.; Capria, A.; Rovella, V.; Tesauro, M.; Splendiani, G.; Annicchiarico-Petruzzelli, M.; Manzuoli, M.; Simonetti, G.; Di Daniele, N. Coronary artery calcifications predict long term cardiovascular events in non diabetic Caucasian hemodialysis patients. Aging 2015, 7, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, A.; Sobrinho, L.; Ferreira, H.G. The Calcium/Phosphorus Homeostasis in Chronic Kidney Disease: From Clinical Epidemiology to Pathophysiology. Acta Med. Port. 2017, 30, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Pastore, A.; Noce, A.; Di Giovamberardino, G.; De Stefano, A.; Calla, C.; Zenobi, R.; Dessi, M.; Di Daniele, N. Homocysteine, cysteine, folate and vitamin B(1)(2) status in type 2 diabetic patients with chronic kidney disease. J. Nephrol. 2015, 28, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Cianciolo, G.; De Pascalis, A.; Di Lullo, L.; Ronco, C.; Zannini, C.; La Manna, G. Folic Acid and Homocysteine in Chronic Kidney Disease and Cardiovascular Disease Progression: Which Comes First? Cardiorenal. Med. 2017, 7, 255–266. [Google Scholar] [CrossRef]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef] [Green Version]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef]
- Noce, A.; Marchetti, M.; Marrone, G.; Di Renzo, L.; Di Lauro, M.; Di Daniele, F.; Albanese, M.; Di Daniele, N.; De Lorenzo, A. Link between gut microbiota dysbiosis and chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2057–2074. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [Green Version]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [Green Version]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Grazioli, E.; Romani, A.; Marrone, G.; Di Lauro, M.; Cerulli, C.; Urciuoli, S.; Murri, A.; Guerriero, C.; Tranchita, E.; Tesauro, M.; et al. Impact of Physical Activity and Natural Bioactive Compounds on Endothelial Dysfunction in Chronic Kidney Disease. Life 2021, 11, 841. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef] [Green Version]
- Bocedi, A.; Fabrini, R.; Lai, O.; Alfieri, L.; Roncoroni, C.; Noce, A.; Pedersen, J.Z.; Ricci, G. Erythrocyte glutathione transferase: A general probe for chemical contaminations in mammals. Cell Death Discov. 2016, 2, 16029. [Google Scholar] [CrossRef] [Green Version]
- Vila Cuenca, M.; van Bezu, J.; Beelen, R.H.J.; Vervloet, M.G.; Hordijk, P.L. Stabilization of cell-cell junctions by active vitamin D ameliorates uraemia-induced loss of human endothelial barrier function. Nephrol. Dial. Transplant. 2019, 34, 252–264. [Google Scholar] [CrossRef]
- Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Zingaretti, V.; Limongi, D.; D’Agostini, C.; Sorge, R.; Casasco, M.; Di Daniele, N.; et al. Hemodialysis biomarkers: Total advanced glycation end products (AGEs) against oxidized human serum albumin (HSAox). Acta Diabetol. 2019, 56, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Saglam, M.; Caglar, K.; Cakir, E.; Sonmez, A.; Ozgurtas, T.; Aydin, A.; Eyileten, T.; Ozcan, O.; Acikel, C.; et al. The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am. J. Kidney Dis. 2006, 47, 42–50. [Google Scholar] [CrossRef]
- Ando, R.; Ueda, S.; Yamagishi, S.; Miyazaki, H.; Kaida, Y.; Kaifu, K.; Yokoro, M.; Nakayama, Y.; Obara, N.; Fukami, K.; et al. Involvement of advanced glycation end product-induced asymmetric dimethylarginine generation in endothelial dysfunction. Diab. Vasc. Dis. Res. 2013, 10, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Goligorsky, M.S. Pathogenesis of endothelial cell dysfunction in chronic kidney disease: A retrospective and what the future may hold. Kidney Res. Clin. Pract. 2015, 34, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Buckland, G.; Mayen, A.L.; Agudo, A.; Travier, N.; Navarro, C.; Huerta, J.M.; Chirlaque, M.D.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; et al. Olive oil intake and mortality within the Spanish population (EPIC-Spain). Am. J. Clin. Nutr. 2012, 96, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Cicero, A.F.; Nascetti, S.; Lopez-Sabater, M.C.; Elosua, R.; Salonen, J.T.; Nyyssonen, K.; Poulsen, H.E.; Zunft, H.J.; Kiesewetter, H.; de la Torre, K.; et al. Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: The EUROLIVE study. J. Am. Coll. Nutr. 2008, 27, 314–320. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols from Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef]
- Senesi, R.; Andreani, C.; Baglioni, P.; Batista de Carvalho, L.A.E.; Licoccia, S.; Marques, M.P.M.; Moretti, G.; Noce, A.; Paolesse, R.; Parker, S.F.; et al. Looking for Minor Phenolic Compounds in Extra Virgin Olive Oils Using Neutron and Raman Spectroscopies. Antioxidants 2021, 10, 643. [Google Scholar] [CrossRef]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Chioccioli, S.; Monaco, N.; Peppicelli, S.; Andreucci, E.; Urciuoli, S.; Romani, A.; Luceri, C.; Tortora, K.; Calorini, L.; et al. Oleuropein-Rich Leaf Extract as a Broad Inhibitor of Tumour and Macrophage iNOS in an Apc Mutant Rat Model. Antioxidants 2021, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Bianchini, F.; Andreucci, E.; Urciuoli, S.; Romani, A.; Tortora, K.; Caderni, G.; Nediani, C.; Calorini, L. Cancer Glycolytic Dependence as a New Target of Olive Leaf Extract. Cancers 2020, 12, 317. [Google Scholar] [CrossRef] [Green Version]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; la Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea. europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [Green Version]
- Holden, R.M.; Mustafa, R.A.; Alexander, R.T.; Battistella, M.; Bevilacqua, M.U.; Knoll, G.; Mac-Way, F.; Reslerova, M.; Wald, R.; Acott, P.D.; et al. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Anaya, J.D.P.; Castaneda-Saucedo, M.C.; Olalla-Herrera, M.; Villalon-Mir, M.; Serrana, H.L.; Samaniego-Sanchez, C. Changes in the Antioxidant Properties of Extra Virgin Olive Oil after Cooking Typical Mediterranean Vegetables. Antioxidants 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Gaman, M.A.; Epingeac, M.E.; Diaconu, C.C.; Gaman, A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World. J. Diabetes 2020, 11, 193–201. [Google Scholar] [CrossRef]
- Millan, J.; Pinto, X.; Munoz, A.; Zuniga, M.; Rubies-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernandez-Mijares, A.; Gonzalez-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Bellizzi, V.; Scalfi, L.; Terracciano, V.; De Nicola, L.; Minutolo, R.; Marra, M.; Guida, B.; Cianciaruso, B.; Conte, G.; Di Iorio, B.R. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Wanner, M.; Probst-Hensch, N.; Kriemler, S.; Meier, F.; Autenrieth, C.; Martin, B.W. Validation of the long international physical activity questionnaire: Influence of age and language region. Prev. Med. Rep. 2016, 3, 250–256. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2033 (accessed on 5 August 2020).
- EFSA. Panel on Dietetic Products. EFSA J. 2011, 9, 2033–2058. [Google Scholar]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Smith, A.B., 3rd; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1alpha and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Carnevale, R.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Monticolo, R.; D’Amico, A.; Stefanini, L.; Pagano, F.; Pastori, D.; Cangemi, R.; et al. Antioxidant activity from extra virgin olive oil via inhibition of hydrogen peroxide-mediated NADPH-oxidase 2 activation. Nutrition 2018, 55–56, 36–40. [Google Scholar] [CrossRef]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Verhoeven, V.; Van der Auwera, A.; Van Gaal, L.; Remmen, R.; Apers, S.; Stalpaert, M.; Wens, J.; Hermans, N. Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome?: A double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 2015, 15, 52. [Google Scholar] [CrossRef] [Green Version]
- Hermans, M.P.; Lempereur, P.; Salembier, J.P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts in the Clinical Management of Hypertension and Metabolic Syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Kannel, W.B.; Wilson, P.W. Efficacy of lipid profiles in prediction of coronary disease. Am. Heart J. 1992, 124, 768–774. [Google Scholar] [CrossRef]
- Nam, B.H.; Kannel, W.B.; D’Agostino, R.B. Search for an optimal atherogenic lipid risk profile: From the Framingham Study. Am. J. Cardiol. 2006, 97, 372–375. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, E.A.; Rifai, N.; Buring, J.; Manson, J.E.; Ridker, P.M. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1668–1673. [Google Scholar] [CrossRef] [Green Version]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Tousoulis, D.; Antoniades, C.; Stefanadis, C. Assessing inflammatory status in cardiovascular disease. Heart 2007, 93, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Nezu, T.; Hosomi, N.; Aoki, S.; Matsumoto, M. Carotid Intima-Media Thickness for Atherosclerosis. J. Atheroscler. Thromb. 2016, 23, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Afari, M.E.; Bhat, T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: An update. Expert Rev. Cardiovasc. Ther. 2016, 14, 573–577. [Google Scholar] [CrossRef]
- Kurtul, A.; Ornek, E. Platelet to Lymphocyte Ratio in Cardiovascular Diseases: A Systematic Review. Angiology 2019, 70, 802–818. [Google Scholar] [CrossRef]
- Malm, C.J.; Hansson, E.C.; Akesson, J.; Andersson, M.; Hesse, C.; Shams Hakimi, C.; Jeppsson, A. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: A prospective observational study. Br. J. Anaesth. 2016, 117, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Li, D.; Xu, Y.; Wang, H.; Gao, J.; Zhang, Z.; Chen, K. Prognostic significance of metastatic lymph node ratio in patients with gastric cancer after curative gastrectomy: A single-center retrospective study. Scand. J. Gastroenterol. 2022, 57, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Ma, Y.; Han, S.; Zhou, L. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: A systematic review and meta-analysis. Kardiol. Pol. 2017, 75, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Gao, X.; Xu, F.; Shang, Z.; Li, S.; Chen, W.; Yang, J.; Li, J. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine 2018, 97, e12813. [Google Scholar] [CrossRef]
- Touboul, P.J.; Labreuche, J.; Bruckert, E.; Schargrodsky, H.; Prati, P.; Tosetto, A.; Hernandez-Hernandez, R.; Woo, K.S.; Silva, H.; Vicaut, E.; et al. HDL-C, triglycerides and carotid IMT: A meta-analysis of 21,000 patients with automated edge detection IMT measurement. Atherosclerosis 2014, 232, 65–71. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart. J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef] [Green Version]
- Perez Banasco, V.; Gil-Cunquero, J.M.; Borrego, F.J.; Grasso, M.; Segura, P.; Warletta, F.; Lozano, J.L.; Costa, L.A.; Torres, J.; Gaforio, J.J.; et al. Preliminary study on efficacy and tolerance of a “coupage” of olive oil in patients with chronic kidney disease. Nutritonal evaluation. Nefrologia 2007, 27, 472–481. [Google Scholar]
- Rondanelli, M.; Talluri, J.; Peroni, G.; Donelli, C.; Guerriero, F.; Ferrini, K.; Riggi, E.; Sauta, E.; Perna, S.; Guido, D. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a useful prognostic factor to describe nutritional, inflammation and muscle mass status in hospitalized elderly?: Body Cell Mass Index links in elderly. Clin. Nutr. 2018, 37, 934–939. [Google Scholar] [CrossRef]

| Compounds | EVOO mg/L |
|---|---|
| Hydroxytyrosol | 3.10 ± 0.09 |
| Tyrosol | 1.02 ± 0.03 |
| Elenolic Acid Derivatives | 9.31 ± 0.28 |
| Elenolic acid | 150.06 ± 4.50 |
| Oleacin (10-hydroxy-oleocanthal) | 315.46 ± 9.46 |
| Oleocanthal | 197.84 ± 5.04 |
| Secoiridoid derivatives | 96.43 ± 2.89 |
| Lignans | 208.17 ± 6.25 |
| Oleuropein aglycone | 164.58 ± 4.94 |
| Total MPCs | 1145.97 ± 34.38 |
| MPCs excluding elenolic acid and derivatives | 986.60 ± 29.60 |
| EVOO | |
|---|---|
| Acidity (% acid oleic) | 0.17 |
| Peroxides (meqO2/kg) | 4.98 |
| Polyphenols (mg tyrosol/kg) | 890 |
| Olea Extract | mg/g |
|---|---|
| Hydroxytyrosol | 7.93 ± 0.24 |
| Tyrosol | 7.12 ± 0.22 |
| Elenolic acid | 89.19 ± 2.68 |
| Oleacin (10-hydroxy-oleocanthal) | 411.73 ± 12.35 |
| Oleocanthal | 331.73 ± 9.95 |
| Oleuropein aglycone | 75.87 ± 2.27 |
| Ligstroside | 4.93 ± 0.15 |
| Secoiridoid derivatives | 59.03 ± 1.77 |
| Total | 987.53 ± 29.63 |
| N° | 40 |
|---|---|
| Age (years) | 70.7 ± 10.6 |
| Sex (M/F) | 24/16 |
| BMI (kg/m2) | 27.6 ± 5.2 |
| T0 | T1 | p-Value | |
|---|---|---|---|
| Creatinine (mg/dL) | 2.17 ± 0.17 | 2.08 ± 0.16 | 0.013 |
| e-GFR (mL/min/1.73 m2) | 33.73 ± 2.81 | 35.35 ± 2.96 | 0.040 |
| Albuminuria (mg/dL) | 223.43 ± 60.95 | 120.88 ± 32.35 | 0.027 |
| Albumin (g/dL) | 4.22 ± 0.26 | 4.38 ± 0.31 | 0.001 |
| Azotaemia (mg/dL) | 79.03 ± 5.86 | 69.82 ± 4.69 | 0.017 |
| Sodium (mEq/L) | 141.24 ± 2.24 | 136.62 ± 2.34 | n.s. |
| Potassium (mEq/L) | 4.59 ± 0.64 | 4.69 ± 0.37 | n.s. |
| Calcium (mg/dL) | 9.51 ± 0.53 | 9.46 ± 0.50 | n.s. |
| Phosphorus (mg/dL) | 3.66 ± 0.63 | 3.67 ± 0.61 | n.s. |
| TC (mg/dL) | 176.20 ± 40.51 | 175.91 ± 38.64 | n.s. |
| HDL-C (mg/dL) | 45.26 ± 1.81 | 49.02 ± 2.29 | 0.004 |
| LDL-C (mg/dL) | 105.74 ± 37.22 | 103.71 ± 32.33 | n.s. |
| Triglycerides (mg/dL) | 124.45 ± 9.71 | 111.03 ± 8.27 | 0.002 |
| PTHI (mg/dL) | 90.15 ± 28.80 | 75.63 ± 11.77 | n.s. |
| Glycaemia (mg/dL) | 95.39 ± 27.01 | 100.97 ± 30.17 | n.s. |
| Uric acid (mg/dL) | 6.34 ± 0.31 | 5.68 ± 0.29 | 0.039 |
| T0 | T1 | p-Value | |
|---|---|---|---|
| FORT (U) | 397.60 ± 27.74 | 303.17 ± 25.65 | 0.013 |
| FORD (mmol/L Trolox) | 1.37 ± 0.49 | 1.51 ± 0.50 | n.s. |
| CRP (mg/L) | 5.39 ± 1.22 | 3.13 ± 0.82 | 0.046 |
| ESR (mm/h) | 46.43 ± 4.70 | 39.22 ± 3.96 | 0.0006 |
| TNF-α (pg/mL) | 89.70 ± 66.53 | 36.65 ± 32.83 | 0.0001 |
| IL-6 (pg/mL) | 71.22 ± 23.81 | 10.13 ± 2.50 | 0.019 |
| T0 | T1 | p-Value | |
|---|---|---|---|
| TC/HDL-C | 3.90 (2.62–6.58) | 3.42 (2.31–6.57) | 0.0171 |
| LDL-C/HDL-C | 2.27 (1.20–5.47) | 1.91 (1.20–4.15) | 0.0098 |
| log(triglycerides/HDL-C) | 0.05 (0.03–0.11) | 0.04 (0.02–0.09) | 0.0067 |
| T0 | T1 | p-Value | |
|---|---|---|---|
| Platelet-to-lymphocyte ratio | 135.09 (51.08–337.84) a | 116.01 (55.08–227.00) a | 0.0003 |
| Neutrophil-to-lymphocyte ratio | 2.22 (0.75–7.00) a | 2.14 (0.86–7.45) a | n.s. |
| Lymphocyte-to-monocyte ratio | 3.75 (0.27–7.74) a | 3.50 (0.15–8.21) a | 0.0406 |
| Lymphocytes (n/mm3) | 2.03 ± 0.70 b | 1.78 ± 0.65 b | 0.0007 |
| T0 | T1 | p-Value | |
|---|---|---|---|
| Carotid Intima-Media Thickness (mm) | 1.10 ± 0.44 | 1.02 ± 0.35 | 0.017 |
| T0 | T1 | p-Value | |
|---|---|---|---|
| Weight (kg) | 74.1 ± 13.8 | 73.7 ± 13.7 | n.s. |
| BMI (kg/m2) | 27.76 ± 5.27 | 28.62 ± 4.50 | n.s. |
| Resistance (ohm) | 496.0 ± 80.3 | 493.6 ± 104.8 | n.s. |
| Reactance (ohm) | 40.78 ± 1.71 | 43.63 ± 1.80 | 0.007 |
| Phase angle (°) | 4.80 ± 0.86 | 4.90 ± 1.01 | n.s. |
| TBW (%) | 52.6 ± 9.1 | 72.4 ± 10.6 | n.s. |
| ICW (%) | 47.2 ± 5.3 | 49.1 ± 8.5 | n.s. |
| ECW (%) | 52.8 ± 5.3 | 50.9 ± 8.5 | n.s. |
| FM (%) | 28.6 ± 9.7 | 29.4 ± 10.6 | n.s. |
| FFM (%) | 69.6 ± 11.6 | 70.7 ± 9.4 | n.s. |
| BCM (%) | 44.6 ± 9.2 | 47.0 ± 6.4 | 0.025 |
| BCMI | 9.0 ± 1.7 | 9.1 ± 1.8 | n.s. |
| BMR (Kcal) | 1452.3 ± 154.7 | 1453.3 ± 157–5 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, G.; Urciuoli, S.; Di Lauro, M.; Ruzzolini, J.; Ieri, F.; Vignolini, P.; Di Daniele, F.; Guerriero, C.; Nediani, C.; Di Daniele, N.; et al. Extra Virgin Olive Oil and Cardiovascular Protection in Chronic Kidney Disease. Nutrients 2022, 14, 4265. https://doi.org/10.3390/nu14204265
Marrone G, Urciuoli S, Di Lauro M, Ruzzolini J, Ieri F, Vignolini P, Di Daniele F, Guerriero C, Nediani C, Di Daniele N, et al. Extra Virgin Olive Oil and Cardiovascular Protection in Chronic Kidney Disease. Nutrients. 2022; 14(20):4265. https://doi.org/10.3390/nu14204265
Chicago/Turabian StyleMarrone, Giulia, Silvia Urciuoli, Manuela Di Lauro, Jessica Ruzzolini, Francesca Ieri, Pamela Vignolini, Francesca Di Daniele, Cristina Guerriero, Chiara Nediani, Nicola Di Daniele, and et al. 2022. "Extra Virgin Olive Oil and Cardiovascular Protection in Chronic Kidney Disease" Nutrients 14, no. 20: 4265. https://doi.org/10.3390/nu14204265





