Adherence to Mediterranean Diet and Soluble Klotho Level: The Value of Food Synergy in Aging
Abstract
1. Introduction
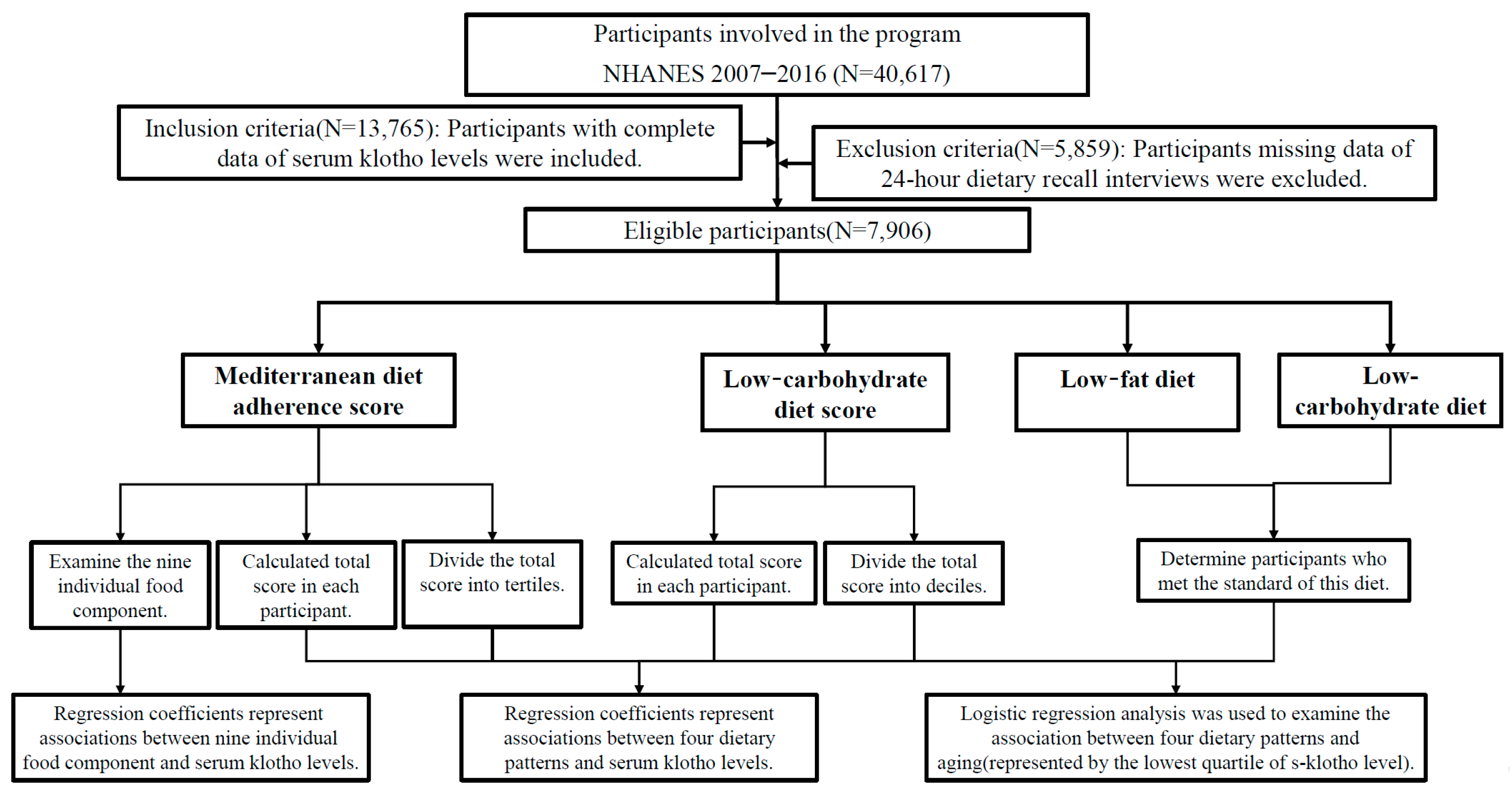
2. Materials and Methods
2.1. Study Design and Participants
2.2. Dietary Data
2.3. Mediterranean Diet Adherence Score
2.4. Low-Carbohydrate-Diet Score
2.5. Low-Fat Diet and Low-Carbohydrate Diet
2.6. Measurement of s-Klotho Levels
2.7. Covariates
2.8. Statistical Analyses
3. Results
3.1. Characteristics of Participants
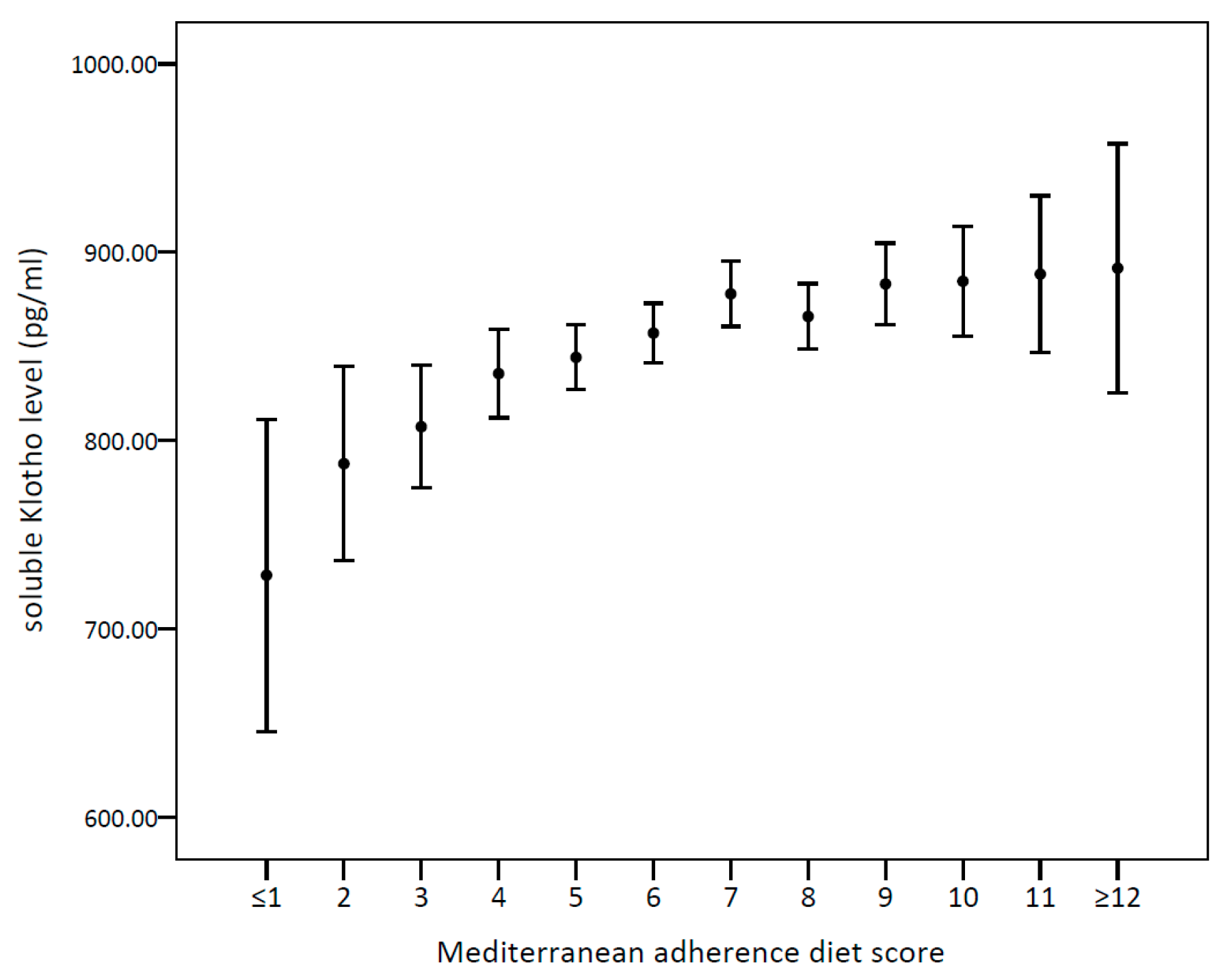
3.2. Associations between Different Dietary Patterns and s-Klotho Levels
3.3. Association between Different Dietary Patterns and Aging
3.4. Associations across Tertiles of MDS and s-Klotho Levels in Subgroups
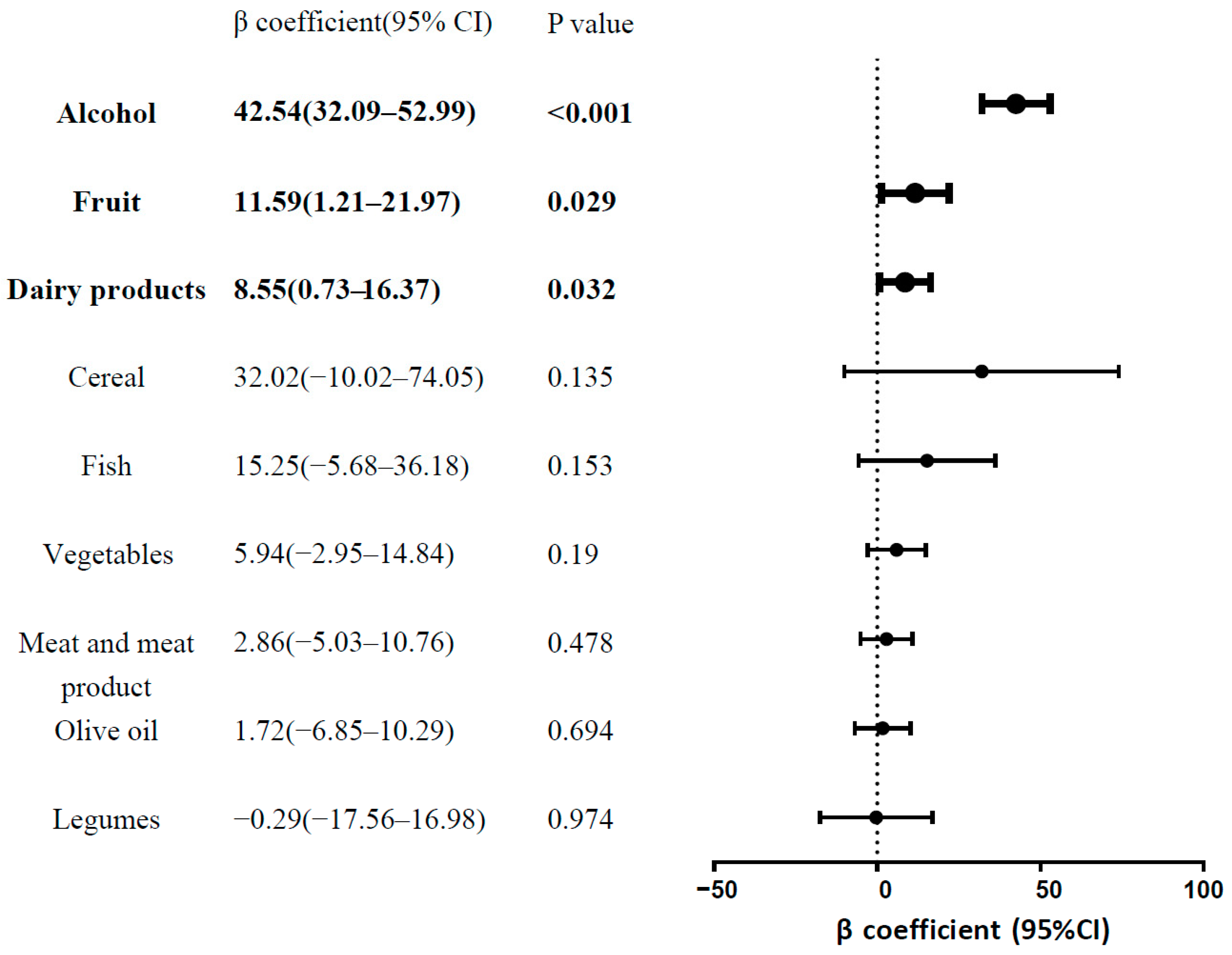
3.5. Associations between Nine Individual Food Components of MDS and s-Klotho Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Mediterranean Adherence Diet Score (MDS) | Low-Carbohydrate-Diet Score (LCD) | Low-Fat Diet | Low-Carbohydrate Diet | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Definition | A score ranging from 0–18 by adding up nine components. | A score ranging from 0–30 by adding up individual scores of three macronutrients, carbohydrate, protein, and fat. | <30% of total daily calorie intake from fat | <20% of total daily calorie intake from carbohydrate | |||||
| Each component is assigned a score of “0”, “1”, or “2” according to the amount of intake. | Each macronutrient was calculated as a percentage (%) of total daily energy intake, and further divided into 11 strata. | ||||||||
| Calculation method | Scores | 0 | 1 | 2 | Scores | Carbohydrate | Protein/Fat | ||
| Fruit | <1 CE/d | ≥1 CE/d | ≥2 CE/d | 0 | 11th stratum | 1st stratum | |||
| Vegetables | <0.5 CE/d | ≥0.5 CE/d | ≥1 CE/d | 1 | 10th stratum | 2nd stratum | |||
| 1 portion = 70 g = 2.5 oz | 2 | 9th stratum | 3rd stratum | ||||||
| Cereals | <4.6 oz/d | 4.6–6.9 oz/d | >6.9 oz/d | 3 | 8th stratum | 4th stratum | |||
| 1 portion = 130 g = 4.6 oz | 4 | 7th stratum | 5th stratum | ||||||
| Fish | <3.5 oz/wk | 3.5–8.75 oz/wk | >8.75 oz/wk | 5 | 6th stratum | 6th stratum | |||
| 1 portion = 100 g = 3.5 oz | 6 | 5th stratum | 7th stratum | ||||||
| Meat product | >4.2 oz/d | 2.8–4.2 oz/d | <2.8 oz/d | 7 | 4th stratum | 8th stratum | |||
| 1 portion = 80 g = 2.8 oz | 8 | 3rd stratum | 9th stratum | ||||||
| Dairy products | >1.5 CE/d | 1–1.5 CE/d | <1 CE/d | 9 | 2nd stratum | 10th stratum | |||
| Alcohol (mL/day) | >2 AU/d | <1 AU/d | 1–2 AU/d | 10 | 1st stratum | 11th stratum | |||
| 1 AU = 12 g | |||||||||
| Olive oil (gm) | <14 g/d | ≥14 g/d | ≥28 g/d | ||||||
| CE = cup equivalent intakes; d = day; wk = week; gm = gram; mL = milliliter; oz = ounce | |||||||||
| References | Previous literature by Sofi et al. [23] | Previous literature by Halton et al. [25] | American Heart Association Dietary Guidelines [5] | American Academy of Family Physicians [26] | |||||
References
- Capurso, C.; Bellanti, F.; Lo Buglio, A.; Vendemiale, G. The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review. Nutrients 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Qin, L.Q.; Dong, J.Y. Mediterranean Diet and Mortality in People with Cardiovascular Disease: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 2623. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Tessarolo Silva, F.; Amaral Medeiros, S.; Mekary, R.A.; Radenkovic, D. The Effect of Low-Fat and Low-Carbohydrate Diets on Weight Loss and Lipid Levels: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3774. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Guo, Y.; Hu, F.B.; Liu, L.; Qi, Q. Association of Low-Carbohydrate and Low-Fat Diets With Mortality Among US Adults. JAMA Intern. Med. 2020, 180, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.M.; Zhang, Y.M.; Zheng, Q.; Gu, J. Klotho is a serum factor related to human aging. Chin. Med. J. 2004, 117, 742–747. [Google Scholar]
- Fernández-Fernández, B.; Valiño-Rivas, L.; Sánchez-Niño, M.D.; Ortiz, A. Albuminuria Downregulation of the Anti-Aging Factor Klotho: The Missing Link Potentially Explaining the Association of Pathological Albuminuria with Premature Death. Adv. Ther. 2020, 37, 62–72. [Google Scholar] [CrossRef]
- Olejnik, A.; Franczak, A.; Krzywonos-Zawadzka, A.; Kałużna-Oleksy, M.; Bil-Lula, I. The Biological Role of Klotho Protein in the Development of Cardiovascular Diseases. BioMed Res. Int. 2018, 2018, 5171945. [Google Scholar] [CrossRef]
- Saar-Kovrov, V.; Donners, M.; van der Vorst, E.P.C. Shedding of Klotho: Functional Implications in Chronic Kidney Disease and Associated Vascular Disease. Front. Cardiovasc. Med. 2020, 7, 617842. [Google Scholar] [CrossRef]
- Daneshgar, N.; Dai, D.F. ROS, Klotho and mTOR in cardiorenal aging. Aging 2020, 12, 19830–19831. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la, O.A.; Gutiérrez, Á.; Castillo, M.J. Alcohol consumption and S-Klotho plasma levels in sedentary healthy middle-aged adults: A cross sectional study. Drug Alcohol Depend. 2019, 194, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Fasoli, L.; Castillo, M.J.; Amaro-Gahete, F.J. Dietary Inflammatory Index and S-Klotho Plasma Levels in Middle-Aged Adults. Nutrients 2020, 12, 281. [Google Scholar] [CrossRef]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la, O.A.; Martinez-Tellez, B.; Ruiz, J.R.; Gutiérrez, Á.; Castillo, M.J. Adherence to the Mediterranean diet, dietary factors, and S-Klotho plasma levels in sedentary middle-aged adults. Exp. Gerontol. 2019, 119, 25–32. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes/about_nhanes (accessed on 12 July 2022).
- Beltsville, M.A.R.S.; Food Surveys Research Group. USDA Food and Nutrient Database for Dietary Studies. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/ (accessed on 12 July 2022).
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef]
- Bowman, S.; Clemens, J.; Friday, J.; Thoerig, R.; Shimizu, M.; Barrows, B.; Moshfegh, A. Food Patterns Equivalents Database 2007–2008: Methodology and User Guide; US Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2013.
- Bowman, S.; Clemens, J.; Thoerig, R.; Friday, J.; Shimizu, M.; Moshfegh, A. Food Patterns Equivalents Database 2009–2010: Methodology and User Guide. US Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center. Available online: http://www.ars.usda.gov/ba/bhnrc/fsrg (accessed on 12 July 2022).
- Bowman, S.; Clemens, J.; Friday, J.; Thoerig, R.; Moshfegh, J. Food Patterns Equivalents Database 2011–2012: Methodology and User Guide. Food Surveys Research Group, Beltsville Human Nutrition Research Center; Agricultural Research Service, US Department of Agriculture: Beltsville, MD, USA, 2014.
- Bowman, S.; Clemens, J.; Friday, J.; Lynch, K.; Moshfegh, A. Food Patterns Equivalents Database 2013–2014: Methodology and User Guide; Food Surveys Research Group: Beltsville, MD, USA, 2017.
- Bowman, S.; Clemens, J.; Shimizu, M.; Friday, J.; Moshfegh, A. Food Patterns Equivalents Database 2015–2016: Methodology and User Guide; US Department of Agriculture: Beltsville, MD, USA, 2018.
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef]
- Taylor, M.K.; Mahnken, J.D.; Sullivan, D.K. NHANES 2011-2014 Reveals Cognition of US Older Adults may Benefit from Better Adaptation to the Mediterranean Diet. Nutrients 2020, 12, 1929. [Google Scholar] [CrossRef]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Albert, C.M.; Rexrode, K.; Hu, F.B. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N. Engl. J. Med. 2006, 355, 1991–2002. [Google Scholar] [CrossRef]
- Krauss, R.M.; Eckel, R.H.; Howard, B.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.W., Jr.; Kris-Etherton, P.; Goldberg, I.J.; Kotchen, T.A.; et al. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef]
- Last, A.R.; Wilson, S.A. Low-carbohydrate diets. Am. Fam. Physician 2006, 73, 1942–1948. [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory Procedures Manual. 2011. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_laboratory_procedures_manual.pdf (accessed on 12 July 2022).
- Yamazaki, Y.; Imura, A.; Urakawa, I.; Shimada, T.; Murakami, J.; Aono, Y.; Hasegawa, H.; Yamashita, T.; Nakatani, K.; Saito, Y.; et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 2010, 398, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Pedersen, S.M.; Brasen, C.L.; Rasmussen, L.M. Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin. Biochem. 2013, 46, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.T.; de Groot, L.C.; Kromhout, D.; Perrin, A.E.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA 2004, 292, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the PREDIMED. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef]
- Formisano, E.; Pasta, A.; Cremonini, A.L.; Di Lorenzo, I.; Sukkar, S.G.; Pisciotta, L. Effects of a Mediterranean Diet, Dairy, and Meat Products on Different Phenotypes of Dyslipidemia: A Preliminary Retrospective Analysis. Nutrients 2021, 13, 1161. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef]
- Milenkovic, T.; Bozhinovska, N.; Macut, D.; Bjekic-Macut, J.; Rahelic, D.; Velija Asimi, Z.; Burekovic, A. Mediterranean Diet and Type 2 Diabetes Mellitus: A Perpetual Inspiration for the Scientific World. A Review. Nutrients 2021, 13, 1307. [Google Scholar] [CrossRef]
- Lagiou, P.; Sandin, S.; Weiderpass, E.; Lagiou, A.; Mucci, L.; Trichopoulos, D.; Adami, H.O. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J. Intern. Med. 2007, 261, 366–374. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Hsieh, C.C.; Trichopoulos, D. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. Eur. J. Clin. Nutr. 2007, 61, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.M.; Winkvist, A.; Eliasson, M.; Jansson, J.H.; Hallmans, G.; Johansson, I.; Lindahl, B.; Lenner, P.; Van Guelpen, B. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur. J. Clin. Nutr. 2012, 66, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Murphy, C.T.; Kenyon, C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009, 10, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Hoxha, S.; Carvalho, G.B.; Yamada, R.; Wang, H.D.; Karayan, P.; He, S.; Brummel, T.; Kapahi, P.; Ja, W.W. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp. Gerontol. 2013, 48, 1129–1135. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Walters, K.A.; Simanainen, U.K.; McMahon, A.C.; Ruohonen, K.; Ballard, J.W.; Raubenheimer, D.; Handelsman, D.J.; Le Couteur, D.G.; Simpson, S.J. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl. Acad. Sci. USA 2015, 112, 3481–3486. [Google Scholar] [CrossRef]
- Kendall, A.; Levitsky, D.A.; Strupp, B.J.; Lissner, L. Weight loss on a low-fat diet: Consequence of the imprecision of the control of food intake in humans. Am. J. Clin. Nutr. 1991, 53, 1124–1129. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Leidig, M.M.; Feldman, H.A.; Lovesky, M.M.; Ludwig, D.S. Effects of a low-glycemic load vs low-fat diet in obese young adults: A randomized trial. JAMA 2007, 297, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Noakes, M.; Keogh, J.B.; Foster, P.R.; Clifton, P.M. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am. J. Clin. Nutr. 2005, 81, 1298–1306. [Google Scholar] [CrossRef]
- O’Rourke, E.J.; Kuballa, P.; Xavier, R.; Ruvkun, G. ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013, 27, 429–440. [Google Scholar] [CrossRef]
- Tsuduki, T.; Honma, T.; Nakagawa, K.; Ikeda, I.; Miyazawa, T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition 2011, 27, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J. Metabolism and longevity: Is there a role for membrane fatty acids? Integr. Comp. Biol. 2010, 50, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543s–1548s. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Steffen, L.M. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am. J. Clin. Nutr. 2003, 78, 508s–513s. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Tapsell, L.C. Food, not nutrients, is the fundamental unit in nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef]
- Simpson, S.J.; Le Couteur, D.G.; Raubenheimer, D. Putting the balance back in diet. Cell 2015, 161, 18–23. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sánchez-Villegas, A. The emerging role of Mediterranean diets in cardiovascular epidemiology: Monounsaturated fats, olive oil, red wine or the whole pattern? Eur. J. Epidemiol. 2004, 19, 9–13. [Google Scholar] [CrossRef]
- Imhof, A.; Woodward, M.; Doering, A.; Helbecque, N.; Loewel, H.; Amouyel, P.; Lowe, G.D.; Koenig, W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: Results from three MONICA samples (Augsburg, Glasgow, Lille). Eur. Heart J. 2004, 25, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, M.S.; Ubbink, J.B.; Sillanaukee, P.; Nikkari, S.; Hendriks, H.F. Effect of consumption of red wine, spirits, and beer on serum homocysteine. Lancet 2000, 355, 1522. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A., Jr.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 510, 160–169. [Google Scholar] [CrossRef]
| Characteristics | Total Participants (n = 7906) |
|---|---|
| 2 Continuous variables | |
| Age (years) | 57.77 ± 10.92 |
| 1 BMI (kg/m2) | 29.67 ± 6.54 |
| Klotho (pg/mL) | 861.17 ± 311.55 |
| Mediterranean diet score | 6.72 ± 2.12 |
| Low-carbohydrate diet score | 14.98 ± 7.19 |
| Daily carbohydrate intake (gm)/Percentage of calories from carbohydrate | 245.50 ± 116.27 (49%) |
| Daily protein intake (gm)/Percentage of calories from protein | 78.67 ± 39.30 (16%) |
| Daily fat intake (gm)/Percentage of calories from fat | 76.10 ± 43.96 (34%) |
| Daily calorie intake (kcal) | 2020.97 ± 923.62 |
| 3 Categorical variables | |
| Sex | |
| Men | 3910 (49.5%) |
| Women | 3996 (50.5%) |
| Race | |
| Mexican American | 1225 (15.5%) |
| Other Hispanic | 855 (10.8%) |
| Non-Hispanic White | 3704 (46.9%) |
| Non-Hispanic Black | 1619 (20.5%) |
| Other race, including multi-racial | 503 (6.4%) |
| History of congestive heart failure | 312 (3.9%) |
| History of coronary heart disease | 392 (5%) |
| History of angina | 254 (3.2%) |
| History of diabetes mellitus | 6705 (84.8%) |
| History of hypertension | 5433 (68.7%) |
| Smoking history | 3987 (50.4%) |
| 1 MDS | 2 LCD Score | Low-Fat Diet | 3 Low-Carb Diet | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Score | Tertile | Total Score | Decile | ||||||||
| 1 | 2 | 3 | 1 | 5 | 10 | ||||||
| Model 1 | β coefficient (95% CI) | 9.41 (6.08, 12.74) | Ref. | 36.87 (19.17, 54.57) | 45.92 (28.01, 63.83) | −0.366 (−1.355, 0.623) | Ref. | 1.45 (−19.82, 22.72) | −6.32 (−28.30, 15.67) | −2.16 (−16.97, 12.66) | −96.08 (−202.49, 10.32) |
| p value | <0.001 | Ref. | <0.001 | <0.001 | 0.468 | Ref. | 0.894 | 0.573 | 0.775 | 0.077 | |
| Model 2 | β coefficient (95% CI) | 9.62 (6.29, 12.95) | Ref. | 37.38 (19.76, 55.01) | 46.79 (28.87, 64.72) | −0.116 (−1.106, 0.873) | Ref. | 5.24 (−15.91, 26.40) | −1.16 (−14.58, 31.66) | −2.58 (−17.37, 12.21) | −95.37 (−201.14, 10.39) |
| p value | <0.001 | Ref. | <0.001 | <0.001 | 0.818 | Ref. | 0.627 | 0.917 | 0.732 | 0.077 | |
| Model 3 | β coefficient (95% CI) | 9.01 (5.67, 12.34) | Ref. | 35.92 (18.33, 53.51) | 43.42 (25.50, 61.34) | −0.200 (−1.188, 0.788) | Ref. | 3.76 (−17.34, 24.85) | −2.56 (−24.48, 19.35) | −1.80 (−16.56, 12.97) | −103.54 (−208.98, 1.89) |
| p value | <0.001 | Ref. | <0.001 | <0.001 | 0.691 | Ref. | 0.727 | 0.819 | 0.811 | 0.054 | |
| 1 MDS | 2 LCD Score | Low-Fat Diet | 3 Low-Carb Diet | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile | Decile | ||||||||
| 1 | 2 | 3 | 1 | 5 | 10 | ||||
| Model 1 | OR (95% CI) | Ref. | 0.86 (0.76, 0.98) | 0.77 (0.68, 0.88) | Ref. | 0.97 (0.83, 1.13) | 0.96 (0.82, 1.13) | 1.01 (0.91, 1.13) | 1.50 (0.73, 3.11) |
| p value | Ref. | 0.026 | <0.001 | Ref. | 0.653 | 0.648 | 0.867 | 0.271 | |
| Model 2 | OR (95% CI) | Ref. | 0.85 (0.75, 0.97) | 0.76 (0.66, 0.86) | Ref. | 0.95 (0.81, 1.11) | 0.94 (0.80, 1.11) | 1.01 (0.91, 1.13) | 1.52 (0.73, 3.15) |
| p value | Ref. | 0.017 | <0.001 | Ref. | 0.512 | 0.478 | 0.929 | 0.260 | |
| Model 3 | OR (95% CI) | Ref. | 0.86 (0.75, 0.98) | 0.77 (0.67, 0.88) | Ref. | 0.96 (0.82, 1.12) | 0.95 (0.81, 1.12) | 1.01 (0.89, 1.12) | 1.58 (0.76, 3.29) |
| p value | Ref. | 0.024 | <0.001 | Ref. | 0.956 | 0.949 | 0.933 | 0.217 | |
| Tertile 1 of 1 MDS | Tertile 2 of MDS | Tertile 3 of MDS | ||
|---|---|---|---|---|
| Sorted by sex | ||||
| Men | ||||
| Model 1 | β coefficient (95% CI) | Ref. | 26.62 (4.18, 49.05) | 43.48 (20.39, 66.56) |
| p value | Ref. | 0.020 | <0.001 | |
| Model 2 | β coefficient (95% CI) | Ref. | 29.73 (7.34, 52.13) | 49.59 (26.45, 72.73) |
| p value | Ref. | 0.009 | <0.001 | |
| Model 3 | β coefficient (95% CI) | Ref. | 27.81 (5.47, 50.16) | 45.31 (22.16, 68.47) |
| p value | Ref. | 0.015 | <0.001 | |
| Women | ||||
| Model 1 | β coefficient (95% CI) | Ref. | 41.01 (13.34, 68.69) | 40.28 (12.63, 67.94) |
| p value | Ref. | 0.004 | 0.004 | |
| Model 2 | β coefficient (95% CI) | Ref. | 45.34 (17.79, 72.89) | 45.01 (17.43, 72.59) |
| p value | Ref. | 0.001 | 0.001 | |
| Model 3 | β coefficient (95% CI) | Ref. | 43.22 (15.75, 70.69) | 41.23 (13.67, 68.79) |
| p value | Ref. | 0.002 | 0.003 | |
| Sorted by age | ||||
| <65 years old | ||||
| Model 1 | β coefficient (95% CI) | Ref. | 33.44 (12.83, 54.05) | 48.38 (27.29, 69.48) |
| p value | Ref. | 0.001 | <0.001 | |
| Model 2 | β coefficient (95% CI) | Ref. | 31.58 (11.01, 52.15) | 45.22 (24.09, 66.35) |
| p value | Ref. | 0.003 | <0.001 | |
| Model 3 | β coefficient (95% CI) | Ref. | 30.59 (10.09, 51.09) | 40.19 (19.07, 61.32) |
| p value | Ref. | 0.003 | <0.001 | |
| ≥65 years old | ||||
| Model 1 | β coefficient (95% CI) | Ref. | 55.30 (20.99, 89.62) | 55.75 (21.85, 89.65) |
| p value | Ref. | 0.002 | 0.001 | |
| Model 2 | β coefficient (95% CI) | Ref. | 53.87 (19.54, 88.21) | 52.40 (18.43, 86.38) |
| p value | Ref. | 0.002 | 0.003 | |
| Model 3 | β coefficient (95% CI) | Ref. | 51.03 (16.68, 85.38) | 49.61 (15.54, 83.68) |
| p value | Ref. | 0.004 | 0.004 | |
| Sorted by BMI | ||||
| BMI < 30 | ||||
| Model 1 | β coefficient (95% CI) | Ref. | 29.98 (6.66, 53.32) | 40.56 (16.93, 64.19) |
| p value | Ref. | 0.012 | 0.001 | |
| Model 2 | β coefficient (95% CI) | Ref. | 31.09 (7.86, 54.31) | 40.98 (17.31, 64.66) |
| p value | Ref. | 0.009 | 0.001 | |
| Model 3 | β coefficient (95% CI) | Ref. | 27.93 (4.74, 51.11) | 36.67 (12.97, 60.38) |
| p value | Ref. | 0.018 | 0.002 | |
| BMI ≥ 30 | ||||
| Model 1 | β coefficient (95% CI) | Ref. | 46.82 (19.67, 73.97) | 53.69 (26.25, 81.13) |
| p value | Ref. | 0.001 | <0.001 | |
| Model 2 | β coefficient (95% CI) | Ref. | 45.52 (18.42, 72.61) | 54.14 (26.68, 81.59) |
| p value | Ref. | 0.001 | <0.001 | |
| Model 3 | β coefficient (95% CI) | Ref. | 46.29 (19.27, 73.31) | 51.86 (24.46, 79.26) |
| p value | Ref. | 0.001 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-E.; Chen, Y.-J.; Chen, W.-L. Adherence to Mediterranean Diet and Soluble Klotho Level: The Value of Food Synergy in Aging. Nutrients 2022, 14, 3910. https://doi.org/10.3390/nu14193910
Wu S-E, Chen Y-J, Chen W-L. Adherence to Mediterranean Diet and Soluble Klotho Level: The Value of Food Synergy in Aging. Nutrients. 2022; 14(19):3910. https://doi.org/10.3390/nu14193910
Chicago/Turabian StyleWu, Shou-En, Ying-Jen Chen, and Wei-Liang Chen. 2022. "Adherence to Mediterranean Diet and Soluble Klotho Level: The Value of Food Synergy in Aging" Nutrients 14, no. 19: 3910. https://doi.org/10.3390/nu14193910
APA StyleWu, S.-E., Chen, Y.-J., & Chen, W.-L. (2022). Adherence to Mediterranean Diet and Soluble Klotho Level: The Value of Food Synergy in Aging. Nutrients, 14(19), 3910. https://doi.org/10.3390/nu14193910





