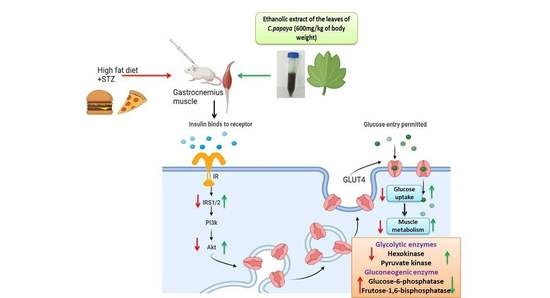
Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Plant Material
2.3. Animals
2.4. Induction of T2DM
2.5. Experimental Design
2.6. Determination of Gluconeogenic Enzymes
2.6.1. Glucose-6-Phosphatase Assay
2.6.2. Fructose-1,6 Bisphosphatase Assay
2.7. Determination of Glycolytic Enzymes
2.8. mRNA Expression Analysis
Total RNA Isolation, cDNA Conversion, and Real-Time PCR
2.9. Immunohistochemical Analysis
2.10. Statistical Analysis
2.11. Molecular Docking
2.11.1. Compound/Ligand Preparation
2.11.2. Protein Preparation
2.11.3. Molecular Docking Procedure
2.12. Molecular Simulation and Dynamics
Molecular Simulation and Dynamics Study of Proposed Compounds and IRS-1 and Akt Complex
3. Results
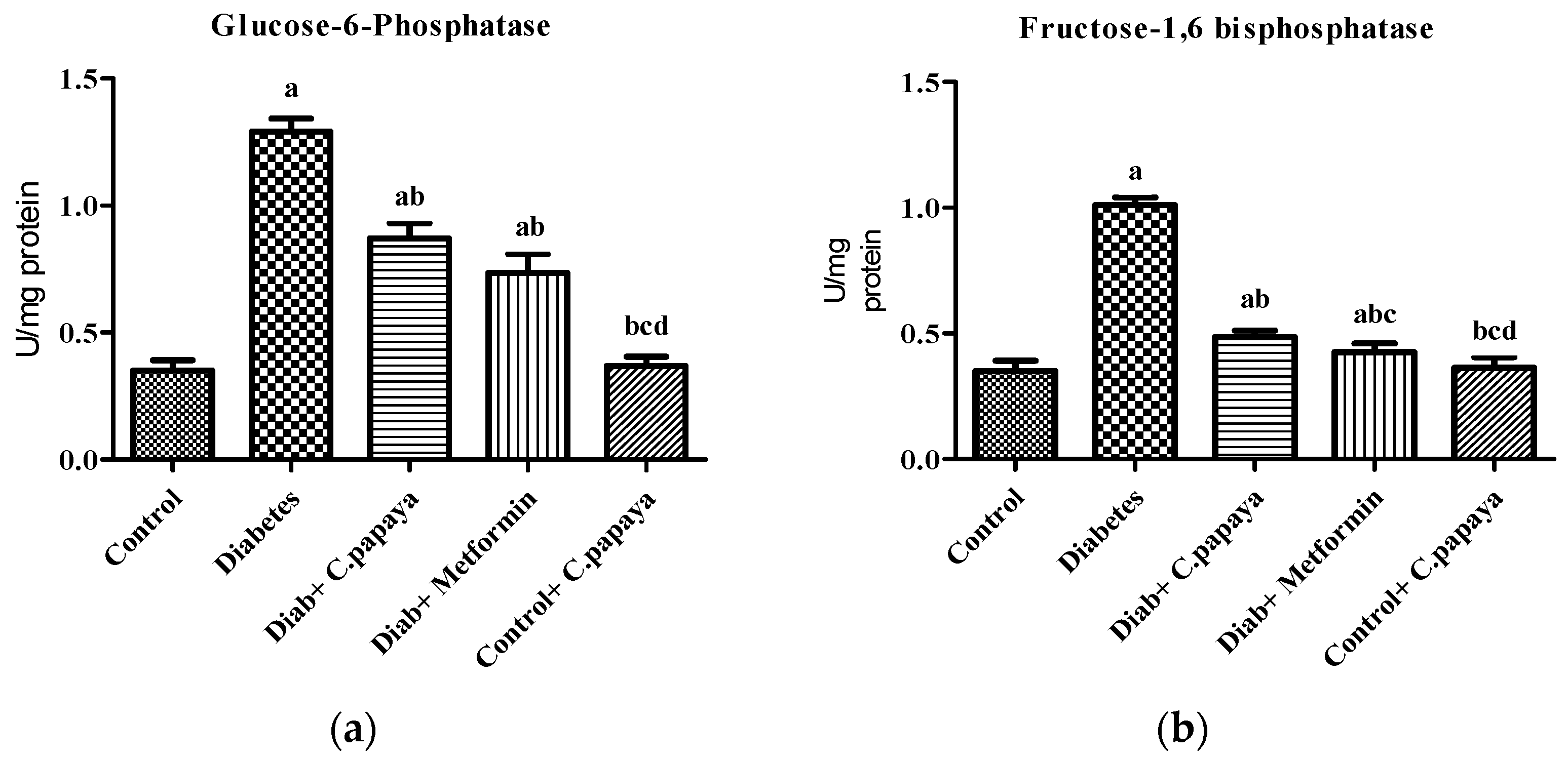
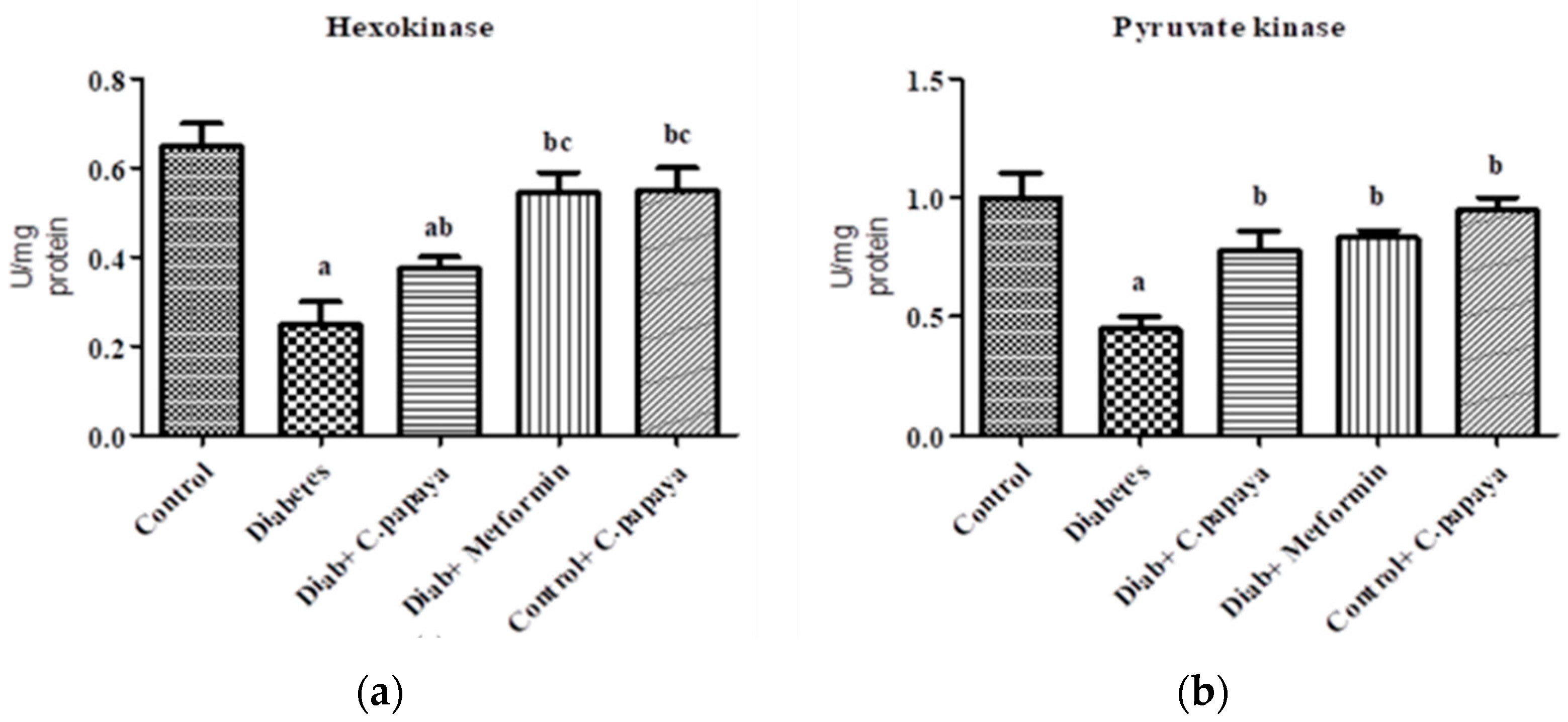
3.1. Estimation of Gluconeogenic Enzymes and Glycolytic Enzymes
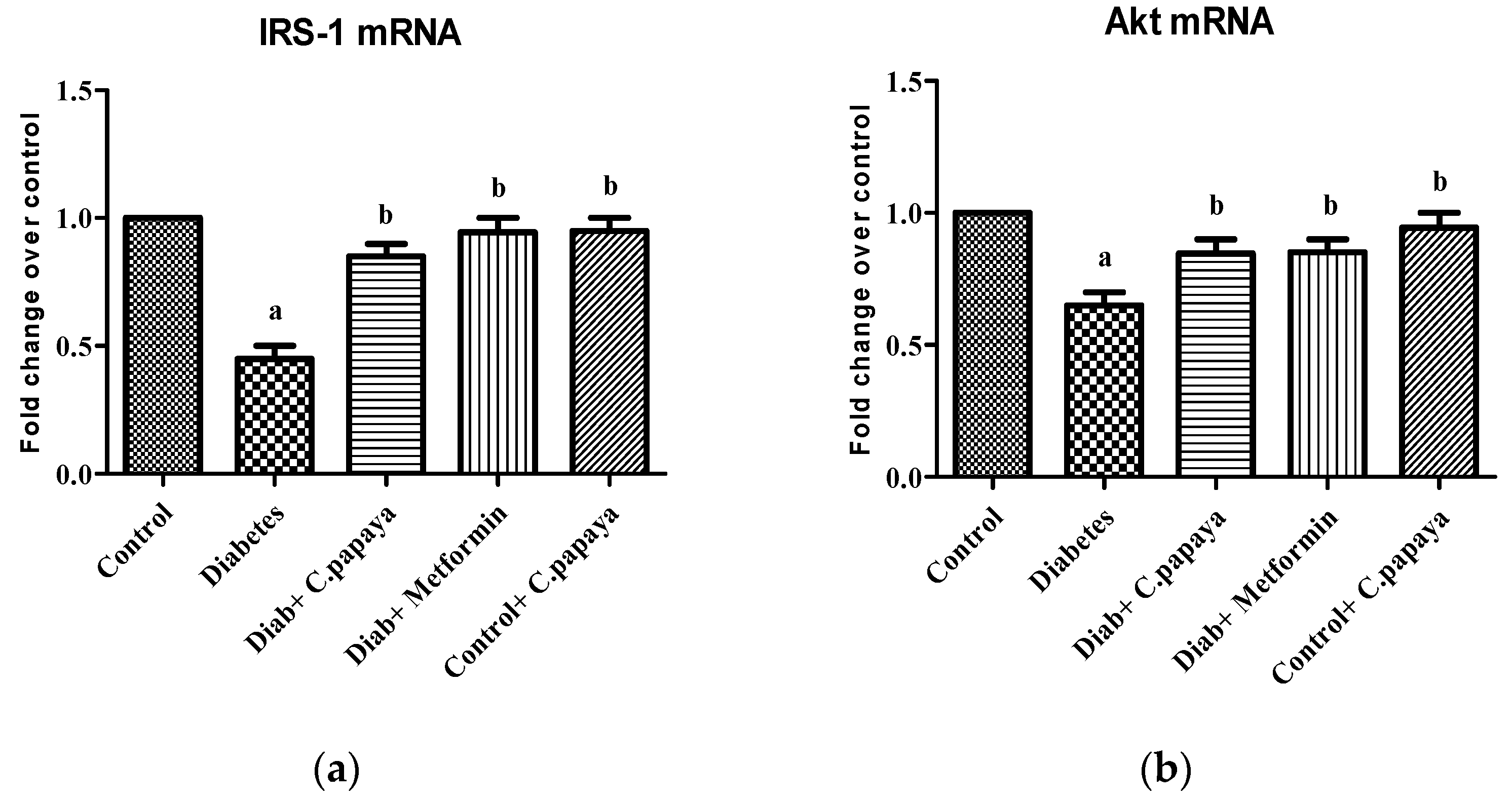
3.2. Effect of C. papaya on mRNA Expression of IRS-1 and Akt
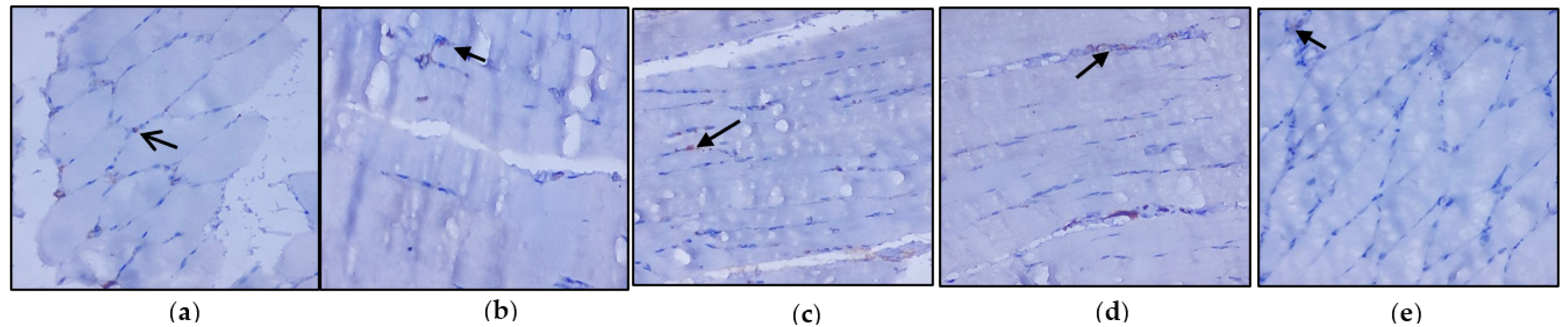
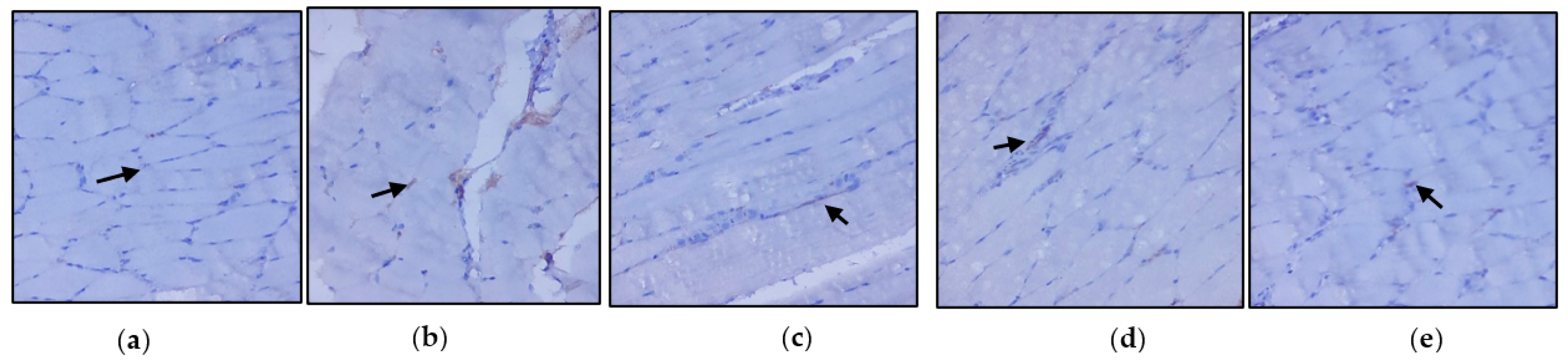
3.3. Evaluation of Immunohistochemical Changes in Skeletal Muscle
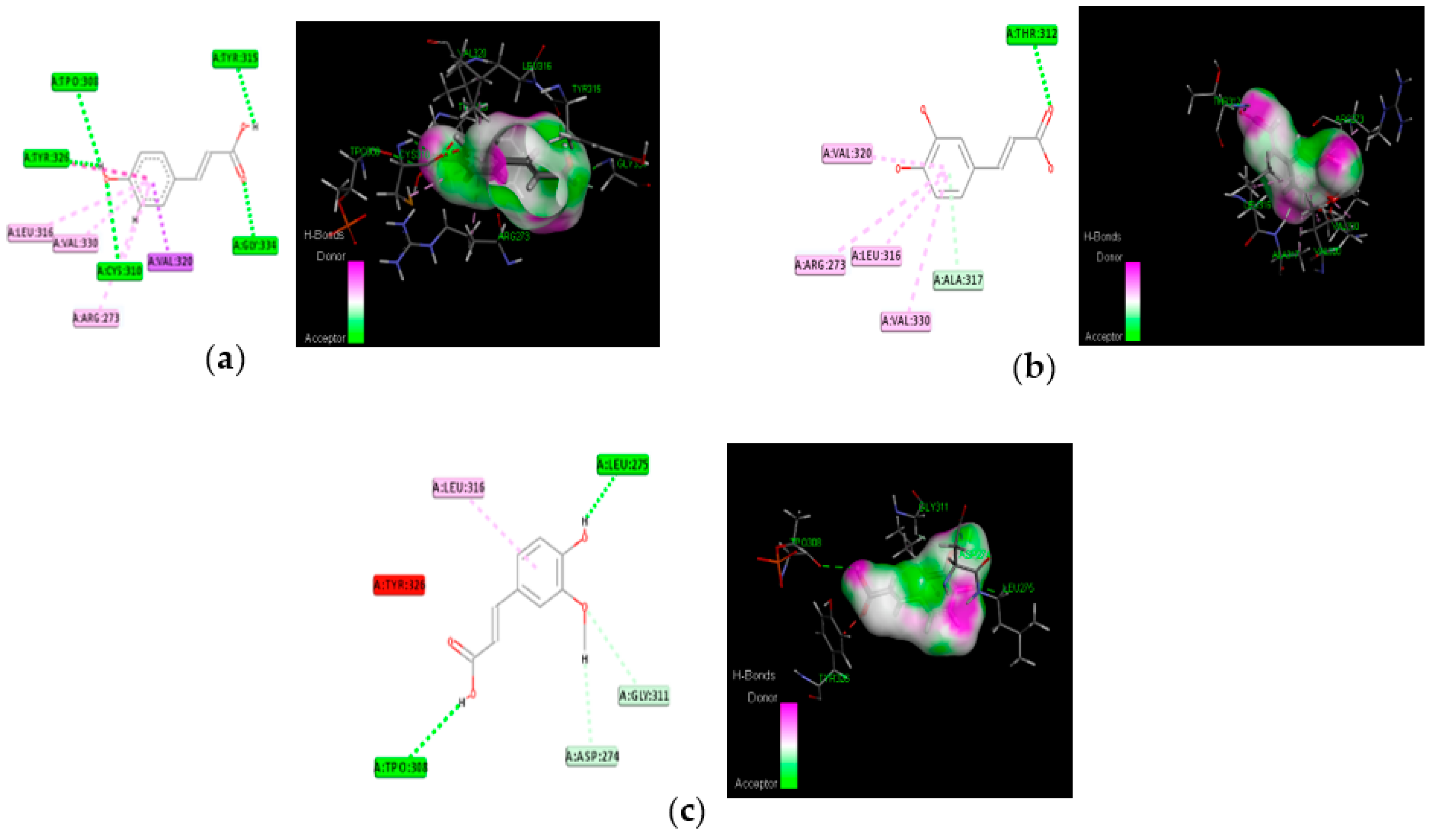
3.4. Molecular Docking
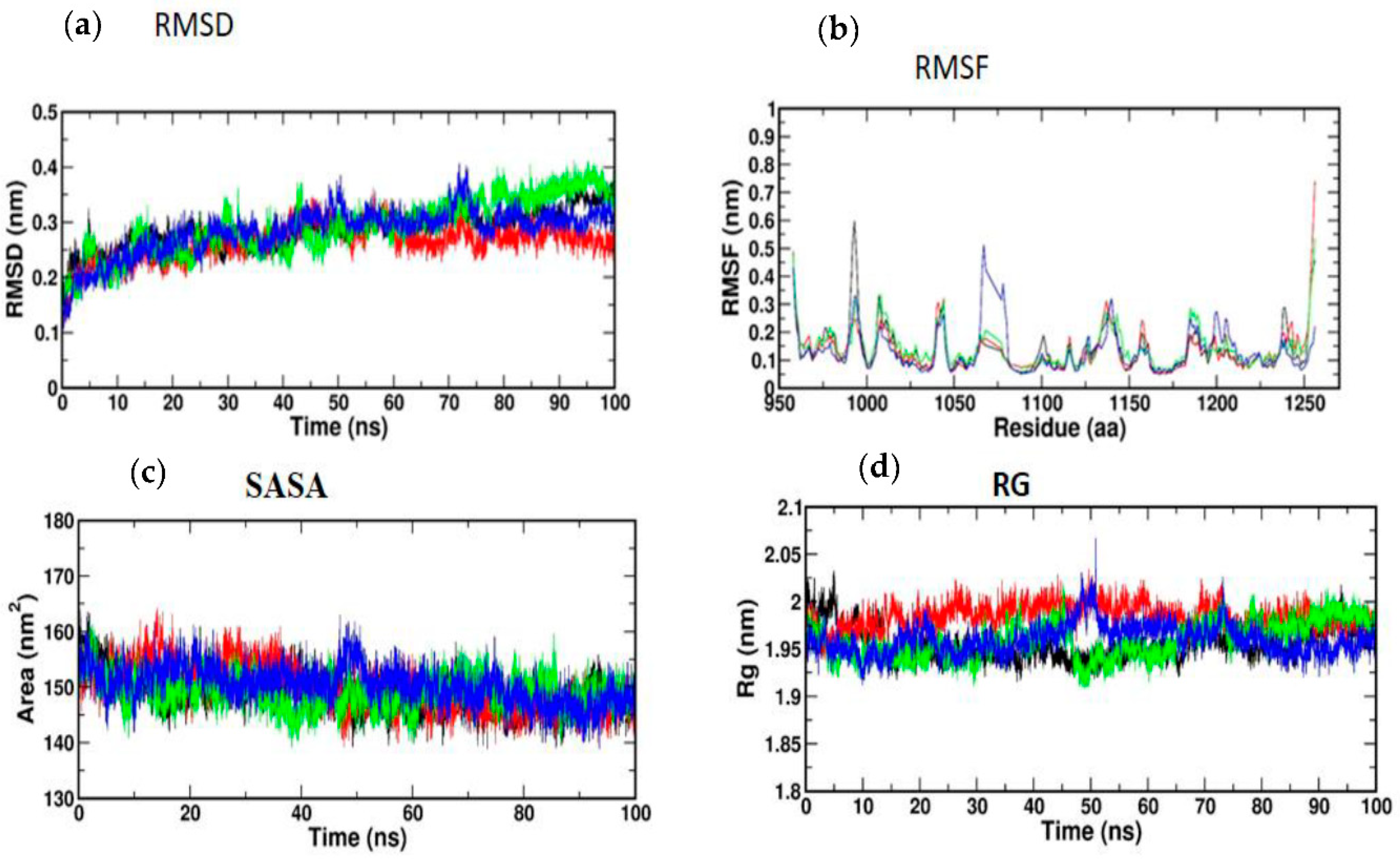
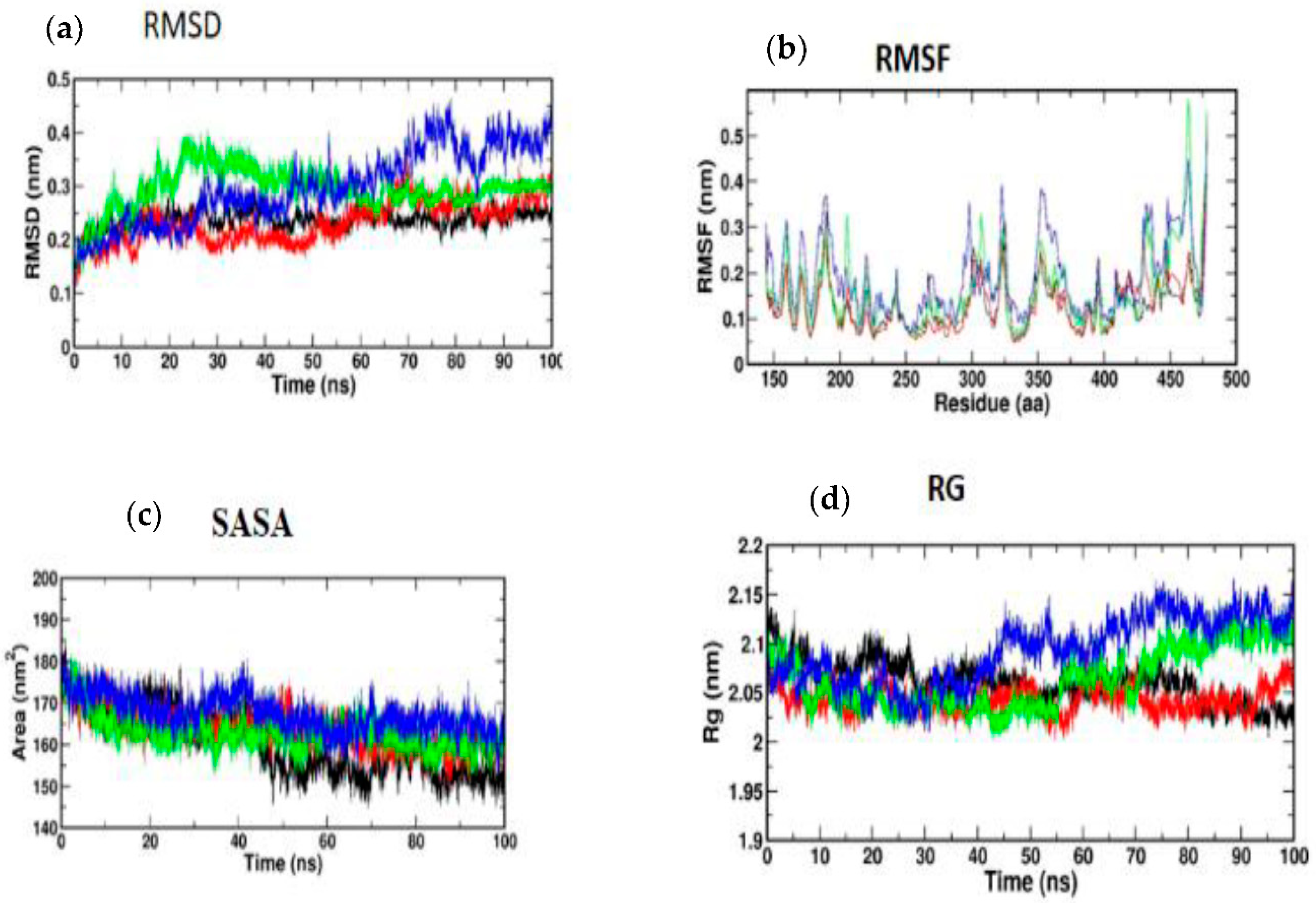
3.5. Molecular Simulation and Dynamics Study of Docked Complex
3.5.1. Molecular Dynamic Simulation of IRS-1
3.5.2. Molecular Dynamic Simulation of Akt
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öhman, T.; Teppo, J.; Datta, N.; Mäkinen, S.; Varjosalo, M.; Koistinen, H.A. Skeletal muscle proteomes reveal downregulation of mitochondrial proteins in transition from prediabetes into type 2 diabetes. iScience 2021, 24, 102712. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; AlKaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Koistinen, H.A.; Zierath, J.R. Regulation of glucose transport in human skeletal muscle. Ann. Med. 2002, 34, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Scalzo, R.L.; Reusch, J.E.B. Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach. Nutrients 2022, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef]
- Petersen, K.F.; Shulman, G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 11G–18G. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Sies, H. Findings in redox biology: From H2O2 to oxidative stress. J. Biol. Chem. 2020, 295, 13458–13473. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Nawaratne, R.; Gray, A.; Jørgensen, C.H.; Downes, C.P.; Siddle, K.; Sethi, J.K. Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: Role of serine 24 phosphorylation. Mol. Endocrinol. 2006, 20, 1838–1852. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Moura, L.P.; Muñoz, V.R.; Silva, A.S.; Gaspar, R.S.; Ropelle, E.R.; Pauli, J.R. Molecular mechanisms of glucose uptake in skeletal muscle at rest and in response to exercise. Mot. Rev. Educ. Fis. 2017, 23, e101609. [Google Scholar] [CrossRef]
- Krook, A.; Björnholm, M.; Galuska, D.; Jiang, X.J.; Fahlman, R.; Myers, M.G., Jr.; Wallberg-Henriksson, H.; Zierath, J.R. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 2000, 49, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.D.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef]
- Phielix, E.; Mensink, M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol. Behav. 2008, 94, 252–258. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef]
- Balbaa, M.; Abdulmalek, S.A.; Khalil, S. Oxidative stress and expression of insulin signaling proteins in the brain of diabetic rats: Role of Nigella sativa oil and antidiabetic drugs. PLoS ONE 2017, 12, e0172429. [Google Scholar] [CrossRef]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar] [CrossRef]
- Sharma, M.; Aggarwal, S.; Nayar, U.; Vikram, N.K.; Misra, A.; Luthra, K. Differential expression of insulin receptor substrate-1(IRS-1) in visceral and subcutaneous adipose depots of morbidly obese subjects undergoing bariatric surgery in a tertiary care center in north India; SNP analysis and correlation with metabolic profile. Diabetes Metab. Syndr. 2021, 15, 981–986. [Google Scholar] [CrossRef]
- Eckstein, S.S.; Weigert, C.; Lehmann, R. Divergent Roles of IRS (Insulin Receptor Substrate) 1 and 2 in Liver and Skeletal Muscle. Curr. Med. Chem. 2017, 24, 1827–1852. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Suer, F.E.O.; Mergen, H.; Bolu, E.; Ozata, M. Molecular scanning for mutations in the insulin receptor substrate-1 (IRS-1) gene in Turkish with type 2 diabetes mellitus. Endocr. J. 2005, 52, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Nandipati, K.C.; Subramanian, S.; Agrawal, D.K. Protein kinases: Mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol. Cell Biochem. 2017, 426, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, X.H.; Yan, Y.G.; Wang, C.; Wang, W.J. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. 2015, 444, 182–192. [Google Scholar] [CrossRef]
- Liu, P.; Gan, W.; Chin, Y.R.; Ogura, K.; Guo, J.; Zhang, J.; Wang, B.; Blenis, J.; Cantley, L.C.; Toker, A.; et al. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015, 5, 1194–1209. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Risso, G.; Blaustein, M.; Pozzi, B.; Mammi, P.; Srebrow, A. Akt/PKB: One kinase, many modifications. Biochem. J. 2015, 468, 203–214. [Google Scholar] [CrossRef]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef]
- Koide, H.; Oda, T. Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin. Chim. Acta 1959, 4, 554–561. [Google Scholar]
- Fiske, C.H.; Subbarow, J. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Gancedo, J.M.; Gancedo, C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch. Mikrobiol. 1971, 76, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Brandstrup, N.; Kirk, J.E.; Bruni, C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J. Gerontol. 1957, 12, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Valentine, W.N.; Tanaka, K.R. Pyruvate kinase: Clinical aspects. Methods Enzymol. 1966, 9, 468–473. [Google Scholar]
- Prasad, M.; Jayaraman, S.; Rajagopal, P.; Veeraraghavan, V.P.; Kumar, P.K.; Piramanayagam, S.; Pari, L. Diosgenin inhibits ER stress-induced inflammation in aorta via iRhom2/TACE mediated signaling in experimental diabetic rats: An in vivo and in silico approach. Chem. Biol. Interact. 2022, 358, 109885. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Alonso, A.; Fernandez, R.; Patterson, A.M. Regulation of insulin receptor substrate-1 in the liver, skeletal muscle and adipose tissue of rats throughout pregnancy. Gynecol. Endocrinol. 2003, 17, 187–197. [Google Scholar]
- Indumathi, D.; Jayashree, S.; Selvaraj, J.; Sathish, S.; Mayilvanan, C.; Akilavalli, N.; Balasubramanian, K. Effect of bisphenol-A on insulin signal transduction and glucose oxidation in skeletal muscle of adult male albino rat. Hum. Exp. Toxicol. 2013, 9, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Christy, J.; Shankari, S.; Majeed, I.; Anand, D.A. Deciphering the Synergistic Mechanism of Cortistatin towards Cancer Targets using Network Pharmacology Approach. Indian J. Pharm. Educ. Res. 2021, 55, 1017–1027. [Google Scholar] [CrossRef]
- Christy, J.; Harini; Vasudevan, S.; Lingesan, P.; Anand, D.A. Deciphering the molecular interplay between pelvic inflammatory disease (PID) and ovarian cancer (OC)—A network biology approach. Gene Rep. 2021, 25, 101405. [Google Scholar] [CrossRef]
- Schüttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Zhao, C.; Yang, C.; Wai, S.; Zhang, Y.; Portillo, M.P.; Paoli, P.; Wu, Y.; Cheang, W.S.; Liu, B.; Carpéné, C.; et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2019, 59, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.; Pari, L. Antihyperglycaemic effect of Cassia auriculata in experimental diabetes and its effects on key metabolic enzymes involved in carbohydrate metabolism. Clin. Exp. Pharmacol. Physiol. 2003, 30, 38–43. [Google Scholar] [CrossRef]
- Kanadi, M.A.; Alhassan, A.J.; Yaradua, A.I.; Nasir, A.; Wudil, A.M. Sub-fractions from Carica Papaya Seed Extracts Can Prevent Potassium Bromate-induced Changes in Activities of Renal Brush Border Membrane Enzymes and Some Enzymes of Carbohydrate Metabolism in the Kidney of Rats. Asian J. Biochem. Genet. Mol. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Langa, S.O.P.; Mukaratirwa, S.; Masola, B. Effects of Centella asiatica on skeletal muscle structure and key enzymes of glucose and glycogen metabolism in type 2 diabetic rats. Biomed. Pharmacother. 2019, 112, 108715. [Google Scholar] [CrossRef]
- Kalaivani, K.; Sankaranarayanan, C. Modulatory effect of isopulegol on hepatic key enzymes of glucose metabolism in high-fat diet/streptozotocin-induced diabetic rats. Arch. Physiol. Biochem. 2021, 127, 318–326. [Google Scholar] [CrossRef]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2018, 82, 112–120. [Google Scholar] [CrossRef]
- Pari, L.; Rajarajeswari, N. Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chem.-Biol. Interact. 2009, 181, 292–296. [Google Scholar] [CrossRef]
- Gothandam, K.; Ganesan, V.S.; Ayyasamy, T.; Ramalingam, S. Antioxidant potential of theaflavin ameliorates the activities of key enzymes of glucose metabolism in high fat diet and streptozotocin–induced diabetic rats. Redox Rep. 2019, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Aguilar-Domínguez, D.E.; Lobato-García, C.E.; Blé-Castillo, J.L.; López-Meraz, L.; Díaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef]
- Chen, C.; Peng, S.; Chen, F.; Liu, L.; Li, Z.; Zeng, G.; Huang, Q. Protective effects of pioglitazone on vascular endothelial cell dysfunction induced by high glucose via inhibition of IKKα/β–NFκB signaling mediated by PPARγ in vitro. Can. J. Physiol. Pharmacol. 2017, 95, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Chuang, W.T.; Yen, C.C.; Huang, C.S.; Chen, H.W.; Lii, C.K. Benzyl Isothiocyanate Ameliorates High-Fat Diet-Induced Hyperglycemia by Enhancing Nrf2-Dependent Antioxidant Defense-Mediated IRS-1/AKT/TBC1D1 Signaling and GLUT4 Expression in Skeletal Muscle. J. Agric. Food Chem. 2020, 68, 15228–15238. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of skeletal muscle in insulin resistance and glucose uptake. Compr. Physiol. 2011, 10, 785–809. [Google Scholar]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Huang, X.; Zhao, Y.; Ren, Q.; Hong, Z.; Huang, M.; Xing, X. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J. Funct. Foods 2018, 48, 515–524. [Google Scholar] [CrossRef]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef]
- Guo, X.; Sun, W.; Luo, G.; Wu, L.; Xu, G.; Hou, D.; Hou, Y.; Guo, X.; Mu, X.; Qin, L.; et al. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS 1–PI 3K–AKT signaling pathway and GLUT 4 expression. FEBS Open Bio 2019, 9, 1008–1019. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.C.; Kim, H.U.; Park, T.; Park, J.; Kim, U.; Kim, M.K.; Jeong, J.H. Asprosin attenuates insulin signaling pathway through PKCδ-activated ER stress and inflammation in skeletal muscle. J. Cell. Physiol. 2019, 234, 20888–20899. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Badrachalam, R.; Shanmugam, S.N.; Balraj, M.; Kasthuri, R.; Danavel, A.; Babu, S. Effect of β-Caryophyllene on insulin resistance in skeletal muscle of high fat diet and fructose-induced type-2 diabetic rats. Bioinformation 2021, 17, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nishina, P.M.; Naggert, J.K. Degradation of IRS1 leads to impaired glucose uptake in adipose tissue of the type 2 diabetes mouse model TALLYHO/Jng. J. Endocrinol. 2009, 203, 65–74. [Google Scholar] [CrossRef]
- Li, H.; Yu, L.; Zhao, C. Dioscin attenuates high fat diet induced insulin resistance of adipose tissue through the IRS 1/PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 19, 1230–1237. [Google Scholar] [CrossRef] [PubMed]

| S. No | Gene Name | Primer Sequence | Reference |
|---|---|---|---|
| 1 | Rat βactin | Sense primer: 5′-AAG TCC CTC ACC CTC CCA AAA G-3′ Antisense primer: 5′-AAG CAA TGC TGT CAC CTT CCC-3′ | [35] |
| 2 | IRS-1 | Sense primer: 5′-GCC AAT CTT CAT CCA GTT GCT-3′ Antisense primer: 5′-CAT CGT GAA GAA GGC ATA GGG-3 | [36] |
| 3 | Akt | Sense primer: 5′-GGA AGC CTT CAG TTT GGA TCC CAA-3′ Antisense primer: 5′-AGT GGA AAT CCA GTT CCG AGC TTG-3′ | [37] |
| S. No. | Compound Name |
|---|---|
| 1 | Caffeic_acid |
| 2 | Chlorogenic_acid |
| 3 | Kaempferol |
| 4 | Quercetin |
| 5 | Rutin |
| 6 | p-coumaric_acid |
| 7 | trans-ferulic_acid |
| S. No | Compound Name | Lig Score1_Drei Ding | Lig Score2_Drei Ding | PLP 1 | PLP 2 | JAIN | PMF | Docking Score |
|---|---|---|---|---|---|---|---|---|
| IK3A | ||||||||
| 1 | Trans-ferulic acid | 1.64 | 3.37 | 38.93 | 36.6 | −1.2 | 34.9 | 37.161 |
| 2 | Quercetin | 2.69 | 3.56 | 52.33 | 58.2 | −0.84 | 52.63 | 49.741 |
| 3 | Kaempferol | 0.32 | 1.75 | 52.03 | 65.41 | 0.75 | 67.22 | 49.413 |
| 4 | Rutin | 3.33 | 4.24 | 109.67 | 113.31 | 1.14 | 73.52 | 103.327 |
| 5 | p-coumaric acid | No interaction | ||||||
| 6 | Chlorogenic acid | 3.96 | 4.6 | 75.2 | 75.63 | −0.37 | 64.06 | 71.235 |
| 7 | Protocatechuic acid | No interaction | ||||||
| 8 | Caffeic acid | No interaction | ||||||
| 3QKM | ||||||||
| 1 | Trans-ferulic acid | 1.02 | 0.14 | 64.19 | 71.24 | 2.36 | −8.12 | 58.136 |
| 2 | Quercetin | −18.41 | −31.47 | 9.64 | 54.3 | 5.87 | −37.75 | 0.656 |
| 3 | Kaempferol | −16.08 | −28.66 | 12.82 | 52.39 | 6.57 | −27.04 | 4.939 |
| 4 | Rutin | No interaction | ||||||
| 5 | p-coumaric acid | 0.26 | −0.99 | 55.11 | 62.57 | 3.43 | 7.61 | 50.999 |
| 6 | Chlorogenic acid | No interaction | ||||||
| 7 | Protocatechuic acid | No interaction | ||||||
| 8 | Caffeic acid | −2.19 | −4.55 | 54.21 | 59.96 | 3.52 | −2.24 | 51.777 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, J.R.; Janaki, C.S.; Jayaraman, S.; Periyasamy, V.; Balaji, T.; Vijayamalathi, M.; Veeraraghavan, V.P. Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach. Nutrients 2022, 14, 4181. https://doi.org/10.3390/nu14194181
Roy JR, Janaki CS, Jayaraman S, Periyasamy V, Balaji T, Vijayamalathi M, Veeraraghavan VP. Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach. Nutrients. 2022; 14(19):4181. https://doi.org/10.3390/nu14194181
Chicago/Turabian StyleRoy, Jeane Rebecca, Coimbatore Sadagopan Janaki, Selvaraj Jayaraman, Vijayalakshmi Periyasamy, Thotakura Balaji, Madhavan Vijayamalathi, and Vishnu Priya Veeraraghavan. 2022. "Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach" Nutrients 14, no. 19: 4181. https://doi.org/10.3390/nu14194181
APA StyleRoy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P. (2022). Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach. Nutrients, 14(19), 4181. https://doi.org/10.3390/nu14194181