Curcumin Offers No Additional Benefit to Lifestyle Intervention on Cardiometabolic Status in Patients with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants and Intervention
2.3. Data Collection
2.4. Definition of the Fatty Liver, Atherosclerosis, and Adipose Tissue-Related Indices
2.5. Statistical Analysis
3. Results
3.1. Participants’ Recruitment Procedure and Baseline Characteristics
3.2. Changes in Fatty liver-Associated Indicators
3.3. Changes in Atherogenicity and Insulin Resistance Markers
3.4. Changes in Adipose Tissue-Related Indicators
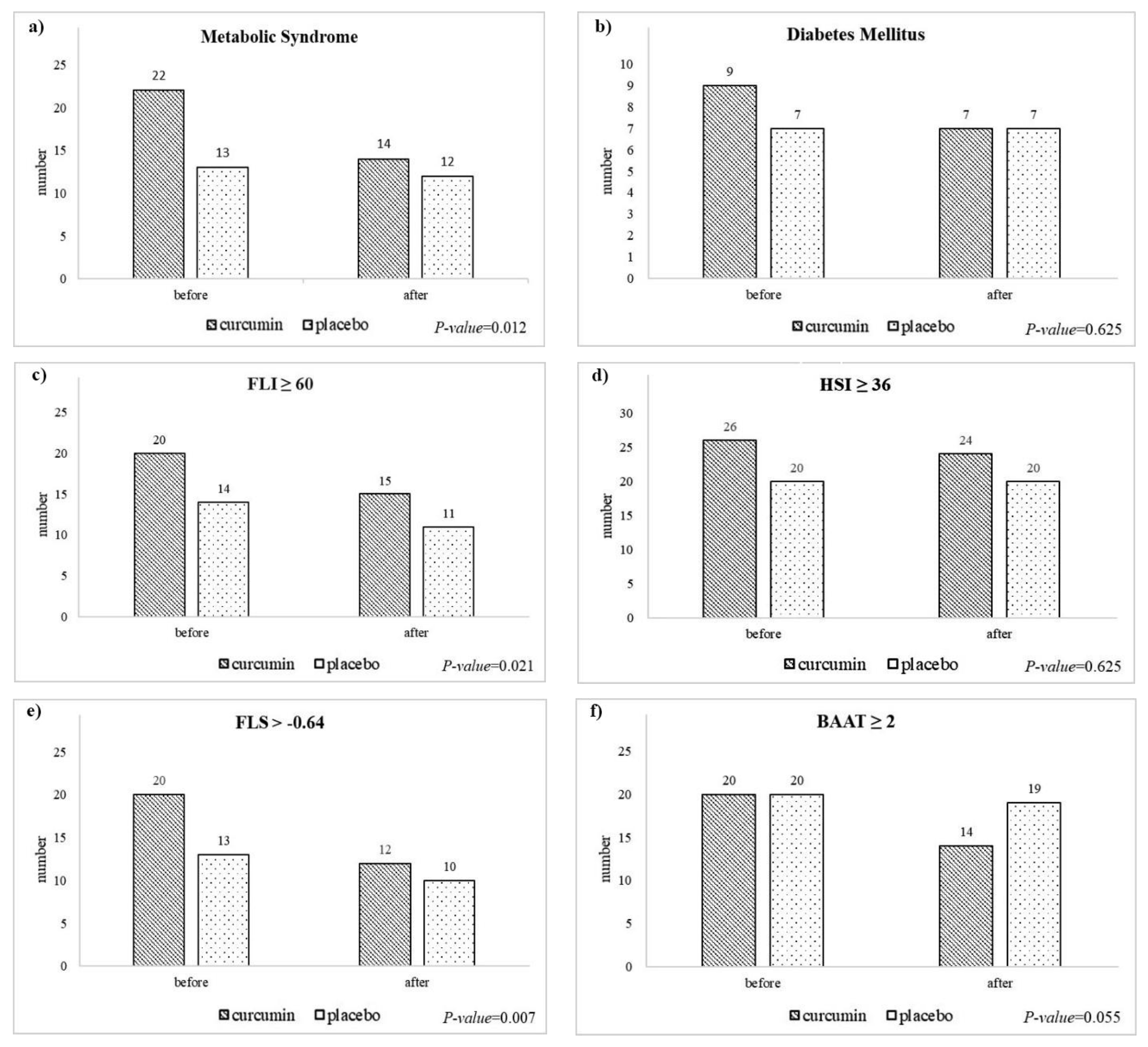
3.5. Changes in the Frequency of Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, X.; Zheng, L.; Wang, M.; Du, Y.; Jiang, J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: A population-based observational study. BMJ Open 2020, 10, e036663. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.C.; Pizzolanti, G.; Torregrossa, V.; Misiano, G.; Milano, S.; Giordano, C. Visceral Adiposity Index (VAI) is predictive of an altered adipokine profile in patients with type 2 diabetes. PLoS ONE 2014, 9, e91969. [Google Scholar] [CrossRef] [Green Version]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.C.; Hung, H.-F.; Lu, C.-W.; Chang, H.-H.; Lee, L.-T.; Huang, K.-C. Association of Non-alcoholic Fatty Liver Disease with Metabolic Syndrome Independently of Central Obesity and Insulin Resistance. Sci. Rep. 2016, 6, 27034. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Dasarathy, S.; Mitchell, M.C.; Barton, B.; McClain, C.J.; Szabo, G.; Nagy, L.E.; Radaeva, S.; McCullough, A.J. Design and rationale of a multicenter defeat alcoholic steatohepatitis trial: (DASH) randomized clinical trial to treat alcohol-associated hepatitis. Contemp. Clin. Trials 2020, 96, 106094. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2021, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xu, L.; Li, J.; Zhao, S. Nonalcoholic fatty liver disease as a potential risk factor of cardiovascular disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 193–199. [Google Scholar] [CrossRef]
- Bang, K.B.; Cho, Y.K. Comorbidities and metabolic derangement of NAFLD. J. Lifestyle Med. 2015, 5, 7–13. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Guaraldi, G.; Nascimbeni, F.; Romagnoli, D.; Zona, S.; Targher, G. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease—Evidence from three different disease models: NAFLD, HCV and HIV. World J. Gastroenterol. 2016, 22, 9674–9693. [Google Scholar] [CrossRef]
- Amato, A.; Caldara, G.-F.; Nuzzo, D.; Baldassano, S.; Picone, P.; Rizzo, M.; Mulè, F.; Di Carlo, M. NAFLD and atherosclerosis are prevented by a natural dietary supplement containing curcumin, silymarin, guggul, chlorogenic acid and inulin in mice fed a high-fat diet. Nutrients 2017, 9, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Verbeek, J.; Tsochatzis, E.A. Non-alcoholic fatty liver disease: Current therapeutic options. Curr. Opin. Pharmacol. 2021, 61, 98–105. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26, 4036. [Google Scholar] [CrossRef]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, A.; Dastani, M.; Zargaran, B.; Ghasemi, A.H.; Rahimi, H.R. Beneficial Effect of curcumin on cardiovascular disease and its risk factors. Rev. Clin. Med. 2019, 6, 12–19. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Banach, M.; Majeed, M.; Sahebkar, A. P5330 Evaluating lipid-lowering and anti-atherogenic effect of injectable curcumin in a rabbit model of atherosclerosis. Eur. Heart J. 2019, 40, ehz746-0299. [Google Scholar] [CrossRef]
- Cesaro, A.; Schiavo, A.; Moscarella, E.; Coletta, S.; Conte, M.; Gragnano, F.; Fimiani, F.; Monda, E.; Caiazza, M.; Limongelli, G.; et al. Lipoprotein(a): A genetic marker for cardiovascular disease and target for emerging therapies. J. Cardiovasc. Med. 2021, 22, 151–161. [Google Scholar] [CrossRef]
- Gragnano, F.; Fimiani, F.; Di Maio, M.; Cesaro, A.; Limongelli, G.; Cattano, D.; Calabrò, P. Impact of lipoprotein(a) levels on recurrent cardiovascular events in patients with premature coronary artery disease. Intern. Emerg. Med. 2019, 14, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, F.; Xiaoqi, C.; YuJun, C.; Zijie, L. Atherogenic index of plasma is an independent risk factor for coronary artery disease and a higher SYNTAX score. Angiology 2020, 72, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; Viveros-Ruiz, T.L.; Almeda-Valdes, P.; Gomez-Velasco, D.; Mehta, R.; Elias-López, D.; Cruz-Bautista, I.; Roldán-Valadez, E.; et al. Metabolic Score for Visceral Fat (METS-VF), a novel estimator of intra-abdominal fat content and cardio-metabolic health. Clin. Nutr. 2020, 39, 1613–1621. [Google Scholar] [CrossRef]
- Ismaiel, A.; Jaaouani, A.; Leucuta, D.-C.; Popa, S.-L.; Dumitrascu, D.L. The Visceral Adiposity Index in Non-Alcoholic Fatty Liver Disease and Liver Fibrosis—Systematic Review and Meta-Analysis. Biomedicines 2021, 9, 1890. [Google Scholar] [CrossRef]
- Cerqueira, M.S.; Santos, C.A.D.; Silva, D.A.S.; Amorim, P.R.D.S.; Marins, J.C.B.; Franceschini, S.D.C.C. Validity of the Body Adiposity Index in Predicting Body Fat in Adults: A Systematic Review. Adv. Nutr. Int. Rev. J. 2018, 9, 617–624. [Google Scholar] [CrossRef]
- Hanlon, C.L.; Yuan, L. Nonalcoholic fatty liver disease: The role of visceral adipose tissue. Clin. Liver Dis. 2022, 19, 106–110. [Google Scholar] [CrossRef]
- Rachakonda, V.; Wills, R.; Delany, J.P.; Kershaw, E.E.; Behari, J. Differential Impact of Weight Loss on Nonalcoholic Fatty Liver Resolution in a North American Cohort with Obesity. Obesity 2017, 25, 1360–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, G.; Xie, Q.; Wang, R.; Hu, C.; Zhong, M.; Zou, Y. Waist-to-height ratio and non-alcoholic fatty liver disease in adults. BMC Gastroenterol. 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.-D.; Chen, Z.-R.; Chen, J.-N.; Lu, Y.-H.; Chen, J. Role of body mass index, waist-to-height and waist-to-hip ratio in prediction of nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2012, 2012, 362147. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zheng, D.; Liu, J.; Fang, L.; Li, Q. Atherogenic index of plasma is a novel predictor of non-alcoholic fatty liver disease in obese participants: A cross-sectional study. Lipids Health Dis. 2018, 17, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, S.; Bhattacharjee, J.; Bhatnagar, M.; Tyagi, S.; Delhi, N. Atherogenic index of plasma, Castelli risk index and atherogenic coefficient-new parameters in assessing cardiovascular risk. Int. J. Pharm. Biol. Sci. 2013, 3, 359–364. [Google Scholar]
- Sujatha, R.; Kavitha, S. Atherogenic indices in stroke patients: A retrospective study. Iran. J. Neurol. 2017, 16, 78–82. [Google Scholar]
- Bedogni, G.; Kahn, H.S.; Bellentani, S.; Tiribelli, C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.; Gitto, S.; Fogacci, F.; Rosticci, M.; Giovannini, M.; D’Addato, S.; Andreone, P.; Borghi, C. Fatty liver index is associated to pulse wave velocity in healthy subjects: Data from the Brisighella Heart Study. Eur. J. Intern. Med. 2018, 53, 29–33. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Panagiotakos, D.B.; Georgousopoulou, E.N.; Chrysohoou, C.; Skoumas, J.; Pan, W.; Tousoulis, D.; Pitsavos, C. The anti-inflammatory potential of diet and nonalcoholic fatty liver disease: The ATTICA study. Ther. Adv. Gastroenterol. 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Saadati, S.; Hekmatdoost, A.; Hatami, B.; Mansour, A.; Zahra, Z.; Hedayati, M.; Sadeghi, A. Comparing different non-invasive methods in assessment of the effects of curcumin on hepatic fibrosis in patients with non-alcoholic fatty liver disease. Gastroenterol. Hepatol. Bed Bench 2018, 11, S8–S13. [Google Scholar] [PubMed]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W. Sex-specific contribution of lipid accumulation product and cardiometabolic index in the identification of nonalcoholic fatty liver disease among Chinese adults. Lipids Health Dis. 2022, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmanesh, M.; Hadaegh, F.; Azizi, F. Diabetes prediction, lipid accumulation product, and adiposity measures; 6-year follow-up: Tehran lipid and glucose study. Lipids Health Dis. 2010, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Motamed, N.; Razmjou, S.; Hemmasi, G.; Maadi, M.; Zamani, F. Lipid accumulation product and metabolic syndrome: A population-based study in northern Iran, Amol. J. Endocrinol. Investig. 2016, 39, 375–382. [Google Scholar] [CrossRef]
- Taverna, M.J.; Martínez-Larrad, M.T.; Frechtel, G.D.; Serrano-Ríos, M. Lipid accumulation product: A powerful marker of metabolic syndrome in healthy population. Eur. J. Endocrinol. 2011, 164, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozorgmanesh, M.; Hadaegh, F.; Azizi, F. Predictive performances of lipid accumulation product vs. adiposity measures for cardiovascular diseases and all-cause mortality, 8.6-year follow-up: Tehran lipid and glucose study. Lipids Health Dis. 2010, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Xia, W.; Zhong, X.; Xu, T.; Li, H.; Zhang, M.; Wang, A.; Sun, Y.; Zhang, Y. Lipid accumulation product and hypertension related to stroke: A 9.2-year prospective study among Mongolians in China. J. Atheroscler. Thromb. 2016, 23, 830–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angoorani, P.; Heshmat, R.; Ejtahed, H.-S.; Motlagh, M.E.; Ziaodini, H.; Taheri, M.; Aminaee, T.; Goodarzi, A.; Qorbani, M.; Kelishadi, R. Validity of triglyceride–glucose index as an indicator for metabolic syndrome in children and adolescents: The CASPIAN-V study. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2018, 23, 877–883. [Google Scholar] [CrossRef]
- Oguntola, S.O.; Hassan, M.O.; Duarte, R.; Dix-Peek, T.; Dickens, C.; Olorunfemi, G.; Vachiat, A.; Paget, G.; Manga, P.; Naicker, S. Atherosclerotic vascular disease and its correlates in stable black South African kidney transplant recipients. Int. J. Nephrol. Renov. Dis. 2018, 11, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Tecer, D.; Sunar, I.; Ozdemirel, A.E.; Tural, R.; Kucuksahin, O.; Dincel, A.S.; Ataman, S. Usefullnes of atherogenic indices and Ca-LDL level to predict subclinical atherosclerosis in patients with psoriatic arthritis? Adv. Rheumatol. 2019, 59, 49. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Zhang, W.; Ming, J.; Jia, A.; Xu, S.; Li, Q.; Ji, Q. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: A nationwide study. J. Diabetes Investig. 2018, 10, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef] [PubMed]
- Akpınar, O.; Bozkurt, A.; Acartürk, E.; Şeydaoğlu, G. A new index (CHOLINDEX) in detecting coronary artery disease risk. Anatol. J. Cardiol. 2013, 13, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Olamoyegun, M.A.; Oluyombo, R.; Asaolu, S.O. Evaluation of dyslipidemia, lipid ratios, and atherogenic index as cardiovascular risk factors among semi-urban dwellers in Nigeria. Ann. Afr. Med. 2016, 15, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.-L.; Wu, W.-C.; Fang, K.-C.; Wang, Y.-C.; Huo, T.-I.; Huang, Y.-H.; Yang, H.-I.; Su, C.-W.; Lin, H.-C.; Lee, F.-Y.; et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS ONE 2015, 10, e0120443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Benjak Horvat, I.; Šuša, L.; Rahelić, D.; Klobučar Majanović, S. Metabolic and Hepatic Effects of Energy-Reduced Anti-Inflammatory Diet in Younger Adults with Obesity. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6649142. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, K.; Chang, J.; Choi, S.; Kim, S.M.; Son, J.S.; Lee, G.; Kim, W.; Park, S.M. Development of a simple nonalcoholic fatty liver disease scoring system indicative of metabolic risks and insulin resistance. Ann. Transl. Med. 2020, 8, 1414. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H. Bonferroni and Šidák corrections for multiple comparisons. Encycl. Meas. Stat. 2007, 3, 103–117. [Google Scholar]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.-M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2020, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Haddad, T.M.; Hamdeh, S.; Kanmanthareddy, A.; Alla, V.M. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2017, 11, S209–S216. [Google Scholar] [CrossRef]
- Carneros, D.; López-Lluch, G.; Bustos, M. Physiopathology of Lifestyle Interventions in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 3472. [Google Scholar] [CrossRef]
- Nourian, M.; Askari, G.; Golshiri, P.; Miraghajani, M.; Shokri, S.; Arab, A. Effect of lifestyle modification education based on health belief model in overweight/obese patients with non-alcoholic fatty liver disease: A parallel randomized controlled clinical trial. Clin. Nutr. ESPEN 2020, 38, 236–241. [Google Scholar] [CrossRef]
- Keating, S.E.; Hackett, D.A.; Parker, H.M.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Baker, M.K.; Chuter, V.H.; Caterson, I.D.; George, J.; et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015, 63, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Sachinwalla, T.; Walton, D.W.; Smith, K.; Armstrong, A.; Thompson, M.W.; George, J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009, 50, 1105–1112. [Google Scholar] [CrossRef]
- Aller, R.; De Luis, D.A.; Izaola, O.; De La Fuente, B.; Bachiller, R. Effect of a high monounsaturated vs. high polyunsaturated fat hypocaloric diets in nonalcoholic fatty liver disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1041–1047. [Google Scholar]
- Qin, Z.; Zhou, K.; Li, Y.; Cheng, W.; Wang, Z.; Wang, J.; Gao, F.; Yang, L.; Xu, Y.; Wu, Y.; et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: Results from an observational cohort study in China. Cardiovasc. Diabetol. 2020, 19, 23. [Google Scholar] [CrossRef]
- Abbasi, F.; Reaven, G.M. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: Triglycerides× glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism 2011, 60, 1673–1676. [Google Scholar] [CrossRef]
- Lambrinoudaki, I.; Kazani, M.V.; Armeni, E.; Georgiopoulos, G.; Tampakis, K.; Rizos, D.; Augoulea, A.; Kaparos, G.; Alexandrou, A.; Stamatelopoulos, K. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2017, 27, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-C.; He, G.-D.; Lo, K.; Huang, Y.-Q.; Feng, Y.-Q. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front. Cardiovasc. Med. 2021, 382, 628109. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Multiple Testing and Protection Against a Type 1 (False Positive) Error Using the Bonferroni and Hochberg Corrections. Indian J. Psychol. Med. 2019, 41, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Kelishadi, M.R.; Bagheri, R.; Moosavian, S.; Wong, A.; Davoodi, S.; Khalili, P.; Dutheil, F.; Suzuki, K.; Asbaghi, O. The Effects of Nano-Curcumin Supplementation on Risk Factors for Cardiovascular Disease: A GRADE-Assessed Systematic Review and Meta-Analysis of Clinical Trials. Antioxidants 2021, 10, 1015. [Google Scholar] [CrossRef]
- de Melo, I.S.V.; Dos Santos, A.F.; Bueno, N.B. Curcumin or combined curcuminoids are effective in lowering the fasting blood glucose concentrations of individuals with dysglycemia: Systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2018, 128, 137–144. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Gh, N.M.; Fatemeh, B. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.N.M.; Abdollahi, F. Effect of phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102322. [Google Scholar] [CrossRef]
- Jalali, M.; Mahmoodi, M.; Mosallanezhad, Z.; Jalali, R.; Imanieh, M.H.; Moosavian, S.P. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102283. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2018, 33, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ngo, C.; Willcox, J.C.; Lappas, M. Anti-inflammatory effects of phenolic acids punicalagin and curcumin in human placenta and adipose tissue. Placenta 2020, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Curcumin Group (n = 27) | Placebo Group (n = 23) | p-Value * | Adjusted p-Value ¥ | |
|---|---|---|---|---|
| Fatty Liver Index (FLI) | ||||
| Baseline | 74.87 ± 17.69 | 74.62 ± 20.87 | 0.9 | >0.999 |
| After 12 weeks | 63.51 ± 26.39 | 66.28 ± 28.24 | 0.7 | >0.999 |
| Differences 95% CI | −11.36 (−18.2, −4.4) | −8.3 (−14.4, −2.2) | 0.5 | >0.999 |
| p-value ⁑ | 0.002 | 0.01 | 0.7 | |
| Hepatic Steatosis Index (HSI) | ||||
| Baseline | 47.49 ± 7.84 | 49.37 ± 8.78 | 0.4 | >0.999 |
| After 12 weeks | 45.06 ± 9.05 | 49.35 ± 17.28 | 0.3 | 0.9 |
| Differences 95% CI | −2.42 (−7.19, 2.3) | −0.1 (−8.9, 8.9) | 0.6 | 0.2 |
| p-value ⁑ | 0.3 | 0.9 | 0.4 ⁋ | |
| Fatty Liver Score (FLS) | ||||
| Baseline | 0.19 ± 1.28 | 0.65 ± 1.66 | 0.3 | 0.9 |
| After 12 weeks | −0.68 ± 1.3 | −0.12 ± 1.6 | 0.2 | 0.6 |
| Differences 95% CI | −0.87 (−1.3, −0.5) | −0.78 (−1.4, −0.13) | 0.8 | >0.999 |
| p-value ⁑ | <0.001 | 0.021 | 0.7 ⁋ | |
| BMI, Age, ALT, TG score (BAAT) | ||||
| Baseline | 2 ± 0.73 | 1.47 ± 1.12 | 0.065 | 0.2 |
| After 12 weeks | 1.63 ± 1.08 | 1.47 ± 0.94 | 0.6 | >0.999 |
| Differences 95% CI | −0.37 (−0.78, 0.04) | 0 (−0.5, 0.5) | 0.2 | 0.7 |
| p-value ⁑ | 0.07 | 1 | 0.9 ⁋ | |
| Curcumin Group (n = 27) | Placebo Group (n = 23) | p-Value * | Adjusted p-Value ¥ | |
|---|---|---|---|---|
| Castelli Risk Index-I (CRI-I) | ||||
| Baseline | 4.59 ± 0.75 | 4.80 ± 0.82 | 0.4 | >0.999 |
| After 12 weeks | 4.62 ± 1.57 | 4.69 ± 0.95 | 0.8 | >0.999 |
| Differences 95% CI | 0.02 (−0.6, 0.6) | −0.11 (−0.47, 0.25) | 0.7 | >0.999 |
| p-value ⁑ | 0.9 | 0.5 | 0.8 ⁋ | |
| Castelli Risk Index-II (CRI-II) | ||||
| Baseline | 2.77 ± 0.72 | 3.06 ± 0.85 | 0.2 | 0.7 |
| After 12 weeks | 2.78 ± 0.96 | 2.86 ± 0.91 | 0.8 | >0.999 |
| Differences 95% CI | 0.01 (−0.38, 0.4) | −0.19 (−0.5, 0.1) | 0.4 | >0.999 |
| p-value ⁑ | 0.9 | 0.2 | 0.4 ⁋ | |
| TG/HDL-C | ||||
| Baseline | 1.79 ± 0.81 | 1.62 ± 0.84 | 0.5 | >0.999 |
| After 12 weeks | 1.90 ± 1.75 | 1.81 ± 0.89 | 0.8 | >0.999 |
| Differences 95% CI | 0.11 (−0.57, 0.79) | 0.19 (−0.07, 0.4) | 0.8 | >0.999 |
| p-value ⁑ | 0.7 | 0.1 | 0.8 ⁋ | |
| TyG index | ||||
| Baseline | 8.9 ± 0.39 | 8.78 ± 0.55 | 0.4 | >0.999 |
| After 12 weeks | 8.73 ± 0.44 | 8.87 ± 0.53 | 0.3 | >0.999 |
| Differences 95% CI | −0.16 (−0.35, 0.02) | 0.09 (−0.05, 0.23) | 0.029 | 0.1 |
| p-value ⁑ | 0.08 | 0.2 | 0.056 ⁋ | |
| Atherogenic Coefficient (AC) | ||||
| Baseline | 3.59 ± 0.75 | 3.8 ± 0.82 | 0.4 | >0.999 |
| After 12 weeks | 3.62 ± 1.57 | 3.69 ± 0.95 | 0.8 | >0.999 |
| Differences 95% CI | 0.02 (−0.6, 0.6) | −0.11 (−0.47, 0.25) | 0.7 | >0.999 |
| p-value ⁑ | 0.9 | 0.5 | 0.8 ⁋ | |
| Atherogenic Index of Plasma (AIP) | ||||
| Baseline | 0.22 ± 0.17 | 0.16 ± 0.21 | 0.3 | 0.9 |
| After 12 weeks | 0.19 ± 0.24 | 0.21 ± 0.2 | 0.7 | >0.999 |
| Differences 95% CI | −0.02 (−0.11, 0.06) | 0.05 (0, 0.1) | 0.2 | 0.6 |
| p-value ⁑ | 0.5 | 0.07 | 0.3 ⁋ | |
| Lipoprotein Combine Index (LCI) | ||||
| Baseline | 25.51 ± 15.42 | 26.1 ± 15.19 | 0.9 | >0.999 |
| After 12 weeks | 20.95 ± 12.92 | 27.43 ± 15.51 | 0.1 | 0.4 |
| Differences 95% CI | −4.56 (−10.7, 1.6) | 1.33 (−5.2, 7.86) | 0.2 | 0.6 |
| p-value ⁑ | 0.1 | 0.6 | 0.2 ⁋ | |
| Cholesterol Index (CHOLINDEX) | ||||
| Baseline | 1.77 ± 0.72 | 2.06 ± 0.85 | 0.2 | 0.7 |
| After 12 weeks | 1.78 ± 0.96 | 1.86 ± 0.91 | 0.8 | >0.999 |
| Differences 95% CI | 0.01 (−0.4, 0.4) | −0.19 (−0.54, 0.15) | 0.4 | >0.999 |
| p-value ⁑ | 0.9 | 0.2 | 0.4 ⁋ | |
| Curcumin Group (n = 27) | Placebo Group (n = 23) | p-Value * | Adjusted p-Value ¥ | |
|---|---|---|---|---|
| Lipid Accumulation Product (LAP) | ||||
| Baseline | 75.01 ± 37.16 | 69.80 ± 35.36 | 0.6 | >0.999 |
| After 12 weeks | 59.79 ± 33.78 | 71.27 ± 39.25 | 0.3 | 0.9 |
| Differences 95% CI | −15.21 (−28.3, −2) | 1.47 (−12.4, 15.3) | 0.07 | 0.2 |
| p-value ⁑ | 0.025 | 0.8 | 0.09 ⁋ | |
| Body adiposity index (BAI) | ||||
| Baseline | 37.15 ± 7.42 | 34.52 ± 7.23 | 0.2 | 0.8 |
| After 12 weeks | 35.53 ± 7.47 | 32.86 ± 7.51 | 0.2 | 0.7 |
| Differences 95% CI | −1.6 (−2.8, −0.4) | −1.5 (−2.89, −0.19) | 0.9 | 0.9 |
| p-value ⁑ | 0.011 | 0.028 | 0.7 ⁋ | |
| visceral adiposity index (VAI) | ||||
| Baseline | 2.93 ± 1.48 | 2.49 ± 1.12 | 0.3 | 0.9 |
| After 12 weeks | 3.14 ± 3.43 | 2.78 ± 1.22 | 0.6 | >0.999 |
| Differences 95% CI | 0.21 (−1.05, 1.48) | 0.28 (−0.09, 0.66) | 0.9 | >0.999 |
| p-value ⁑ | 0.7 | 0.1 | 0.9 ⁋ | |
| METS-VF | ||||
| Baseline | 7.17 ± 0.35 | 7.17 ± 0.41 | 0.9 | >0.999 |
| After 12 weeks | 7.01 ± 0.48 | 7 ± 0.56 | 0.9 | >0.999 |
| Differences 95% CI | −0.15 (−0.23, −0.77) | −0.16 (−0.25, −0.06) | 0.9 | >0.999 |
| p-value | <0.001 | 0.003 | 0.7 ⁋ | |
| Visceral adipose tissue (VAT) (cm3) | ||||
| Baseline | 4764.9 ± 1476 | 4736.15 ± 1372.3 | 0.9 | >0.999 |
| After 12 weeks | 4273.7 ± 1517.9 | 4382.4 ± 1684.5 | 0.8 | >0.999 |
| Differences 95% CI | −491.2 ± 417.8 | −437.2 ± 562.1 | 0.7 | >0.999 |
| p-value ⁑ | <0.001 | 0.004 | 0.8 ⁋ | |
| Waist to height ratio (WHtR) | ||||
| Baseline | 0.63 ± 0.07 | 0.62 ± 0.07 | 0.5 | >0.999 |
| After 12 weeks | 0.59 ± 0.07 | 0.6 ± 0.09 | 0.9 | >0.999 |
| Differences 95% CI | −0.03 (−0.04, −0.02) | −0.02 (−0.04, −0.002) | 0.2 | 0.9 |
| p-value ⁑ | <0.001 | 0.029 | 0.3 ⁋ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseri, K.; Saadati, S.; Yari, Z.; Askari, B.; Mafi, D.; Hoseinian, P.; Asbaghi, O.; Hekmatdoost, A.; de Courten, B. Curcumin Offers No Additional Benefit to Lifestyle Intervention on Cardiometabolic Status in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3224. https://doi.org/10.3390/nu14153224
Naseri K, Saadati S, Yari Z, Askari B, Mafi D, Hoseinian P, Asbaghi O, Hekmatdoost A, de Courten B. Curcumin Offers No Additional Benefit to Lifestyle Intervention on Cardiometabolic Status in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. 2022; 14(15):3224. https://doi.org/10.3390/nu14153224
Chicago/Turabian StyleNaseri, Kaveh, Saeede Saadati, Zahra Yari, Behzad Askari, Davood Mafi, Pooria Hoseinian, Omid Asbaghi, Azita Hekmatdoost, and Barbora de Courten. 2022. "Curcumin Offers No Additional Benefit to Lifestyle Intervention on Cardiometabolic Status in Patients with Non-Alcoholic Fatty Liver Disease" Nutrients 14, no. 15: 3224. https://doi.org/10.3390/nu14153224
APA StyleNaseri, K., Saadati, S., Yari, Z., Askari, B., Mafi, D., Hoseinian, P., Asbaghi, O., Hekmatdoost, A., & de Courten, B. (2022). Curcumin Offers No Additional Benefit to Lifestyle Intervention on Cardiometabolic Status in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients, 14(15), 3224. https://doi.org/10.3390/nu14153224







