Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
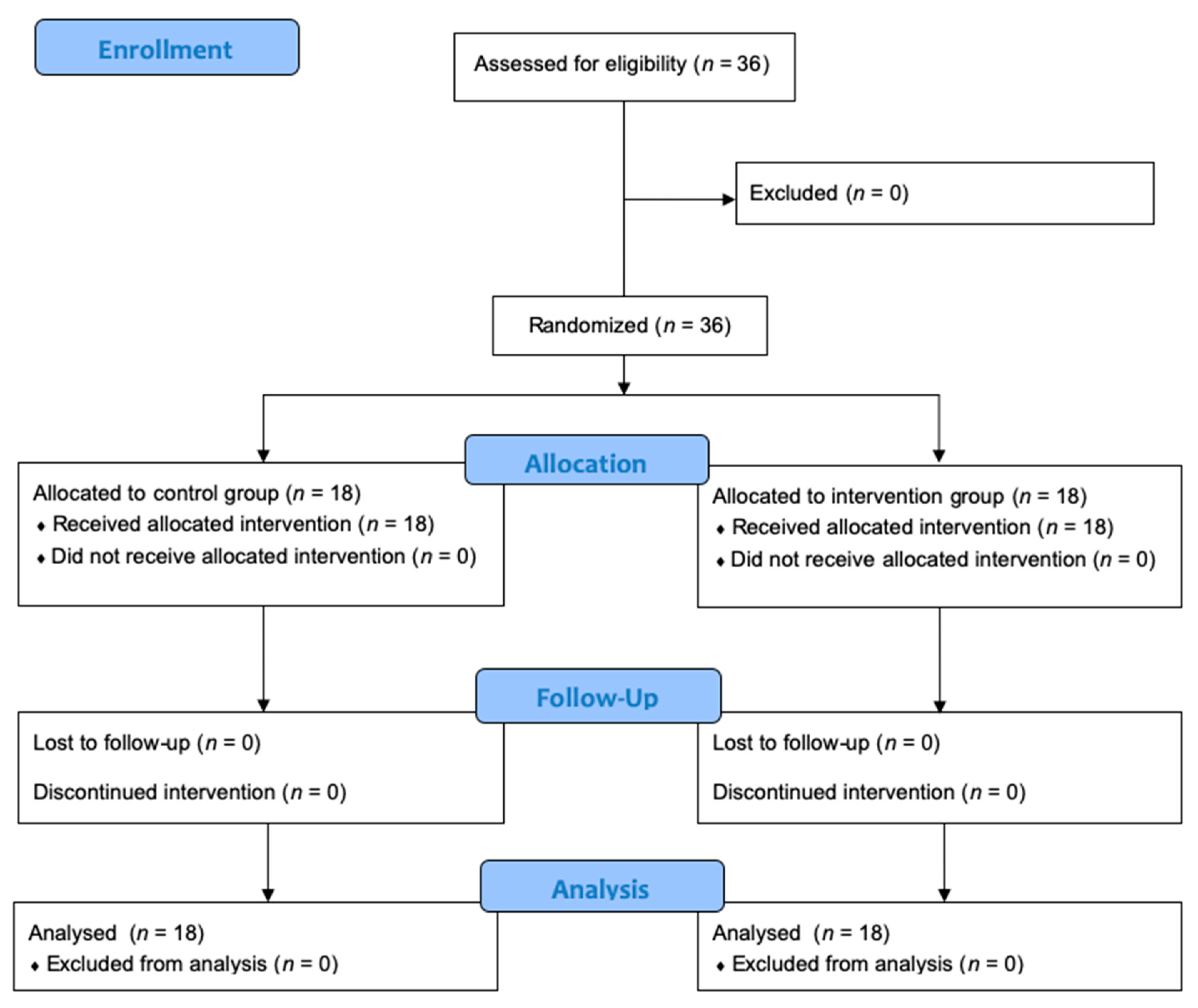
2.2. Study Design
2.3. Aqueous Cinnamon Extract Preparation
2.4. Body Composition and Clinical Assessment
2.5. Dietary Intake Assessment
2.6. Blood Glucose Level Analysis
2.7. Characterization of Antioxidant Capacity
2.7.1. Chemical Analysis Reagents
2.7.2. Total Phenolic Content Determination
2.7.3. Antioxidant Activity Assay
2.8. Statistical Analysis
3. Results
3.1. Baseline Sample Characterization
3.2. Postprandial Blood Glucose Levels
3.3. Antioxidant Activity of Aqueous Cinnamon Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Jamali, N.; Jalali, M.; Saffari-chaleshtori, J. Effect of cinnamon supplementation on blood pressure and anthropometric parameters in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 119–125. [Google Scholar] [CrossRef]
- Davari, M.; Hashemi, R.; Mirmiran, P.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and Sirtuin-1 (SIRT1) in type 2 diabetes: A randomized, double blind, and controlled clinical trial. Nutr. J. 2020, 19, 1. [Google Scholar] [CrossRef]
- Talaei, B.; Amouzegar, A.; Sahranavard, S.; Hedayati, M.; Mirmiran, P.; Azizi, F. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients 2017, 9, 991. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Behl, S. Treating Endocrine and Metabolic Disorders with Herbal Medicines; IGI Global: Hershey, PA, USA, 2020; ISBN 1799848094. [Google Scholar]
- Crawford, P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: A randomized, controlled trial. J. Am. Board Fam. Med. 2009, 22, 507–512. [Google Scholar] [CrossRef]
- Gruenwald, J.; Freder, J.; Armbruester, N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2010, 50, 822–834. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Babu, K.N.; Shylaja, M. Cinnamon and Cassia: The Genus Cinnamonum; CRC Press: Boca Raton, FL, USA, 2004; ISBN 041531755X. [Google Scholar]
- Silva, M.L.; Bernardo, M.A.; Singh, J.; Mesquita, M.F. Beneficial Uses of Cinnamon in Health and Diseases: An Interdisciplinary Approach. In The Role of Functional Food Security in Global Health; Academic Press: Cambridge, MA, USA, 2019; pp. 565–576. ISBN 9780128131480. [Google Scholar]
- Bernardo, M.A.; Silva, M.L. Effect of cinnamon on postprandial glucose concentration. J. Diabetes Res. 2015, 564, 56–89. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, M.A.; Amaral, C.; Moncada, M.M.; Silva, M.L.; De Mesquita, M.F. Effect of Cinnamon Addition to an High-Sugar Meal on the Postprandial Blood Glucose Response of Healthy Subjects. Int. J. Clin. Res. Trials 2017, 2, 113. [Google Scholar] [CrossRef]
- Moncada, M.M.; Bernardo, M.A.; Silva, M.L.; Jorge, A.; Pereira, P.; Brito, J.; Singh, J.; Mesquita, M.F. Effect of cinnamon powder addition to a Portuguese custard tart (Pastel de Nata) on healthy adults’ postprandial glycemia. World Heart J. 2017, 9, 135–144. [Google Scholar]
- Soni, R.; Bhatnagar, V. Effect of Cinnamon (Cinnamomum Cassia) intervention on Blood Glucose of Middle Aged Adult Male with Non Insulin Dependent Diabetes Mellitus (NIDDM). Ethno-Medicine 2009, 3, 141–144. [Google Scholar] [CrossRef]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Sengsuk, C.; Sanguanwong, S.; Tangvarasittichai, O.; Tangvarasittichai, S. Effect of cinnamon supplementation on glucose, lipids levels, glomerular filtration rate, and blood pressure of subjects with type 2 diabetes mellitus. Diabetol. Int. 2016, 7, 124–132. [Google Scholar] [CrossRef]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef] [Green Version]
- Mang, B.; Wolters, M.; Schmitt, B.; Kelb, K.; Lichtinghagen, R.; Stichtenoth, D.O.; Hahn, A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur. J. Clin. Investig. 2006, 36, 340–344. [Google Scholar] [CrossRef]
- Sahib, A.S. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J. Intercult. Ethnopharmacol. 2013, 5, 108–113. [Google Scholar] [CrossRef]
- Namazi, N.; Khodamoradi, K.; Khamechi, S.P.; Heshmati, J.; Ayati, M.H.; Larijani, B. The impact of cinnamon on anthropometric indices and glycemic status in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2019, 43, 92–101. [Google Scholar] [CrossRef]
- ADA Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [CrossRef] [Green Version]
- Shen, Y.; Fukushima, M.; Ito, Y.; Muraki, E.; Hosono, T.; Seki, T.; Ariga, T. Verification of the Antidiabetic Effects of Cinnamon (Cinnamomum zeylanicum) Using Insulin-Uncontrolled Type 1 Diabetic Rats and Cultured Adipocytes. Biosci. Biotechnol. Biochem. 2010, 74, 2418–2425. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.; Almeida, M.; Pinho, O. Manual de Quantificação de Alimentos; Faculdade de Ciências da Nutrição e Alimentação da Universidade do Porto: Porto, Portugal, 1996; ISBN 972-96840-0-6. [Google Scholar]
- Prabha, M.; Vasantha, K. Antioxidant, Cytotoxicity and Polyphenolic Content of Calotropis procera (Ait.) R. Br. Flowers. J. Appl. Pharm. Sci. 2011, 1, 136–140. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points; StatPearls: Treasure Island, FL, USA, 2021; pp. 1–6. [Google Scholar]
- Vanschoonbeek, K.; Thomassen, B.J.W.; Senden, J.M.; Wodzig, W.K.W.H.; Van Loon, L.J.C. Cinnamon Supplementation Does Not Improve Glycemic Control in Postmenopausal Type 2 Diabetes Patients. J. Nutr. 2006, 136, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blevins, S.; Leyva, M.; Brown, J.; Wright, J.; Scofield, R.; Aston, C. Effect of Cinnamon on Glucose and Lipid Levels in Non–Insulin-Dependent Type 2 Diabetes. Diabetes Care 2007, 30, 2236–2237. [Google Scholar] [CrossRef] [Green Version]
- Hasanzade, F.; Toliat, M.; Emami, S.A.; Emamimoghaadam, Z. The Effect of Cinnamon on Glucose of Type II Diabetes Patients. J. Tradt. Complement. Med. 2013, 3, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Hoehn, A.N.; Stockert, A.L. The Effects of Cinnamomum Cassia on Blood Glucose Values are Greater than those of Dietary Changes Alone. Nutr. Metab. Insights 2012, 5, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Hayward, N.J.; McDougall, G.J.; Farag, S.; Allwood, J.W.; Austin, C.; Campbell, F.; Horgan, G.; Ranawana, V. Cinnamon Shows Antidiabetic Properties that Are Species-Specific: Effects on Enzyme Activity Inhibition and Starch Digestion. Plant Foods Hum. Nutr. 2019, 74, 544–552. [Google Scholar] [CrossRef] [Green Version]
- Wickenberg, J.; Lindstedt, S.; Berntorp, K.; Nilsson, J.; Hlebowicz, J. Ceylon cinnamon does not affect postprandial plasma glucose or insulin in subjects with impaired glucose tolerance. Br. J. Nutr. 2012, 107, 1845–1849. [Google Scholar] [CrossRef]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Ann, M.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Graves, D.J.; Anderson, R.A. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomed. Int. J. Phyther. Phytopharm. 2010, 17, 1027–1032. [Google Scholar] [CrossRef]
- Anand, P.; Murali, K.Y.; Tandon, V.; Murthy, P.S.; Chandra, R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, L.; Fan, S.; Wang, J.; Luo, T.; Tang, Y.; Chen, Z.; Yu, L. Cinnamomum cassia Presl: A review of its traditional uses, phytochemistry, pharmacology and toxicology. Molecules 2019, 24, 3473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.; Yin, J.-J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Avula, B.; Nanayakkara, N.; Zhao, J. Cassia Cinnamon as a Source of Coumarin in Cinnamon-Flavored Food and Food Supplements in the United States. J. Agric. Food Chem. 2013, 61, 4470–4476. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Cho, E.J.; Park, C.H.; Kim, J.H. Protective Effect of Proanthocyanidin against Diabetic Oxidative Stress. Evid.-Based Complement Altern. Med. 2012, 2012, 623879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Fitoterapia Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [Google Scholar] [CrossRef]
- Cao, H.; Polansky, M.M.; Anderson, R.A. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2007, 459, 214–222. [Google Scholar] [CrossRef]
- Geng, S.; Cui, Z.; Huang, X.; Chen, Y.; Xu, D.; Xiong, P. Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind. Crops Prod. 2011, 33, 248–252. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Zhu, C.; Yan, H.; Zheng, Y.; Santos, H.O.; Macit, M.S.; Zhao, K. Impact of Cinnamon Supplementation on cardiometabolic Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 53, 102517. [Google Scholar] [CrossRef]
- Roussel, A.-M.; Hininger, I.; Benaraba, R.; Ziegenfuss, T.N.; Anderson, R.A. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J. Am. Coll. Nutr. 2009, 28, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Intervention Group Mean ± SEM | Control Group Mean ± SEM | p-Value |
|---|---|---|---|
| Age, years | 63.5 ± 1.6 | 62.06 ± 2.4 | 0.625 1 |
| Body weight, kg | 84.3 ± 3.1 | 79.8 ± 3.6 | 0.319 2 |
| Height, m | 1.6 ± 0.02 | 1.6 ± 0.02 | 0.824 2 |
| Waist circumference, cm | 110.2 ± 1.7 | 105.3 ± 2.5 | 0.051 2 |
| Body mass index, kg/m2 | 32.5 ± 1.0 | 31.0 ± 1.3 | 0.223 2 |
| Body fat mass, % | 38.8 ± 1.3 | 37.7 ± 1.9 | 0.354 1 |
| Skeletal muscle mass, kg | 48.3 ± 1.9 | 46.9 ± 2.0 | 0.725 1 |
| Visceral fat, cm3 | 15.0 ± 1.4 | 13.2 ± 0.9 | 0.657 2 |
| Pharmacological therapy | n (%) | n (%) | |
| Biguanide | 14 (77.8) | 16 (88.9) | |
| Sulfonylurea | 7 (38.9) | 7 (38.9) | |
| Alpha-glucosidase inhibitor | 0 | 1 (5.6) | |
| Dipeptidyl peptidase 4 (DPP4) inhibitors | 2 (11.1) | 0 | |
| Biguanide + Dipeptidyl peptidase 4 (DPP4) inhibitors | 2 (11.1) | 7 (38.9) |
| Dietary Parameters | Intervention Group Mean ± SEM | Control Group Mean ± SEM | p-Value |
|---|---|---|---|
| Total energy, Kcal | 2090.24 ± 223.28 | 2340.46 ± 249.09 | 0.467 2 |
| Total protein, g | 88.56 ± 12.52 | 104.53 ± 11.59 | 0.174 2 |
| Total fat, g | 52.67 ± 6.50 | 50.39 ± 7.64 | 0.613 2 |
| Total carbohydrate, g | 302.43 ± 30.99 | 356.06 ± 41.40 | 0.383 1 |
| Dietary fiber, g | 34.44 ± 4.72 | 44.78 ± 6.14 | 0.192 1 |
| Soluble fiber, g | 2.55 (± 0.71) | 1.14 ± 0.27 | 0.383 2 |
| Glycemic index | 48.10 ± 4.60 | 43.68 ± 2.23 | 0.939 2 |
| Glycemic load | 19.00 ± 6.07 | 10.09 ± 2.46 | 0.544 2 |
| Time | Intervention Group Mean ± SEM (mmol/L) | Control Group Mean ± SEM (mmol/L) |
|---|---|---|
| t0 | 8.10 ± 0.75 | 7.02 ± 0.45 |
| t30 | 14.27 ± 0.98 | 12.92 ± 0.75 |
| t60 | 16.51 ± 1.02 | 16.07 ± 1.05 |
| t90 | 17.39 ± 1.30 | 16.30 ± 1.21 |
| t120 | 15.27 ± 1.38 | 14.49 ± 1.17 |
| Parameters | Intervention Group Mean ± SEM | Control Group Mean ± SEM | p-Value |
|---|---|---|---|
| AUCi0–120min (mmol/L) | 781.60 ± 53.81 | 798.45 ± 58.70 | 0.834 1 |
| Cmax (mmol/L) | 18.19 ± 1.16 | 17.21 ± 0.99 | 0.527 1 |
| ∆Cmax | 10.09 ± 0.72 | 10.25 ± 0.67 | 0.873 1 |
| Chemical Parameters | Aqueous Cinnamon Extract |
|---|---|
| Total Phenols (n = 3), mg/L Equivalent of Gallic acid 1 | 1554.9 ± 72.8 |
| Antioxidant capacity—FRAP assay (n = 4), µmol Trolox/L 2 | 3658.8 ± 16.7 |
| Antioxidant capacity—DPPH assay (n = 6), µmol Trolox/L 3 | 5125.0 ± 74.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachid, A.P.; Moncada, M.; Mesquita, M.F.d.; Brito, J.; Bernardo, M.A.; Silva, M.L. Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Nutrients 2022, 14, 1576. https://doi.org/10.3390/nu14081576
Rachid AP, Moncada M, Mesquita MFd, Brito J, Bernardo MA, Silva ML. Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Nutrients. 2022; 14(8):1576. https://doi.org/10.3390/nu14081576
Chicago/Turabian StyleRachid, Ana Paula, Margarida Moncada, Maria Fernanda de Mesquita, José Brito, Maria Alexandra Bernardo, and Maria Leonor Silva. 2022. "Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial" Nutrients 14, no. 8: 1576. https://doi.org/10.3390/nu14081576
APA StyleRachid, A. P., Moncada, M., Mesquita, M. F. d., Brito, J., Bernardo, M. A., & Silva, M. L. (2022). Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Nutrients, 14(8), 1576. https://doi.org/10.3390/nu14081576








