Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of Vitamin D3 According to the “Coimbra Protocol”
Abstract
1. Introduction
2. Patients and Methods
- To drink at least 2.5 liters of fluid per day (with a calcium level less than 200 mg/L). In the case of fever, profuse sweating (for whatever reason) or gastrointestinal infection, this amount must be increased or, in the event of illness, supplemented with infusions if necessary. This volume must be maintained even during long-haul flights, longer car trips, bus and train journeys, etc.
- To avoid foods rich in calcium (maximum intake ca. 500 mg per day). A low-calcium diet, which avoids milk and dairy products (some butter is allowed), food and dietary supplements with added calcium, is necessary in order to prevent excessive absorption of calcium from the intestine that could cause hypercalciuria and hypercalcemia as an automatic consequence of a high-dose vitamin D therapy and to protect renal function. In addition, patients were told not to eat peanuts, walnuts, almonds, chestnuts, cashews, nut-granola bars, pistachios, hazelnuts, etc., and dried fruits with seeds as well as sesame paste (tahini), hummus, baba ghanoush, sardines and anchovies. They were also recommended to avoid the consumption of green smoothies (with a high concentration of dark green vegetables, such as kale and spinach). Calcium supplementation was not allowed.
- To regularly attend visits for laboratory investigations.
3. Results
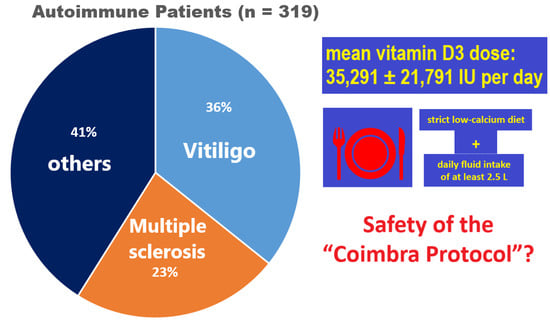
3.1. Population
3.1.1. Sex, Age, Diagnosis
3.1.2. Vitamin D and Parathormone
3.2. Safety Data during the Treatment
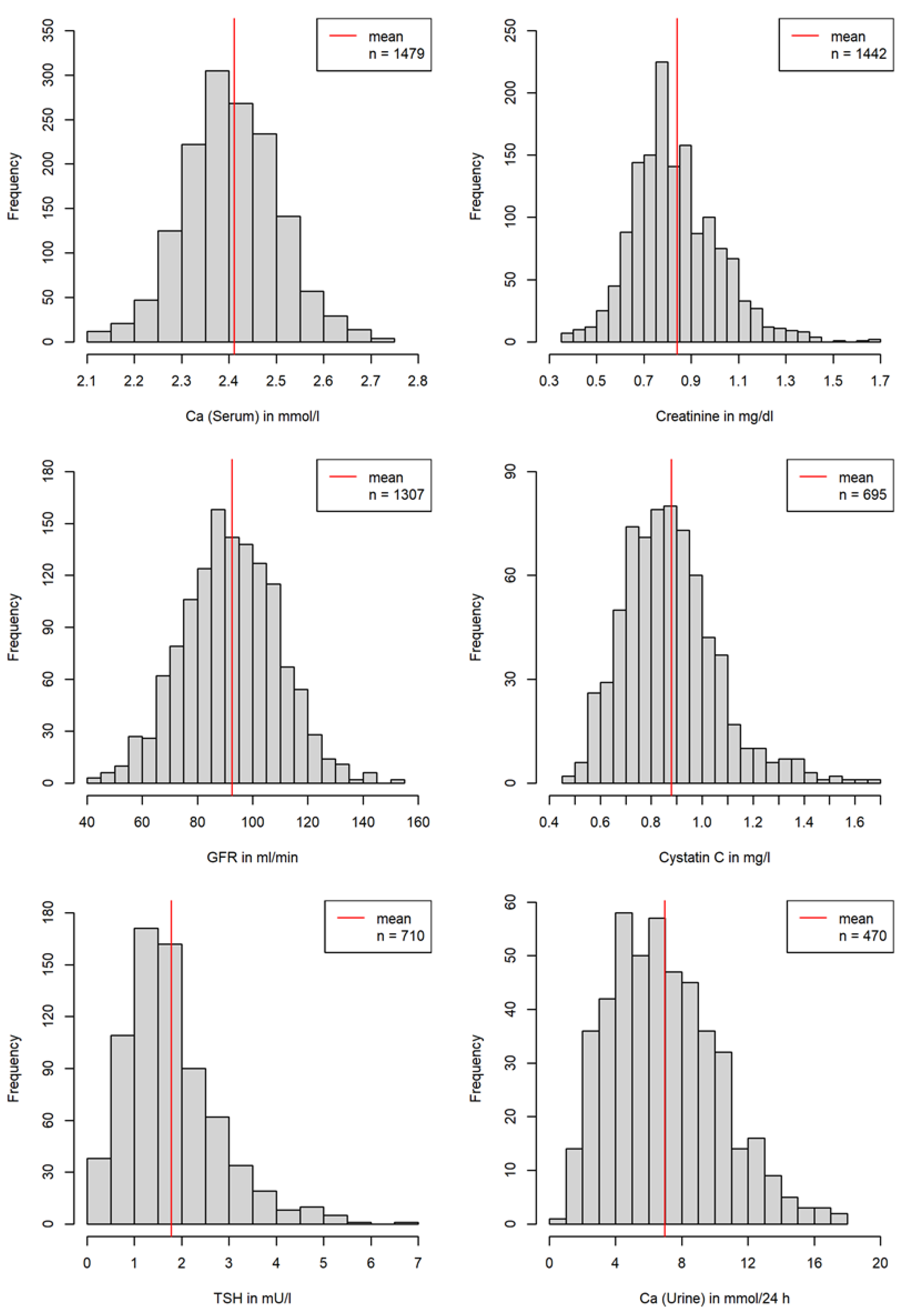
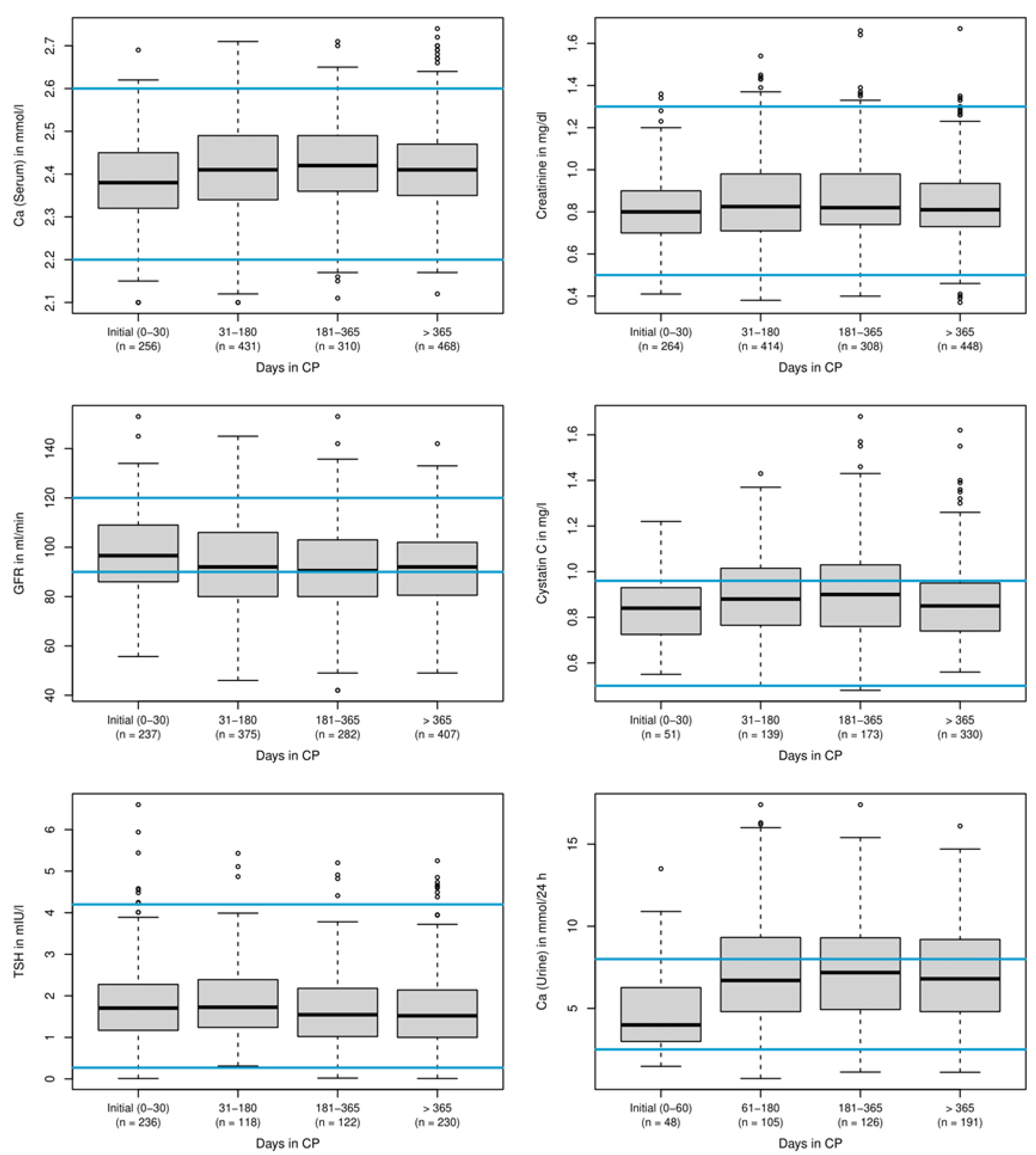
3.2.1. Laboratory Values
3.2.2. Discontinuation of Treatment
3.3. Parathormone Levels and Vitamin D3 Dosage during Treatment
3.4. Single Nucleotide Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, G.S.; Stroehla, B.C. The Epidemiology of Autoimmune Diseases. Autoimmun. Rev. 2003, 2, 119–125. [Google Scholar] [CrossRef]
- Rose, N.R. Prediction and Prevention of Autoimmune Disease in the 21st Century: A Review and Preview. Am. J. Epidemiol. 2016, 183, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.D.; Gratz, I.K.; Paw, J.S.; Abbas, A.K. Treating Human Autoimmunity: Current Practice and Future Prospects. Sci. Transl. Med. 2012, 4, 125sr1. [Google Scholar] [CrossRef] [PubMed]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western Diet” in Inflammatory Autoimmune Diseases. Curr. Allergy Asthma Rep. 2013, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Pollard, K.M.; Campbell, A.W. Environmental Triggers and Autoimmunity. Autoimmune Dis. 2014, 2014, 798029. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T Cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M. Vitamin D–Effects on Skeletal and Extraskeletal Health and the Need for Supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. The Role of the Parent Compound Vitamin D with Respect to Metabolism and Function: Why Clinical Dose Intervals Can Affect Clinical Outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef]
- Amon, U.; Baier, L.; Yaguboglu, R.; Ennis, M.; Holick, M.F.; Amon, J. Serum 25-Hydroxyvitamin D Levels in Patients with Skin Diseases Including Psoriasis, Infections, and Atopic Dermatitis. Dermato-Endocrinology 2018, 10, e1442159. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Bouillon, R. Vitamin D Nutritional Policy Needs a Vision for the Future. Exp. Biol. Med. 2010, 235, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 Positivity Rates Associated with Circulating 25-Hydroxyvitamin D Levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.A.; Dumas, K.J.; Bhaumik, D.; Schilling, B.; Davis, S.; Oron, T.; Sorensen, D.J.; Lucanic, M.; Brem, R.B.; Melov, S.; et al. Vitamin D Promotes Protein Homeostasis and Longevity via the Stress Response Pathway Genes Skn-1, Ire-1, and Xbp-1. Cell Rep. 2016, 17, 1227–1237. [Google Scholar] [CrossRef]
- Caristia, S.; Filigheddu, N.; Barone-Adesi, F.; Sarro, A.; Testa, T.; Magnani, C.; Aimaretti, G.; Faggiano, F.; Marzullo, P. Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients 2019, 11, 2384. [Google Scholar] [CrossRef]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.M.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A Pilot Study Assessing the Effect of Prolonged Administration of High Daily Doses of Vitamin D on the Clinical Course of Vitiligo and Psoriasis. Dermato-Endocrinology 2013, 5, 222–234. [Google Scholar] [CrossRef]
- Available online: https://www.coimbraprotocol.com/the-protocol-1 (accessed on 19 February 2022).
- Available online: https://coimbraprotokoll.de/coimbra/ (accessed on 19 February 2022).
- Lemke, D.; Klement, R.J.; Schweiger, F.; Schweiger, B.; Spitz, J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front. Immunol. 2021, 12, 655739. [Google Scholar] [CrossRef]
- Available online: https://coimbraprotokoll.de/coimbraprotokoll_aerzte/ (accessed on 19 February 2022).
- Charlon, T.; Martínez-Bueno, M.; Bossini-Castillo, L.; Carmona, F.D.; Di Cara, A.; Wojcik, J.; Voloshynovskiy, S.; Martín, J.; Alarcón-Riquelme, M.E. Single Nucleotide Polymorphism Clustering in Systemic Autoimmune Diseases. PLoS ONE 2016, 11, e0160270. [Google Scholar] [CrossRef]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef]
- Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 19 February 2022).
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Whang, R. Magnesium Deficiency: Pathogenesis, Prevalence, and Clinical Implications. Am. J. Med. 1987, 82, 24–29. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin Effects on the Immune System: Vitamins a and D Take Centre Stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.D.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef] [PubMed]
- Hufnagl, K.; Jensen-Jarolim, E. Vitamin a and D in Allergy: From Experimental Animal Models and Cellular Studies to Human Disease. Allergo J. Int. 2018, 27, 72–78. [Google Scholar] [CrossRef]
- Gröber, U. Vitamin a (Retinol). Z. Für Orthomol. Med. 2019, 17, 44–49. [Google Scholar] [CrossRef]
- Reza Dorosty-Motlagh, A.; Mohammadzadeh Honarvar, N.; Sedighiyan, M.; Abdolahi, M. The Molecular Mechanisms of Vitamin a Deficiency in Multiple Sclerosis. J. Mol. Neurosci. 2016, 60, 82–90. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Stoney, P.N.; McCaffery, P.J. The Evidence for a Beneficial Role of Vitamin a in Multiple Sclerosis. CNS Drugs 2014, 28, 291–299. [Google Scholar] [CrossRef]
- Gerster, H. Vitamin A--functions, dietary requirements and safety in humans. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. De Vitaminol. Et De Nutr. 1997, 67, 71–90. [Google Scholar]
- Rheaume-Bleue, K. Vitamin K2 and the Calcium Paradox: How a Little-Known Vitamin Could Save Your Life; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Beulens, J.W.J.; Booth, S.L.; van den Heuvel, E.G.H.M.; Stoecklin, E.; Baka, A.; Vermeer, C. The Role of Menaquinones (Vitamin K2) in Human Health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef]
- Epstein, M. Matrix Gla-Protein (MGP) Not Only Inhibits Calcification in Large Arteries but Also May Be Renoprotective: Connecting the Dots. EBioMedicine 2016, 4, 16–17. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Wang, H.; Rosenberg, P.A. Vitamin K Prevents Oxidative Cell Death by Inhibiting Activation of 12-Lipoxygenase in Developing Oligodendrocytes. J. Neurosci. Res. 2009, 87, 1997–2005. [Google Scholar] [CrossRef]
- Lasemi, R.; Kundi, M.; Moghadam, N.B.; Moshammer, H.; Hainfellner, J.A. Vitamin K2 in Multiple Sclerosis Patients. Wien. Klin. Wochenschr. 2018, 130, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin-Induced Artery Calcification Is Accelerated by Growth and Vitamin D. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Scazzone, C.; Agnello, L.; Bivona, G.; Lo Sasso, B.; Ciaccio, M. Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem. Genet. 2020, 59, 1–30. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs2060793 (accessed on 19 February 2022).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs703842 (accessed on 19 February 2022).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs2296241 (accessed on 19 February 2022).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs7041 (accessed on 19 February 2022).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs1155563 (accessed on 19 February 2022).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs4588 (accessed on 19 February 2022).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs1544410 (accessed on 19 February 2022).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs731236 (accessed on 19 February 2022).
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D Status and Ill Health: A Systematic Review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer. Res. 2016, 36, 345–1356. [Google Scholar]
- Holick, M.F. Can You Have Your Cake and Eat It Too? The Sunlight D-Lema. Br. J. Dermatol. 2016, 175, 1129–1131. [Google Scholar] [CrossRef]
- Mokry, L.E.; Ross, S.; Ahmad, O.S.; Forgetta, V.; Smith, G.D.; Leong, A.; Greenwood, C.M.T.; Thanassoulis, G.; Richards, J.B. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLOS Med. 2015, 12, e1001866. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Vollmer, T.; Lee, H. Reliability and Validity of Two Self-Report Measures of Impairment and Disability for MS. Neurology 1999, 52, 63. [Google Scholar] [CrossRef]
- Available online: https://www.nationalmssociety.org/Treating-MS/Complementary-Alternative-Medicines (accessed on 20 February 2022).
- Available online: https://www.facebook.com/coimbraprotocol.vitamind (accessed on 20 February 2022).
- Available online: https://www.coimbraprotocol.com/testimonials-1 (accessed on 20 February 2022).
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-Calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, P.; Pham, T.-M.; Ekwaru, J. Optimal Vitamin D Supplementation Doses That Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population. Nutrients 2015, 7, 10189–10208. [Google Scholar] [CrossRef] [PubMed]
- Lugg, S.T.; Howells, P.A.; Thickett, D.R. Optimal Vitamin D Supplementation Levels for Cardiovascular Disease Protection. Disease Markers 2015, 2015, 864370. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Carlberg, C.; Haq, A. The Concept of the Personal Vitamin D Response Index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of Vitamin D Status and Vitamin D3 Supplementation on Genome Wide Expression of White Blood Cells: A Randomized Double-Blind Clinical Trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef]
- Mendes, M.M.; Hart, K.H.; Lanham-New, S.A.; Botelho, P.B. Suppression of Parathyroid Hormone as a Proxy for Optimal Vitamin D Status: Further Analysis of Two Parallel Studies in Opposite Latitudes. Nutrients 2020, 12, 942. [Google Scholar] [CrossRef]
- Pani, M.; Regulla, K.; Segni, M.; Krause, M.; Hofmann, S.; Hufner, M.; Herwig, J.; Pasquino, A.; Usadel, K.; Badenhoop, K. Vitamin D 1alpha-Hydroxylase (CYP1alpha) Polymorphism in Graves’ Disease, Hashimoto’s Thyroiditis and Type 1 Diabetes Mellitus. Eur. J. Endocrinol. 2002, 146, 777–781. [Google Scholar] [CrossRef]
- Sundqvist, E.; Bäärnhielm, M.; Alfredsson, L.; Hillert, J.; Olsson, T.; Kockum, I. Confirmation of Association between Multiple Sclerosis and CYP27B1. Eur. J. Hum. Genet. 2010, 18, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily Oral Dosing of Vitamin D3 Using 5000 to 50,000 International Units a Day in Long-Term Hospitalized Patients: Insights from a Seven Year Experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Song, Y.; Manson, J.E.; Signorello, L.B.; Zhang, S.M.; Shrubsole, M.J.; Ness, R.M.; Seidner, D.L.; Dai, Q. Magnesium, Vitamin D Status and Mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, C.G.; (University of São Paulo, São Paulo, Brazil). Personal Communication. 2018. Recording of the lecture at the Congress of Human Medicine, Frankfurt, 14 April 2018. Available online: https://www.youtube.com/watch?v=w1XT0btvVSg (accessed on 31 January 2022).
- Krafka, J., Jr. Simple Treatment for Psoriasis. J. Lab. Clin. Med. 1936, 21, 1147–1148. [Google Scholar]
- Relhan, V.; Goel, K.; Kochhar, A.; Garg, V.; Wadhwa, B. Vitamin D and Skin Diseases: A Review. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 344. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D. Dermato-Endocrinology 2013, 5, 331–347. [Google Scholar] [CrossRef]
- Perez, A.; Raab, R.; Chen, T.C.; Turner, A.; Holick, M.F. Safety and Efficacy of Oral Calcitriol (1, 25 -Dihydroxyvitamin D3) for the Treatment of Psoriasis. Br. J. Dermatol. 1996, 134, 1070–1078. [Google Scholar] [CrossRef]
- Morimoto, S.; Yoshikawa, K.; Kozoka, T.; Kitano, Y.; Imanaka, S.; Fukuo, K.; Koh, E.; Kumahara, Y. An Open Study of Vitamin D3 Treatment in Psoriasis Vulgaris. Br. J. Dermatol. 1986, 115, 421–429. [Google Scholar] [CrossRef]
- Smith, E.L.; Pincus, S.H.; Donovan, L.; Holick, M.F. A Novel Approach for the Evaluation and Treatment of Psoriasis. J. Am. Acad. Dermatol. 1988, 19, 516–528. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Sanguankeo, A. Low 25-Hydroxyvitamin D Levels Are Associated with Vitiligo: A Systematic Review and Meta-Analysis. Photodermatol. Photoimmunol. Photomed. 2016, 32, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Z.; Wang, M.; Ding, Y.; Gao, F.; Feng, Y.-Y.; Yakeya, B.; Wang, P.; Wu, X.-J.; Hu, F.-X.; Xian, J.; et al. Vitamin D Receptor Gene Polymorphism, Serum 25-Hydroxyvitamin D Levels, and Risk of Vitiligo. Medicine 2018, 97, e11506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-Wide Association Analysis Identifies Variation in Vitamin D Receptor and Other Host Factors Influencing the Gut Microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef]
- Sipos, M.; Gerszi, D.; Dalloul, H.; Bányai, B.; Sziva, R.E.; Kollarics, R.; Magyar, P.; Török, M.; Ács, N.; Szekeres, M.; et al. Vitamin D Deficiency and Gender Alter Vasoconstrictor and Vasodilator Reactivity in Rat Carotid Artery. Int. J. Mol. Sci. 2021, 22, 8029. [Google Scholar] [CrossRef]
- Rogers, M.P.; Fozdar, M. Psychoneuroimmunology of autoimmune disorders. Adv. Neuroimmunol. 1996, 6, 169–177. [Google Scholar] [CrossRef]
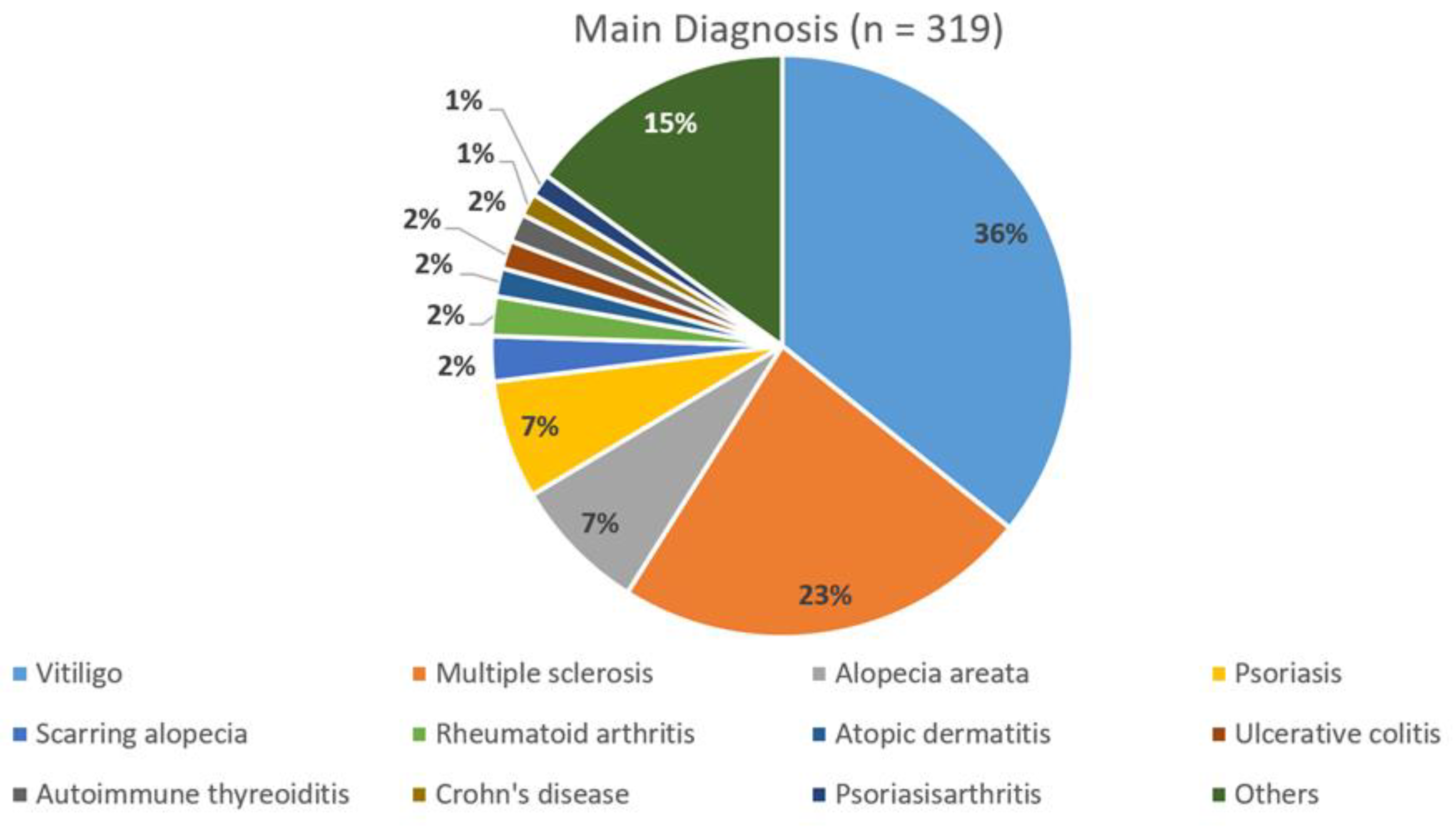
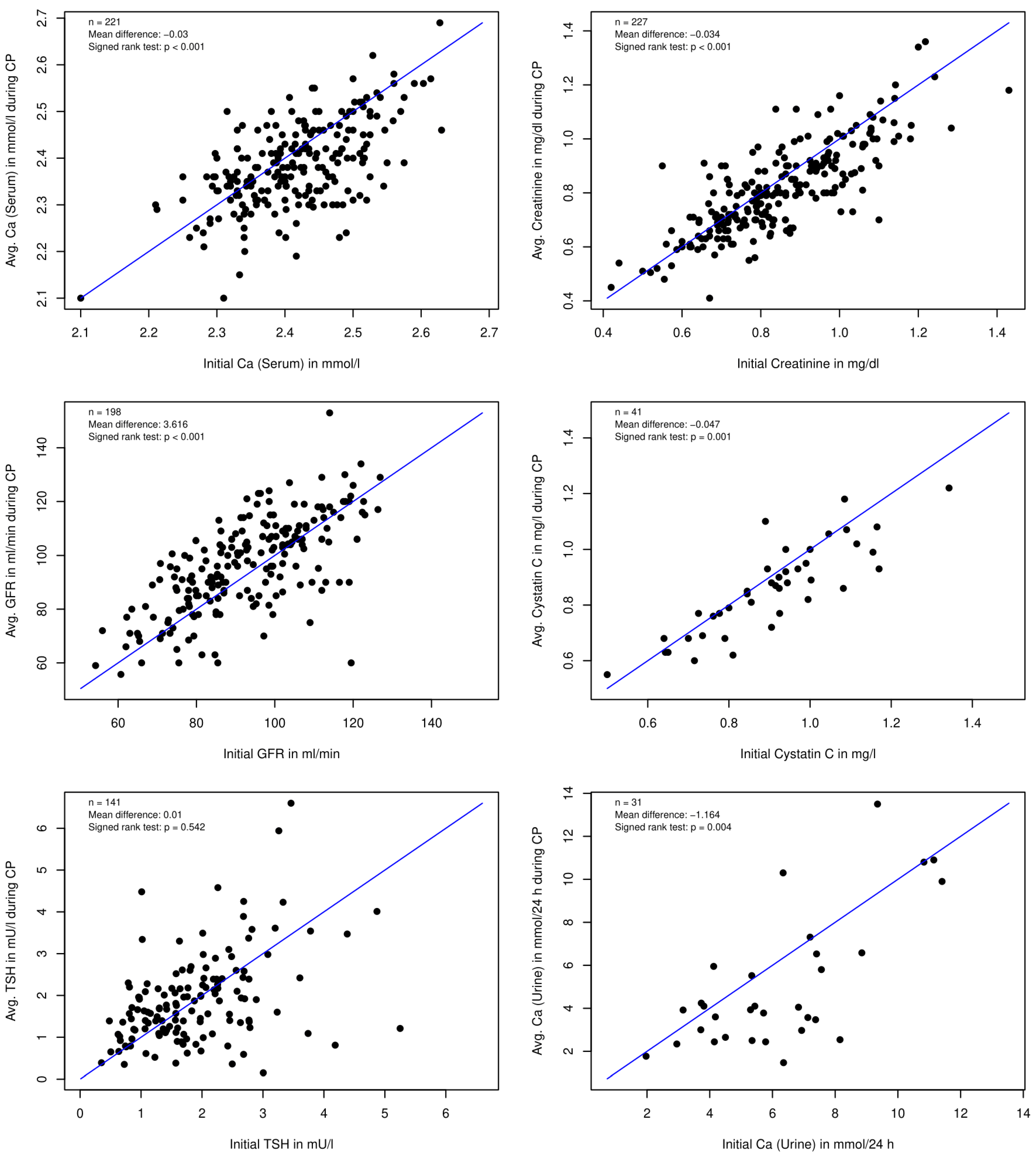
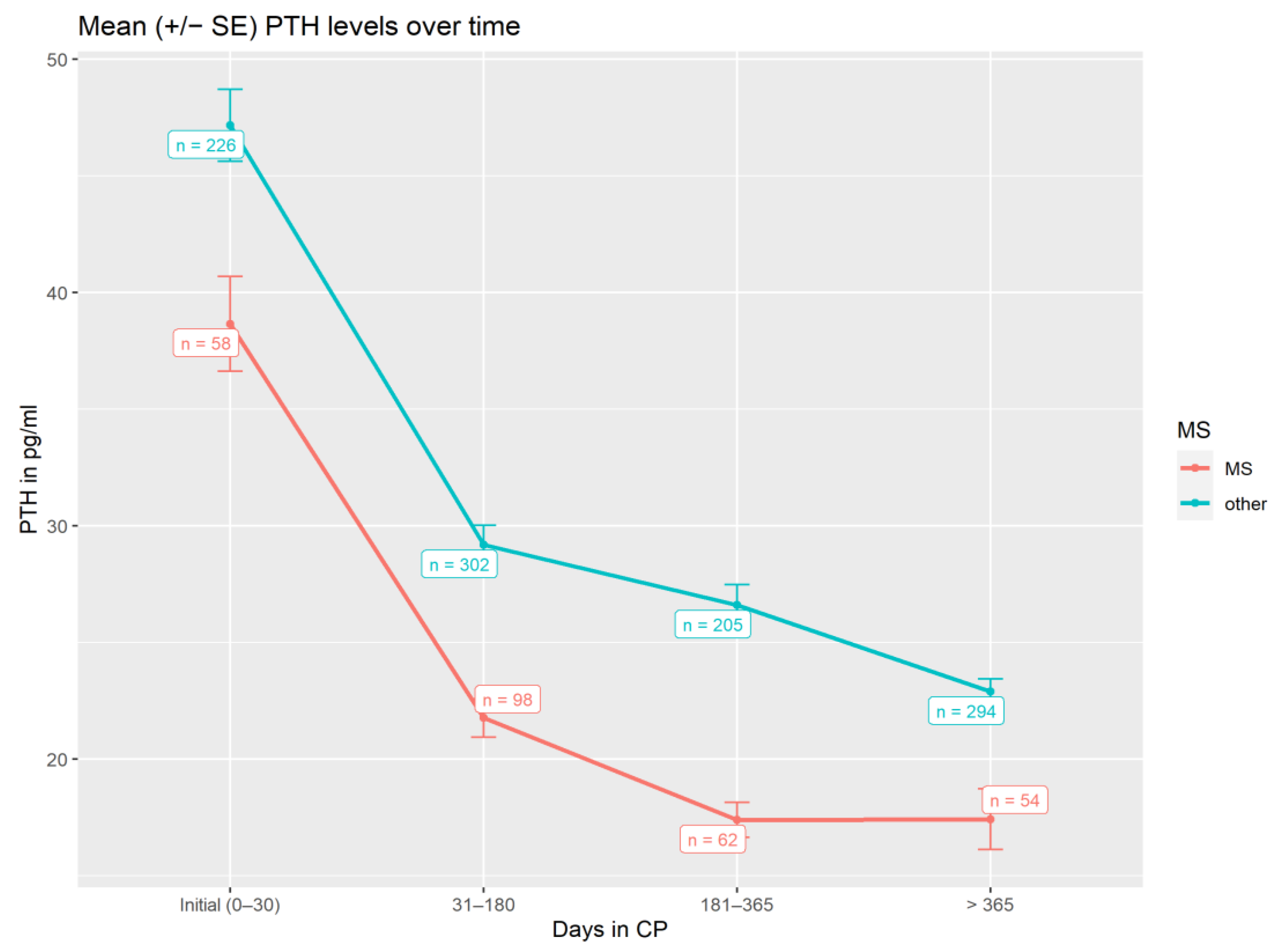
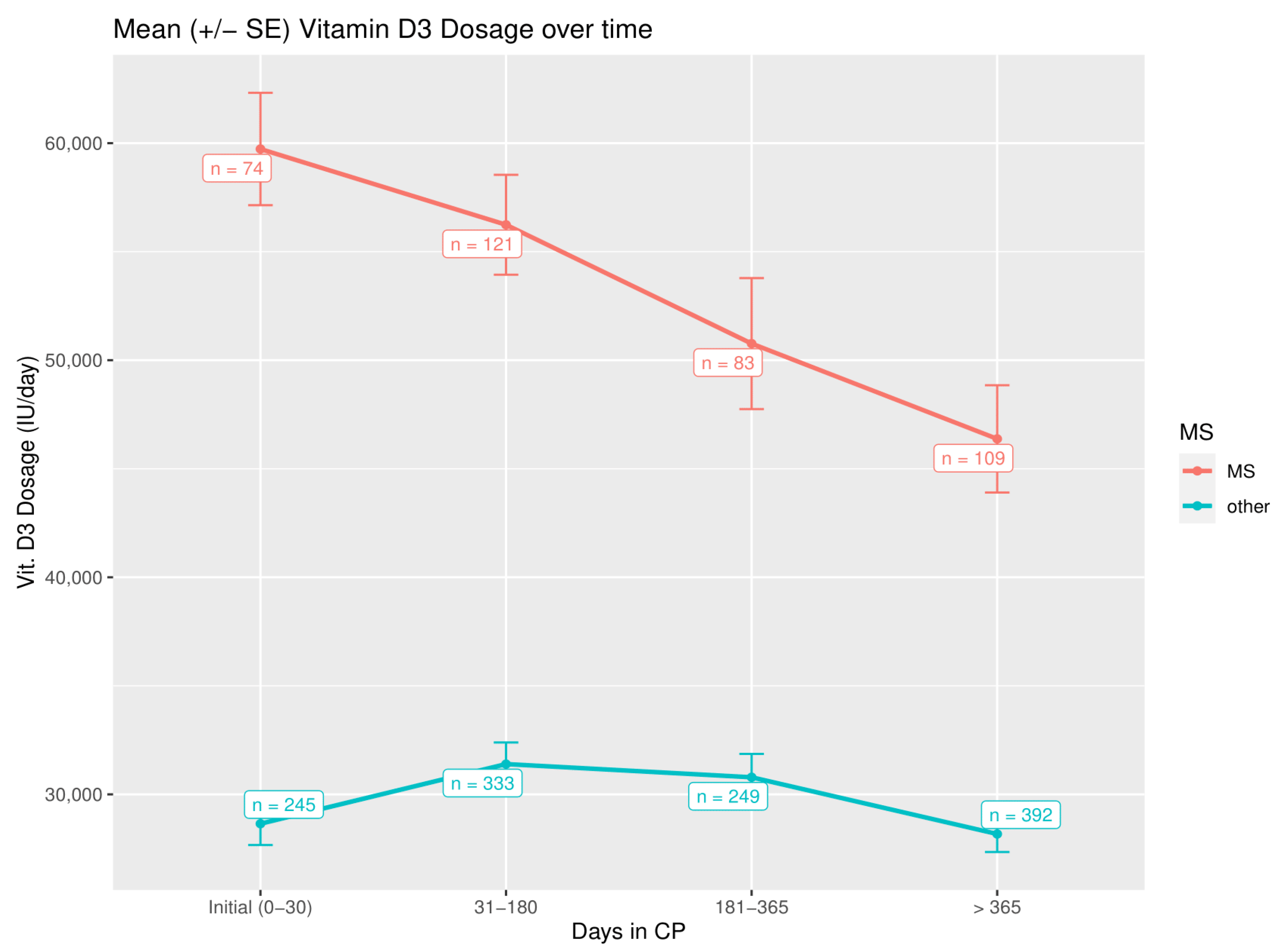
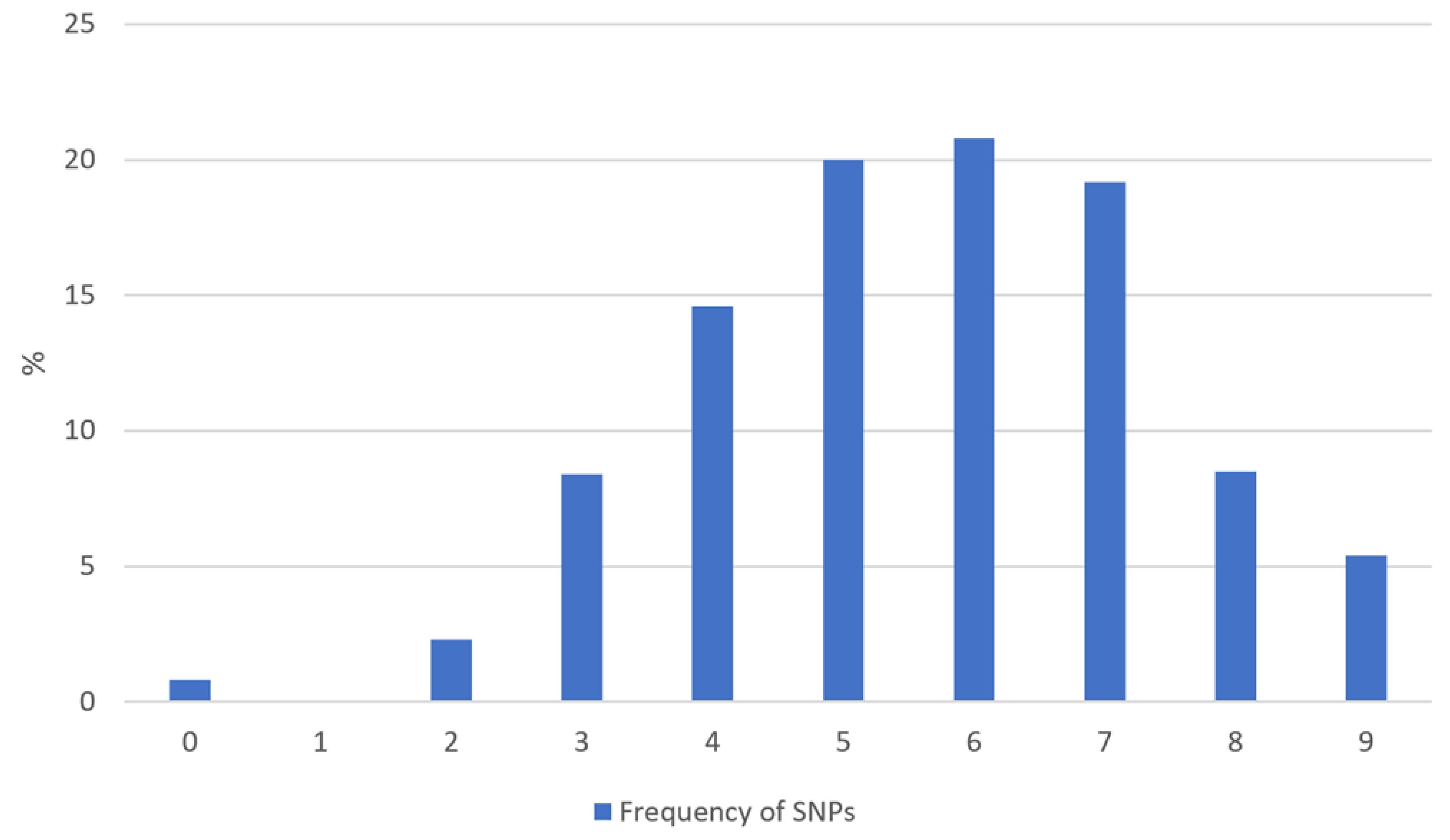
| All Patients (n = 319) | MS Patients (n = 74) | Non-MS Patients (n = 245) | |
|---|---|---|---|
| Number of laboratory investigations | 1606 | 387 | 1219 |
| Minimum/Maximum of daily vitamin D3 dosage (IU per day) | 0/150,000 | 0/150,000 | 0/100,000 |
| Mean dosage of daily vitamin D3 (IU per day ±SD) | 35,291 ± 21,791 | 52,955 ± 25,791 | 29,683 ± 16,861 |
| Spearman correlation dosage vitamin D3 and serum calcium | 0.103, p < 0.001 n = 1204 | 0.151, p = 0.010 n = 291 | 0.050, p = 0.128 n = 913 |
| Spearman correlation dosage vitamin D3 and urinary calcium excretion | 0.162, p = 0.001 n = 433 | 0.058, p = 0.456 n = 166 | 0.233, p < 0.001 n = 267 |
| Mean Difference of Initial PTH and Average PTH 31–180 Days in CP | Mean Difference of Initial Dose and Average Dose 181–365 Days in CP | Mean Difference of Initial Dose and Average Dose >365 Days in CP | |
|---|---|---|---|
| MS patients | −16.1 pg/mL PTH (p < 0.001) | −19.3 pg/mL PTH (p < 0.001) | −25.3 pg/mL PTH (p < 0.001) |
| Non-MS patients | −18.4 pg/mL PTH (p < 0.001) | −22.0 pg/mL PTH (p < 0.001) | −28.3 pg/mL PTH (p < 0.001) |
| Mean Difference of Initial Dose and Average Dose 31–180 Days in CP | Mean Difference of Initial Dose and Average Dose 181–365 Days in CP | Mean Difference of Initial Dose and Average Dose >365 Days in CP | |
|---|---|---|---|
| MS patients | −2881.4 IU (p = 0.039) | −9520.9 IU (p = 0.008) | −16,318.5 IU (p = 0.001) |
| Non-MS patients | −442.9 IU (p = 0.864) | +163.2 IU (p = 0.640) | −2736.8 IU (p = 0.024) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amon, U.; Yaguboglu, R.; Ennis, M.; Holick, M.F.; Amon, J. Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of Vitamin D3 According to the “Coimbra Protocol”. Nutrients 2022, 14, 1575. https://doi.org/10.3390/nu14081575
Amon U, Yaguboglu R, Ennis M, Holick MF, Amon J. Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of Vitamin D3 According to the “Coimbra Protocol”. Nutrients. 2022; 14(8):1575. https://doi.org/10.3390/nu14081575
Chicago/Turabian StyleAmon, Ulrich, Raul Yaguboglu, Madeleine Ennis, Michael F. Holick, and Julian Amon. 2022. "Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of Vitamin D3 According to the “Coimbra Protocol”" Nutrients 14, no. 8: 1575. https://doi.org/10.3390/nu14081575
APA StyleAmon, U., Yaguboglu, R., Ennis, M., Holick, M. F., & Amon, J. (2022). Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of Vitamin D3 According to the “Coimbra Protocol”. Nutrients, 14(8), 1575. https://doi.org/10.3390/nu14081575