Association between the Phytochemical Index and Overweight/Obesity: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources and Methods of Data Retrieval
2.2. Inclusion Criteria and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Meta-Analysis
3.3. Subgroup Analysis
3.4. Sensitivity Analysis
3.5. Meta-Regression
3.6. Publication Bias
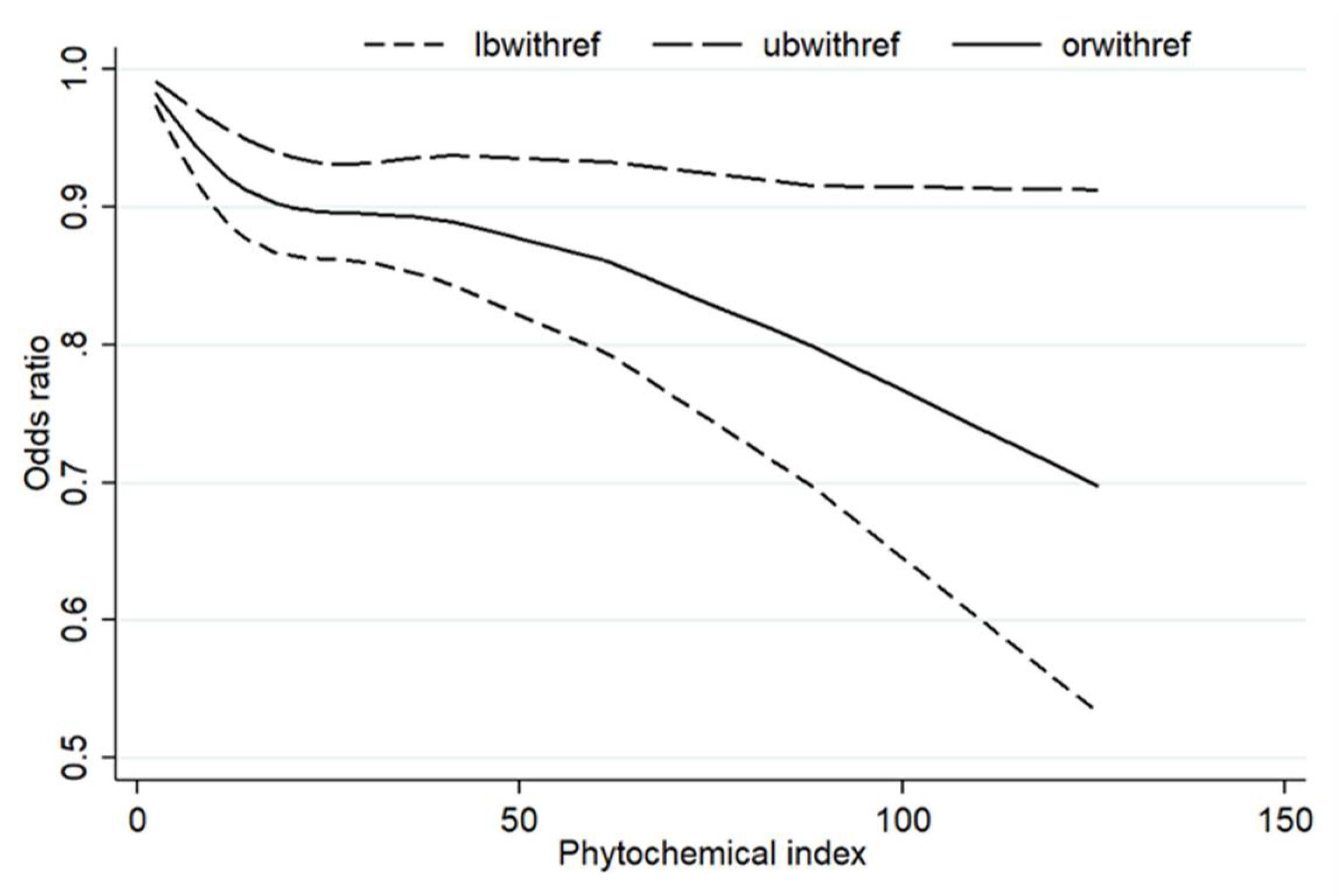
3.7. Dose–Response Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poletto, J.E.; Rizzo, D.T.; Almeida, A.M.N.; Cândido, E.C.; Cazzo, E.; Chaim, É.A. Evolution of anthropometric data and quality of life in active bariatric individuals. Rev. Assoc. Med. Bras. 2021, 67, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Deusinger, S.S.; Deusinger, R.H. Obesity: Overview of prevalence, etiology, and treatment. Phys. Ther. 2003, 83, 276–288. [Google Scholar] [CrossRef]
- Eslami, O.; Khoshgoo, M.; Shidfar, F. Dietary phytochemical index and overweight/obesity in children: A cross-sectional study. BMC Res. Notes 2020, 13, 132. [Google Scholar] [CrossRef]
- Sabaté, J.; Wien, M. Vegetarian diets and childhood obesity prevention. Am. J. Clin. Nutr. 2010, 91, 1525s–1529s. [Google Scholar] [CrossRef]
- Newby, P.K.; Tucker, K.L.; Wolk, A. Risk of overweight and obesity among semivegetarian, lactovegetarian, and vegan women. Am. J. Clin. Nutr. 2005, 81, 1267–1274. [Google Scholar] [CrossRef]
- Brathwaite, N.; Fraser, H.S.; Modeste, N.; Broome, H.; King, R. Obesity, diabetes, hypertension, and vegetarian status among Seventh-Day Adventists in Barbados: Preliminary results. Ethn. Dis. 2003, 13, 34–39. [Google Scholar]
- Thedford, K.; Raj, S. A vegetarian diet for weight management. J. Am. Diet. Assoc. 2011, 111, 816–818. [Google Scholar] [CrossRef]
- Roman, D.D.L.; Aller, R.; Castano, O. Vegetarian diets; effect on health. Rev. Clin. Esp. 2007, 207, 141–143. [Google Scholar] [CrossRef]
- Fraser, G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009, 89, 1607s–1612s. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Proposal for a dietary “phytochemical index”. Med. Hypotheses 2004, 63, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Firdous, S.M. Phytochemicals for treatment of diabetes. EXCLI J. 2014, 13, 451–453. [Google Scholar] [PubMed]
- Tucci, S.A. Phytochemicals in the Control of Human Appetite and Body Weight. Pharmaceuticals 2010, 3, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Golzarand, M.; Mirmiran, P.; Saadati, N.; Azizi, F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2013, 26 (Suppl. 1), 145–153. [Google Scholar] [CrossRef]
- Im, J.; Kim, M.; Park, K. Association between the Phytochemical Index and Lower Prevalence of Obesity/Abdominal Obesity in Korean Adults. Nutrients 2020, 12, 2312. [Google Scholar] [CrossRef]
- Kim, M.; Park, K. Association between phytochemical index and metabolic syndrome. Nutr. Res. Pract. 2020, 14, 252–261. [Google Scholar] [CrossRef]
- Dehghani Firouzabadi, F.; Jayedi, A.; Asgari, E.; Farazi, M.; Noruzi, Z.; Djafarian, K.; Shab-Bidar, S. The Association of Dietary Phytochemical Index with Metabolic Syndrome in Adults. Clin. Nutr. Res. 2021, 10, 161–171. [Google Scholar] [CrossRef]
- Asgari, E.; Jayedi, A.; Dehghani Firouzabadi, F.; Noruzi, Z.; Farazi, M.; Djafarian, K.; Shab-Bidar, S. Association of the dietary phytochemical index with general and central obesity in a sample of Iranian adults. J. Funct. Foods 2021, 83, 104546. [Google Scholar] [CrossRef]
- Chen, J.P.; Chen, G.C.; Wang, X.P.; Qin, L.; Bai, Y. Dietary Fiber and Metabolic Syndrome: A Meta-Analysis and Review of Related Mechanisms. Nutrients 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N.; Li, R.; Wolk, A.; Khudyakov, P.; Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012, 175, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Vasmehjani, A.A.; Darabi, Z.; Nadjarzadeh, A.; Mirzaei, M.; Hosseinzadeh, M. The relation between dietary phytochemical index and metabolic syndrome and its components in a large sample of Iranian adults: A population-based study. BMC Public Health 2021, 21, 1587. [Google Scholar] [CrossRef]
- Azizi-Soleiman, F.; Khoshhali, M.; Heidari-Beni, M.; Qorbani, M.; Ali Pourmirzaei, M.; Kelishadi, R. Higher dietary phytochemical index is associated with anthropometric indices in children and adolescents: The weight disorders survey of the CASPIAN-IV study. Int. J. Vitam. Nutr. Res. 2021, 91, 531–538. [Google Scholar] [CrossRef]
- Delshad Aghdam, S.; Siassi, F.; Nasli Esfahani, E.; Qorbani, M.; Rajab, A.; Sajjadpour, Z.; Bashiri, A.; Aghayan, M.; Sotoudeh, G. Dietary phytochemical index associated with cardiovascular risk factor in patients with type 1 diabetes mellitus. BMC Cardiovasc. Disord. 2021, 21, 293. [Google Scholar] [CrossRef]
- Abshirini, M.; Mahaki, B.; Bagheri, F.; Siassi, F.; Koohdani, F.; Sotoudeh, G. Higher Intake of Phytochemical-Rich Foods is Inversely Related to Prediabetes: A Case-Control Study. Int. J. Prev. Med. 2018, 9, 64. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Karimi, Z.; Houshiar-rad, A.; Mirzayi, H.R.; Rashidkhani, B. Dietary phytochemical index and the risk of breast cancer: A case control study in a population of Iranian women. Asian Pac. J. Cancer Prev. 2013, 14, 2747–2751. [Google Scholar] [CrossRef] [PubMed]
- Aghababayan, S.; Sheikhi Mobarakeh, Z.; Qorbani, M.; Abbasvandi, F.; Tiznobeyk, Z.; Aminianfar, A.; Sotoudeh, G. Dietary Phytochemical Index and Benign Breast Diseases: A Case-Control Study. Nutr. Cancer 2020, 72, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishy, S.M.; Aminianfar, A.; Benisi-Kohansal, S.; Azadbakht, L.; Esmaillzadeh, A. Association between dietary phytochemical index and breast cancer: A case-control study. Breast Cancer 2021, 28, 1283–1291. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Golzarand, M.; Shiva, N.; Azizi, F. Association between dietary phytochemical index and 3-year changes in weight, waist circumference and body adiposity index in adults: Tehran Lipid and Glucose study. Nutr. Metab. 2012, 9, 108. [Google Scholar] [CrossRef]
- Dongwoo, H.; Seong-Ah, K.; Jun, S.; Kang, M.S.; Joung, H. Association between antioxidant vitamin intake and obesity among Korean women: Using the Korea National Health and Nutrition Examination Survey 2007–2016. J. Nutr. Health 2018, 51, 400–413. [Google Scholar]
- Suzuki, K.; Inoue, T.; Hioki, R.; Ochiai, J.; Kusuhara, Y.; Ichino, N.; Osakabe, K.; Hamajima, N.; Ito, Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin. Nutr. 2006, 25, 780–789. [Google Scholar] [CrossRef]
- Kim, M.; Choi, M.-K.; Ja, S.C. The study of pytoestrogen intake and bone mineral density of vegetarian and nonvegetarian postmenopausal women. Korean J. Community Nutr. 2004, 9, 66–72. [Google Scholar]
- Lee, J.; Heo, J.; Park, Y.; Park, H. Survey on the Consumption of the Phytoestrogen Isoflavone in Postmenopausal Korean Women. J. Menopausal Med. 2012, 18, 163–173. [Google Scholar] [CrossRef][Green Version]
- Kim, B.-J. Obesity and Sex Hormones. J. Obes. Metab. Syndr. 2010, 19, 113–118. [Google Scholar]
- Holubková, A.; Penesová, A.; Šturdík, E.; Mošovská, S.; Mikušová, L. Phytochemicals with potential effects in metabolic syndrome prevention and therapy %J Acta Chimica Slovaca. Acta Chim. Slovaca 2012, 5, 186–199. [Google Scholar] [CrossRef]
- Golzarand, M.; Mirmiran, P.; Bahadoran, Z.; Alamdari, S.; Azizi, F. Dietary phytochemical index and subsequent changes of lipid profile: A 3-year follow-up in Tehran Lipid and Glucose Study in Iran. ARYA Atheroscler. 2014, 10, 203–210. [Google Scholar] [PubMed]
- Vincent, H.K.; Bourguignon, C.M.; Taylor, A.G. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2010, 23, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Carnauba, R.A.; Chaves, D.F.; Baptistella, A.B.; Paschoal, V.; Naves, A.; Buehler, A.M. Association between high consumption of phytochemical-rich foods and anthropometric measures: A systematic review. Int. J. Food Sci. Nutr. 2017, 68, 158–166. [Google Scholar] [CrossRef] [PubMed]

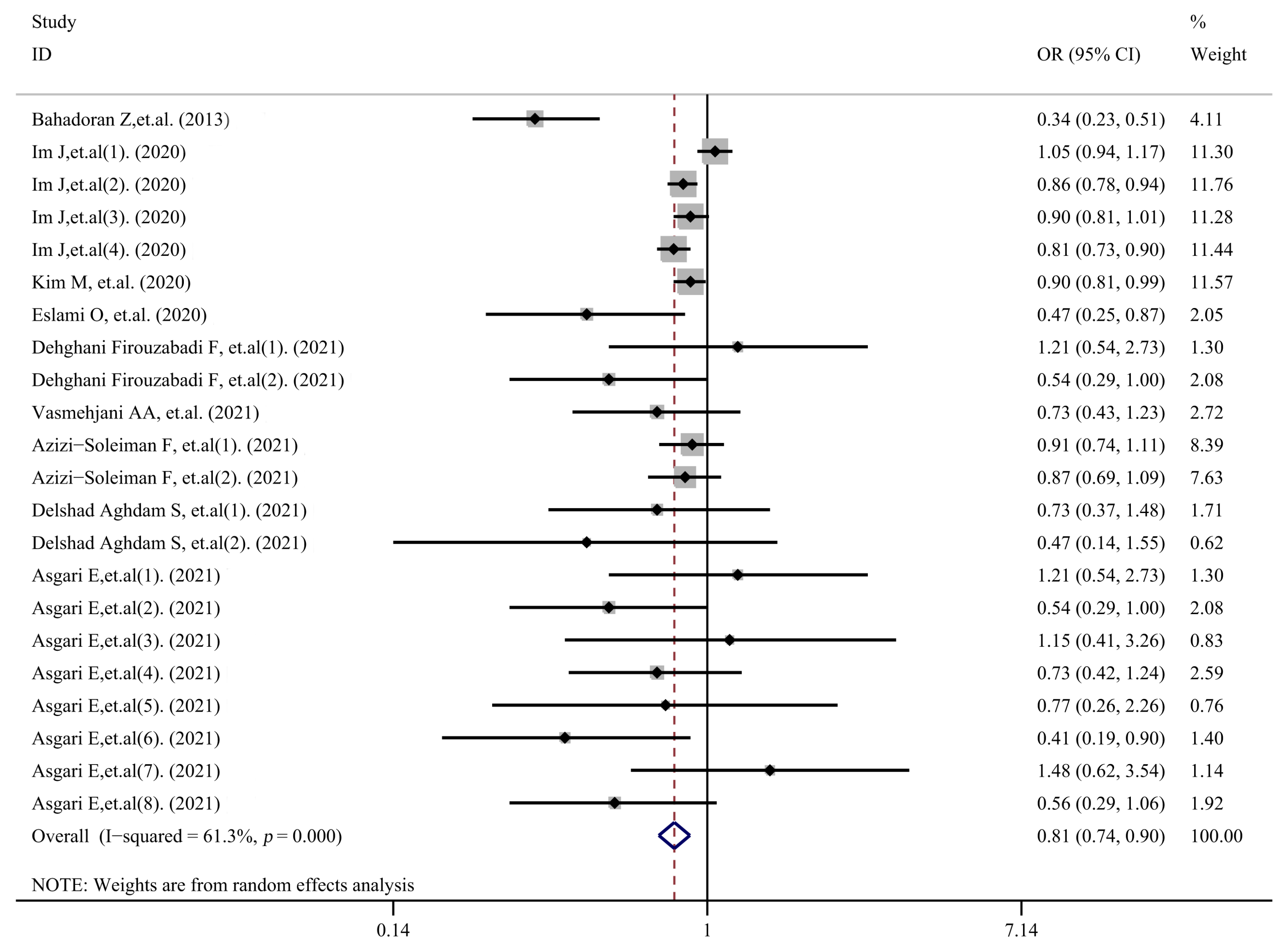




| Study | Country | Age | Subjects | Outcome Variable | Diagnostic Criteria | Score |
|---|---|---|---|---|---|---|
| Bahadoran Z, 2013 [16] | Iran | 19–70 | 2567 | Abdominal obesity | WC ≥ 95 cm | 7 |
| Im J, 2020 [17] | Korea | ≥19 | 57,940 | Obesity, abdominal obesity | BMI ≥ 25 kg/m2, WC ≥ 90 and ≥85 cm for men and women | 7 |
| Kim M, 2020 [18] | Korea | ≥19 | 31,319 | Abdominal obesity | WC ≥ 90 cm in men and ≥80 cm in women | 6 |
| Eslami O, 2020 [5] | Iran | 7–10 | 356 | Overweight and obesity | overweight: BMI percentile ≥ 85 and <95, obese: ≥95 | 6 |
| Dehghani Firouzabadi F, 2021 [19] | Iran | 18–65 | 844 | Central obesity | WC ≥ 102 cm for men and 88 cm for women | 7 |
| Vasmehjani AA, 2021 [27] | Iran | 20–70 | 2326 | Abdominal obesity | WC ≥ 102 cm for men and >88 cm for women | 6 |
| Azizi-Soleiman F, 2021 [28] | China | 6–18 | 4296 | Obesity or overweight, abdominal obesity | BMI > 85th percentile, WHtR ≥ 0.5 | 7 |
| Delshad Aghdam S, 2021 [29] | Iran | 18–35 | 261 | Overweight or obesity, abdominal obesity | BMI > 24.9 kg/m2, WC ≥ 80 cm in women and ≥94 cm in men | 6 |
| Asgari E, 2021 [20] | Iran | 18–59 | 844 | Central obesity, general obesity | BMI ≥ 30 kg/m2, central obesity: WHtR ≥ 0.5; WHR ≥ 0.8 for women and ≥1 for men; WC ≥ 102 cm for men and ≥88 cm for women | 7 |
| Study | OR (95%CI) | I2 | p |
|---|---|---|---|
| Bahadoran Z, et al. (2013) [16] | 0.87 (0.80–0.93) | 38.4% | 0.039 |
| Im J, et al. (1). (2020) [17] | 0.79 (0.72–0.87) | 51.9% | 0.003 |
| Im J, et al. (2). (2020) [17] | 0.80 (0.71–0.89) | 63.1% | 0.001 |
| Im J, et al. (3). (2020) [17] | 0.80 (0.71–0.89) | 63.0% | 0.001 |
| Im J, et al. (4). (2020) [17] | 0.81 (0.72–0.90) | 61.4% | 0.001 |
| Kim M, et al. (2020) [18] | 0.79 (0.71–0.89) | 62.9% | 0.001 |
| Eslami O, et al. (2020) [5] | 0.83 (0.75–0.91) | 60.4% | 0.001 |
| Dehghani Firouzabadi F, et al. (1). (2021) [19] | 0.81 (0.73–0.89) | 62.8% | 0.001 |
| Dehghani Firouzabadi F, et al. (2). (2021) [19] | 0.82 (0.75–0.91) | 61.5% | 0.001 |
| Vasmehjani AA, et al. (2021) [27] | 0.82 (0.74–0.90) | 62.9% | 0.001 |
| Azizi-Soleiman F, et al. (1). (2021) [28] | 0.80 (0.72–0.89) | 63.1% | 0.001 |
| Azizi-Soleiman F, et al. (2). (2021) [28] | 0.81 (0.73–0.89) | 63.2% | 0.001 |
| Delshad Aghdam S, et al. (1). (2021) [29] | 0.81 (0.74–0.90) | 63.0% | 0.001 |
| Delshad Aghdam S, et al. (2). (2021) [29] | 0.82 (0.74–0.90) | 62.5% | 0.001 |
| Asgari E, et al. (1). (2021) [20] | 0.81 (0.73–0.89) | 62.8% | 0.001 |
| Asgari E, et al. (2). (2021) [20] | 0.82 (0.75–0.91) | 61.5% | 0.001 |
| Asgari E, et al. (3). (2021) [20] | 0.81 (0.73–0.89) | 63.0% | 0.001 |
| Asgari E, et al. (4). (2021) [20] | 0.82 (0.74–0.90) | 62.9% | 0.001 |
| Asgari E, et al. (5). (2021) [20] | 0.81 (0.74–0.90) | 63.1% | 0.001 |
| Asgari E, et al. (6). (2021) [20] | 0.82 (0.75–0.91) | 60.5% | 0.001 |
| Asgari E, et al. (7). (2021) [20] | 0.81 (0.73–0.89) | 62.2% | 0.001 |
| Asgari E, et al. (8). (2021) [20] | 0.82 (0.74–0.90) | 61.9% | 0.001 |
| Combined | 0.81(0.74–0.90) | 61.3% | 0.001 |
| Covariate | p |
|---|---|
| Age | 0.895 |
| Gender | 0.023 |
| Diagnostic criteria of obesity | 0.360 |
| Area | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Liu, L.; Liu, R.; Dai, W.; Cui, W.; Li, D. Association between the Phytochemical Index and Overweight/Obesity: A Meta-Analysis. Nutrients 2022, 14, 1429. https://doi.org/10.3390/nu14071429
Wei C, Liu L, Liu R, Dai W, Cui W, Li D. Association between the Phytochemical Index and Overweight/Obesity: A Meta-Analysis. Nutrients. 2022; 14(7):1429. https://doi.org/10.3390/nu14071429
Chicago/Turabian StyleWei, Chaojie, Liping Liu, Renli Liu, Wenwen Dai, Weiwei Cui, and Dong Li. 2022. "Association between the Phytochemical Index and Overweight/Obesity: A Meta-Analysis" Nutrients 14, no. 7: 1429. https://doi.org/10.3390/nu14071429
APA StyleWei, C., Liu, L., Liu, R., Dai, W., Cui, W., & Li, D. (2022). Association between the Phytochemical Index and Overweight/Obesity: A Meta-Analysis. Nutrients, 14(7), 1429. https://doi.org/10.3390/nu14071429








