Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Multi-Centre Population-Based Birth Cohort Study in Spain
Abstract
:1. Introduction
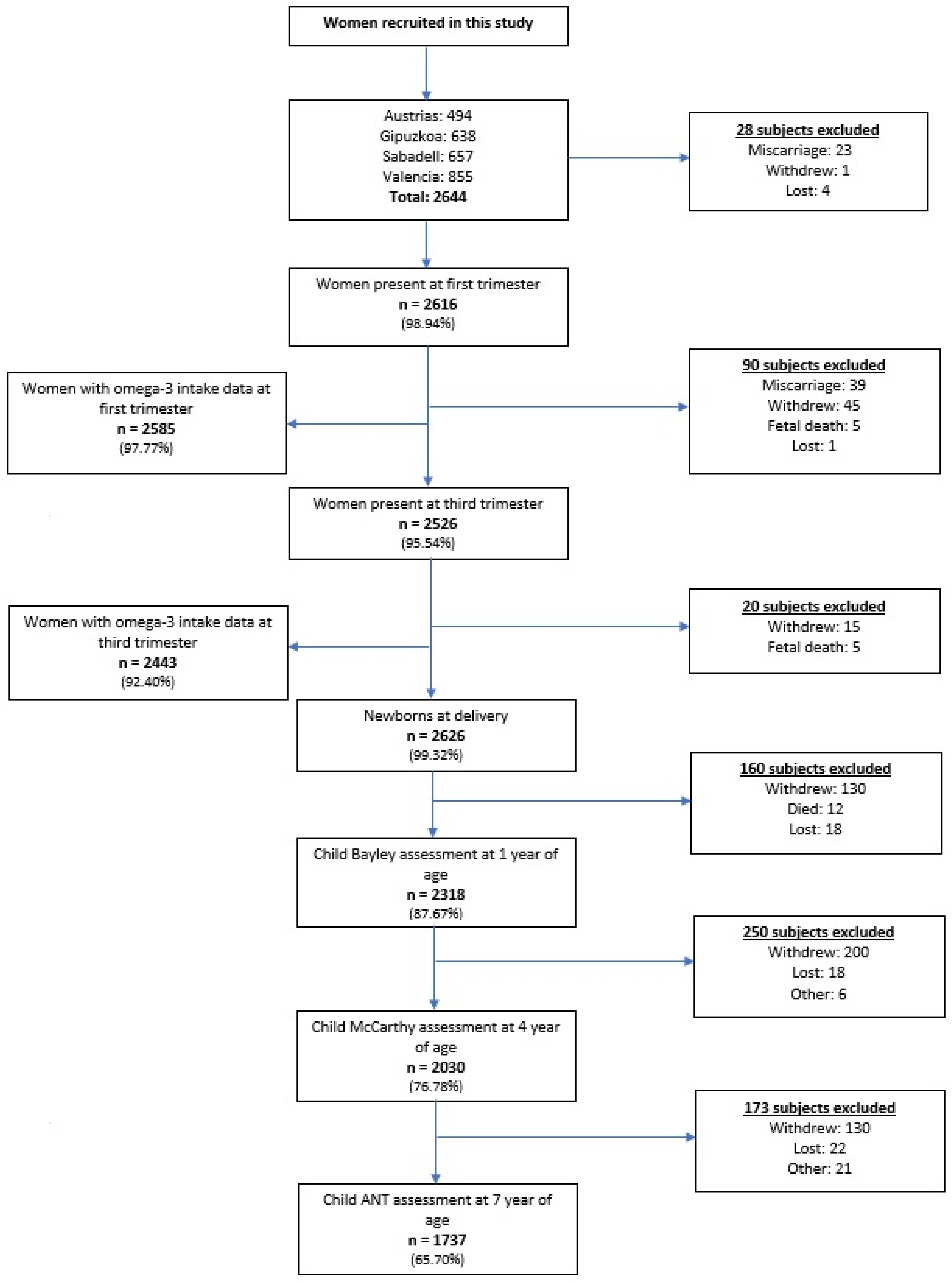
2. Subjects and Methods
2.1. Study Population
2.2. Exposure Information
2.3. Co-Variable Information
2.4. Neuropsychological Assessments
2.5. Statistical Analysis
- (1)
- Minimally adjusted regression models included co-variate adjustments for maternal energy intake (kcal/day), sex of the child, age of the child at the time of testing, cohort location, and quality of the test.
- (2)
- Fully adjusted regression models additionally included co-variate adjustments for the birthweight of the child, gestational age, the duration of breastfeeding, maternal alcohol consumption, maternal education, maternal smoking, maternal social class, and maternal pre-pregnancy BMI, as well as number of pregnancies before the index pregnancy, number of miscarriages, and maternal country origin (Spain, or other).
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Bayley Scales of Infant Development | BSID |
| Confidence interval | CI |
| Docosahexaenoic | DHA |
| Directly acyclic graph | DAG |
| Eicosapentaenoic | EPA |
| Food frequency questionnaire | FFQ |
| Infancia y Medio Ambiente (Environment and Childhood) | INMA |
| McCarthy Scale of Children’s Abilities | MSCA |
| Polyunsaturated fatty acids | PUFA |
| Recommended dietary allowance | RDA |
| Standard deviation | SD |
References
- United Nations. United Nations Sustainable Development Knowledge Platform. Sustainable Development Goals. 2015. Available online: https://sustainabledevelopment.un.org/?menu=1300 (accessed on 14 January 2020).
- World Health Organization. Global Targets 2025. Available online: https://www.who.int/nutrition/global-target-2025/en/ (accessed on 14 January 2020).
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; WHO: Luxemburg, 2016; Available online: https://www.who.int/publications/i/item/9789241549912 (accessed on 6 January 2022).
- European Food Safety Panel on Dietetic Products, Nutrition, and Allergies. Scientific Opinion on Dietary Reference Intakes for fats, including saturated fatty acids, polyunsaturated fatty acids, trans fatty acids, monounsaturated fatty acids, trans fats, and cholesterol. EFSA J. 2010, 8, 1–107. [Google Scholar]
- Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for Energy. Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Clandinin, M.; Chappell, J.; Heim, T.; Swyer, P.; Chance, G. Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum. Dev. 1981, 5, 355–366. [Google Scholar] [CrossRef]
- Innis, S. Essential fatty acid transfer and fetal development. Placenta 2005, 26, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Rombaldi Bernardi, J.; de Souza Escobar, R.; Ferreira, C.F.; Pelufo Silveira, P. Fetal and neonatal levels of n-3 PUFAs: Effects on neurodevelopment, nutrition, and growth. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- The Lancet. Executive Summary of the Lancet Maternal and Child Nutrition Series. Lancet 2013. Available online: https://www.thelancet.com/pb/assets/raw/Lancet/stories/series/nutrition-eng.pdf (accessed on 14 January 2020).
- Danielewicz, H.; Myszczyszyn Debinska, A.; Myszkal, A.; Boznanski, A.; Hirnle, L. Diet in Pregnancy—More than food. Eur. J. Pediatr. 2017, 176, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Gow, R.V.; Hibbeln, J.R. N-3 PUFAs fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 555–590. [Google Scholar] [CrossRef] [Green Version]
- Karr, J.E.; Alexander, J.E.; Winningham, R.G. N-3 PUFAs polyunsaturated fatty acids and cognition throughout the lifespan: A review. Nutr. Neurosci. 2011, 14, 216–225. [Google Scholar] [CrossRef]
- Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 305–314. [Google Scholar] [CrossRef]
- Julvez, J.; Méndez, M.; Fernández-Barrés, S.; Romaguera, D.; Vioque, J.; Llop, S.; Ibarluzea, J.; Guxens, M.; Avella-Garcia, C.; Tardon, A.; et al. Maternal consumption of seafood in pregnancy and child neuropsychological development: A longitudinal study based on a population with high consumption levels. Am. J. Epidemiol. 2016, 183, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Gignac, F.; Romaguera, D.; Fernández-Barrés, S.; Phillipat, C.; Esteban, R.G.; López-Vicente, M.; Vioque, J.; Fernández-Somoano, A.; Tardón, A.; Iñiguez, C.; et al. Maternal nut intake in pregnancy and child neuropsychological development up to 8 years old: A population-based cohort study in Spain. Eur. J. Epidemiol. 2019, 34, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Guxens, M.; Ballester, F.; Espada, M.; Fernández, M.F.; Grimalt, J.O.; Ibarluzea, J.; Olea, N.; Rebagliato, M.; Tardón, A.; Torrent, M.; et al. Cohort profile: The INMA—INfancia y Medio Ambiente—(Environment and Childhood) Project. Int. J. Epidemiol. 2012, 41, 930–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vioque, J.; Navarrete-Muñoz, E.-M.; Gimenez-Monzó, D.; García-De-La-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Vioque et al. Nutr. J. 2013, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Chisaguano, A.M.; Montes, R.; Castellote, A.I.; Morales, E.; Júlvez, J.; Vioque, J.; Sunyer, J.; López-Sabater, M.C. Elaidic, vaccenic, and rumenic acid status during pregnancy: Association with maternal plasmatic LC-PUFAs and atopic manifestations in infants. Pediatr. Res. 2014, 76, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Royston, P.; Wright, E.M. A method for estimating age-specific reference intervals (`normal ranges’) based on fractional polynomials and exponential transformation. J. R. Stat. Soc. 1998, 161, 79–101. [Google Scholar] [CrossRef]
- McCarthy, D. Manual for the McCarthy Scales of Children’s Abilities; Psychological Corp.: New York, NY, USA, 1972. [Google Scholar]
- Forns, J.; Esnaola, M.; López-Vicente, M.; Suades-González, E.; Alvarez-Pedrerol, M.; Julvez, J.; Grellier, J.; Sebastián-Gallés, N.; Sunyer, J. The n-back test and the attentional network task as measures of child neuropsychological development in epidemiological studies. Neuropsychology 2014, 28, 519–529. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology-Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Pediatric Obesity Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef] [PubMed]
- Lora, K.R.; Lewis, N.M.; Eskridge, K.M.; Stanek-Krogstrand, K.; Travnicek, D.A. Correlation of n-3 PUFAs fatty acids intakes with acculturation and socioeconomic status in midwestern Latinas. J. Immigr. Minor. Health 2011, 13, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrier, I.; Platt, R.W. Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol. 2008, 8, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seaman, S.R.; White, I.R. Review of inverse probability weighting for dealing with missing data. Stat. Methods Med. Res. 2011, 22, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Michelle, A.M.; Torrent, M.; Julvez, J.; Ribas-Fito, N.; Kogevinas, M.; Sunyer, J. Maternal fish and other seafood intakes during pregnancy and child neurodevelopment at age 4 years. Public Health Nutr. 2009, 12, 1702–1710. [Google Scholar]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal Supplementation With Very-Long-Chain n-3 Fatty Acids During Pregnancy and Lactation Augments Children’s IQ at 4 Years of Age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [Green Version]
- McNamara, R.K.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P. The aging human orbitofrontal cortex: Decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Larqué, E.; Demmelmair, H. Placental transfer of long-chain polyunsaturated fatty acids (LC-PUFA). J. Perinat. Med. 2007, 35 (Suppl. 1), S5–S11. [Google Scholar] [CrossRef] [Green Version]
- Julvez, J.; Fernández-Barrés, S.; Gignac, F.; López-Vicente, M.; Bustamante, M.; Garcia-Esteban, R.; Vioque, J.; Llop, S.; Ballester, F.; Fernández-Somoano, A.; et al. Maternal seafood consumption during pregnancy and child attention outcomes: A cohort study with gene effect modification by PUFA-related genes. Int. J. Epidemiol. 2020, 49, 559–571. [Google Scholar] [CrossRef]
| Quartiles of Dietary Omega b 3 Intake at 1st Trimester * | p Values | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| (n = 645) a | (n = 645) a | (n = 645) a | (n = 647) a | ||
| Age (years) c, mean (SD) | 31.32 (4.36) | 31.84 (4.33) | 32.25 (3.97) | 32.37 (4.24) | 0.001 |
| Mother education level, n (%) | 0.131 | ||||
| Primary or less | 177 (27.48) | 169 (26.24) | 152 (23.49) | 150 (23.22) | |
| Secondary school | 264 (40.99) | 248 (38.51) | 263 (40.65) | 291 (45.05 | |
| University or more | 203 (31.52) | 227 (35.25) | 232 (35.86) | 205 (31.73) | |
| Social class of mother, n (%) | 0.115 | ||||
| Highly skilled | 123 (19.07) | 142 (22.02) | 143 (22.07) | 144 (22.29) | |
| Non-manual | 152 (23.57) | 158 (24.50) | 186 (28.70) | 168 (26.01) | |
| Manual | 370 (57.36) | 345 (53.49) | 319 (49.23) | 334 (51.70) | |
| Smoking during pregnancy, n (%) | 0.783 | ||||
| Yes | 101 (16.67) | 98 (16.25) | 102 (16.64) | 112 (18.30) | |
| No | 505 (83.33) | 505 (83.75) | 511 (83.36) | 500 (81.70) | |
| Alcohol consumption during first trimester, n (%) | 0.048 | ||||
| Yes | 172 (26.67) | 197 (30.54) | 219 (33.80) | 201 (31.07) | |
| No | 473 (73.33) | 448 (69.46) | 429 (66.20) | 446 (68.93) | |
| Energy intake (kcals/day), mean (SD) | 2115.48 (570.06) | 2147.30 (559.07) | 2127.43 (572.07) | 2089.87 (523.77) | 0.308 |
| Preterm, n (%) | 0.836 | ||||
| Yes | 32 (5.24) | 26 (4.25) | 27 (4.33) | 29 (4.73) | |
| No | 579 (94.76) | 586 (95.75) | 597 (95.67) | 584 (95.27) | |
| Mother BMI pre-pregnancy, mean (SD) | 23.51 (4.39) | 23.46 (4.27) | 23.29 (3.86) | 24.04 (4.70) | 0.012 |
| Number of pregnancies, n (%) | 0.001 | ||||
| 1 | 308 (47.75) | 305 (47.29) | 291 (44.91) | 272 (42.04) | |
| 2 | 226 (35.04) | 246 (38.14) | 224 (34.57) | 214 (33.08) | |
| 3 or more | 111 (17.21) | 94 (14.57) | 133 (20.52) | 161 (24.88) | |
| Number of previous miscarriages, n (%) | 0.151 | ||||
| 0 | 492 (76.28) | 510 (79.07) | 499 (77.13) | 477 (73.72) | |
| 1 or more | 153 (23.72) | 135 (20.93) | 148 (22.87) | 170 (26.28) | |
| Country of birth of mother, n (%) | 0.001 | ||||
| Spain | 550 (85.27) | 588 (91.30) | 614 (94.75) | 612 (94.88) | |
| Other | 95 (14.73) | 56 (8.70) | 34 (5.25) | 33 (5.12) | |
| Cohort location, n (%) | 0.001 | ||||
| Asturias | 103 (15.97) | 95 (14.73) | 115 (17.75) | 169 (26.12) | |
| Gipuzkoa | 183 (28.37) | 170 (26.36) | 155 (23.92) | 119 (18.39) | |
| Sabadell | 123 (19.07) | 149 (23.10) | 187 (28.86) | 195 (30.14) | |
| Valencia | 236 (36.59) | 231 (35.81) | 191 (29.48) | 164 (25.35) | |
| Cord blood omega 6(AA)/omega 3 (EPA + DHA) ratio, mean (SD) | 2.98 (0.83) | 2.94 (0.89) | 2.93 (0.84) | 2.87 (0.77) | 0.560 |
| Breastfeeding (weeks), n (%) | 0.194 | ||||
| 0 | 87 (15.48) | 77 (16.96) | 97 (16.96 | 79 (13.64) | |
| >0–16 | 157 (27.94) | 156 (26.94) | 129 (22.55) | 140 (24.18) | |
| >16–24 | 82 (14.59) | 103 (17.79) | 92 (16.08) | 86 (14.85) | |
| >24 | 236 (41.99) | 243 (41.97) | 254 (44.41) | 274 (47.32) | |
| Child Characteristics | |||||
| Sex, n (%) | 0.459 | ||||
| Female | 300 (48.78) | 300 (48.47) | 317 (50.48) | 285 (45.97) | |
| Male | 315 (51.22) | 319 (51.53) | 311 (49.52) | 335 (54.03) | |
| Birthweight, n (%) | 0.290 | ||||
| <3000 g | 171 (27.94) | 178 (28.94) | 183 (29.33) | 154 (25.00) | |
| 3000–3500 g | 263 (42.97) | 258 (41.95) | 269 (43.11) | 299 (48.54) | |
| >3500 g | 178 (29.08) | 179 (29.11) | 172 (27.56) | 163 (26.46) | |
| Quartiles of Dietary Omega-3 b Intake at 1st Trimester | p Values | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| MSCA | n = 422 | n = 444 | n = 468 | n = 461 | |
| General Cognitive a | 98.59 (14.23) | 100.45 (15.11) | 100.70 (14.12) | 101.11 (15.57) | 0.060 |
| Verbal a | 98.71 (14.19) | 99.69 (14.81) | 100.74 (14.70) | 101.24 (15.72) | 0.056 |
| Executive Function a | 98.60 (14.48) | 100.14 (15.17) | 100.98 (14.33) | 101.08 (15.24) | 0.049 |
| ANT | n = 379 | n = 411 | n = 444 | n = 421 | |
| HRT-SE c | 314.93 (80.45) | 306.11 (83.50) | 306.39 (81.24) | 297.31 (83.57) | 0.027 |
| Neuropsychological Outcome | No. of Subjects | Difference in Child’s Neuropsychological Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimally adjusted a | Fully adjusted b | |||||||
| β | (95% CI) | p * | β | (95% CI) | p * | |||
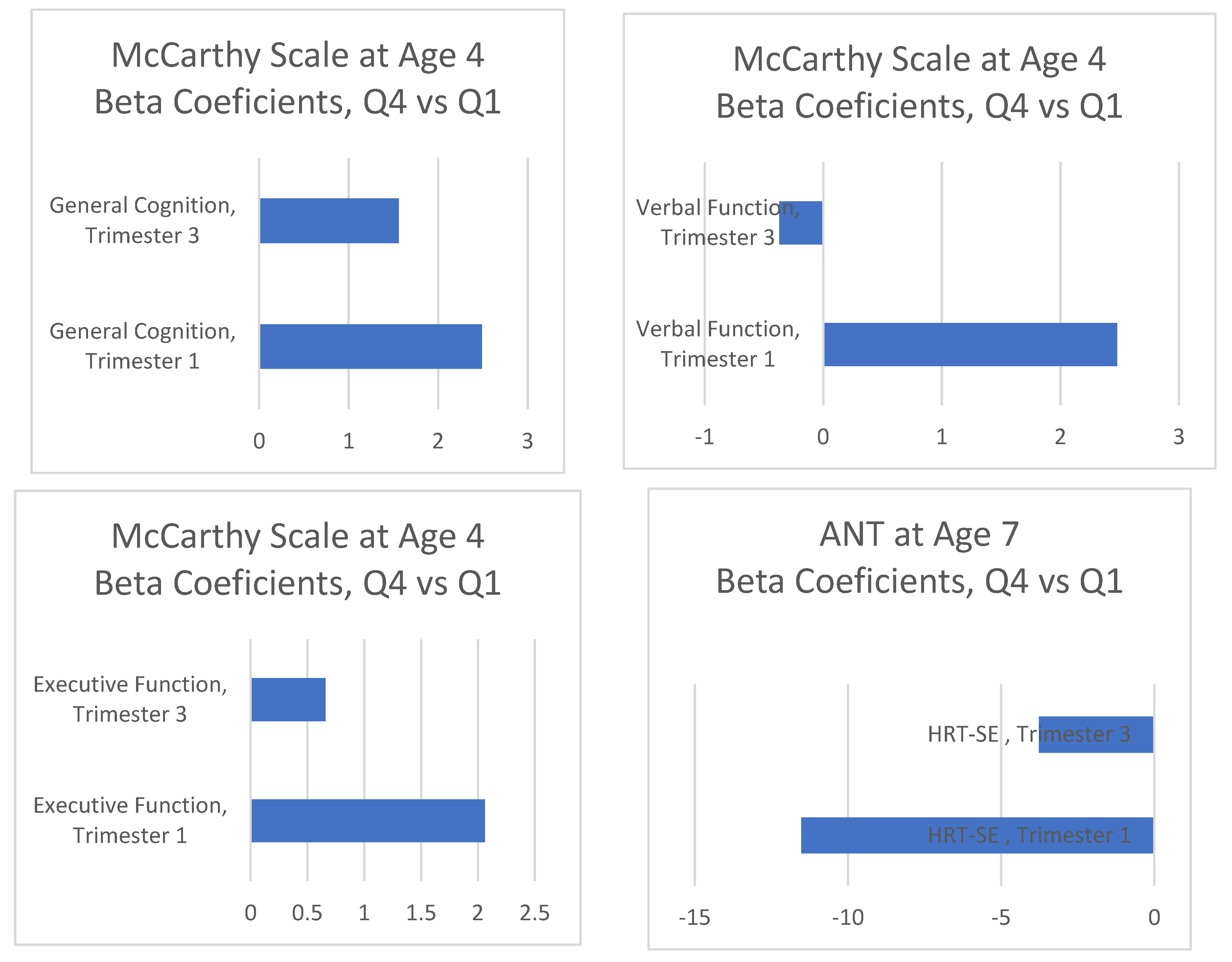
| MSCA | ||||||||
| General Cognitive | ||||||||
| First quartile | 409 | ref | ref | |||||
| Second quartile | 425 | 1.72 | (−0.16, 3.60) | 0.070 | 1.16 | (−0.67, 2.98) | 0.212 | |
| Third quartile | 443 | 2.19 | (0.32, 4.05) | 0.020 | 1.61 | (−0.21 3.43) | 0.084 | |
| Fourth quartile | 436 | 2.64 | (0.76, 4.52) | 0.006 | 2.26 | (0.41, 4.11) | 0.017 | |
| Verbal | ||||||||
| First quartile | 409 | ref | ref | |||||
| Second quartile | 425 | 0.82 | (−1.12, 2.77) | 0.405 | 0.35 | (−1.57, 2.27) | 0.722 | |
| Third quartile | 443 | 2.16 | (0.24, 4.10) | 0.028 | 1.82 | (−0.10, 3.7) | 0.063 | |
| Fourth quartile | 436 | 2.61 | (0.66, 4.55) | 0.009 | 2.48 | (0.53, 4.43) | 0.013 | |
| Executive Function | ||||||||
| First quartile | 409 | ref | ref | |||||
| Second quartile | 425 | 1.39 | (−0.52, 3.31) | 0.153 | 1.00 | (−0.86, 2.87) | 0.291 | |
| Third quartile | 443 | 2.43 | (0.53, 4.33) | 0.012 | 1.88 | (0.01, 3.74) | 0.049 | |
| Fourth quartile | 436 | 2.55 | (0.63, 4.47) | 0.009 | 2.06 | (0.17, 3.95) | 0.033 | |
| ANT | ||||||||
| HRT-SE | ||||||||
| First quartile | 363 | ref | ref | |||||
| Second quartile | 392 | −8.22 | (−19.14, 2.68) | 0.130 | −7.29 | (−18.50, 3.91) | 0.200 | |
| Third quartile | 421 | −9.01 | (−19.77, 1.75) | 0.100 | −8.01 | (−19.10, 3.09) | 0.160 | |
| Fourth quartile | 398 | −12.42 | (−23.41, −1.43) | 0.030 | −11.52 | (−22.95, −0.09) | 0.048 | |
| Neuropsychological Outcome | No. of Subjects | Difference in Child’s Neuropsychological Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Fully adjusted a | Inverse probability weighting b | |||||||
| β | (95% CI) | p | β | (95% CI) | p | |||
| MSCA | 1713 | |||||||
| General Cognitive | ||||||||
| First quartile | ref | ref | ||||||
| Second quartile | 1.16 | (−0.67, 2.98) | 0.212 | 0.95 | (−0.92, 2.81) | 0.320 | ||
| Third quartile | 1.61 | (−0.21 3.43) | 0.084 | 1.98 | (0.19, 3.77) | 0.031 | ||
| Fourth quartile | 2.26 | (0.41, 4.11) | 0.017 | 2.49 | (0.62, 4.36) | 0.009 | ||
| Verbal | ||||||||
| First quartile | ref | ref | ||||||
| Second quartile | 0.35 | (−1.57, 2.27) | 0.722 | 0.20 | (−1.71, 2.10) | 0.841 | ||
| Third quartile | 1.82 | (−0.10, 3.7) | 0.063 | 2.08 | (0.21, 3.94) | 0.029 | ||
| Fourth quartile | 2.48 | (0.53, 4.43) | 0.013 | 2.76 | (0.83, 4.69) | 0.005 | ||
| Executive Function | ||||||||
| First quartile | ref | ref | ||||||
| Second quartile | 1.00 | (−0.86, 2.87) | 0.291 | 0.73 | (−1.15, 2.62) | 0.447 | ||
| Third quartile | 1.88 | (0.01, 3.74) | 0.049 | 2.23 | (0.38, 4.08) | 0.019 | ||
| Fourth quartile | 2.06 | (0.166, 3.95) | 0.033 | 2.54 | (0.65, 4,43) | 0.008 | ||
| ANT | ||||||||
| HRT-SE | 1574 | |||||||
| First quartile | ref | ref | ||||||
| Second quartile | −7.29 | (−18.50, 3.91) | 0.200 | −8.34 | (−23.66, 1.25) | 0.078 | ||
| Third quartile | −8.01 | (−19.10, 3.09) | 0.160 | −8.90 | (−20.53, 3.06) | 0.147 | ||
| Fourth quartile | −11.52 | (−22.95, −0.09) | 0.048 | −14.34 | (−30.76, −6.12) | 0.003 | ||
| Neuropsychological Outcome | Difference in Child’s Neuropsychological Score | |
|---|---|---|
| Minimally adjusted a | Change in % | |
| β (95% CI) | ||
| MSCA | ||
| Executive Function | ||
| Minimially adjusted model | 2.15 (0.15, 4.14) | |
| Maternal social class | 1.78 (−0.18, 3.74) | −17.06 |
| Maternal education | 1.96 (0.03, 3.89) | 10.12 |
| Pre-pregnancy BMI | 2.06 (0.12, 3.99) | 4.93 |
| Child’s birth weight | 2.03 (0.10, 3.96) | −1.37 |
| Alcohol consumption | 2.02 (0.08, 3.95) | −0.61 |
| Smoking during pregnancy | 2.02 (0.09, 3.95) | 0.18 |
| General Cognitive | ||
| Minimially adjusted model | 2.12 (0.17, 4.07) | |
| Maternal social class | 1.72 (−0.18, 3.62) | −18.76 |
| Maternal education | 1.90 (0.03, 3.78) | 10.4 |
| Pre-pregnancy BMI | 1.98 (0.10, 3.85) | 3.91 |
| Child’s birth weight | 1.95 (0.07, 3.82) | −1.44 |
| Alcohol consumption | 1.92 (0.05, 3.80) | −1.35 |
| Smoking during pregnancy | 1.93 (0.05, 3.80) | 0.24 |
| Verbal | ||
| Minimially adjusted model | 2.14 (0.11, 4.17) | |
| Maternal social class | 1.82 (−0.18, 3.83) | −14.85 |
| Maternal education | 1.99 (0.01, 3.97) | 9.14 |
| Pre-pregnancy BMI | 2.11 (0.13, 4.09) | 5.99 |
| Child’s birth weight | 2.10 (0.12, 4.08) | −0.48 |
| Alcohol consumption | 2.10 (0.12, 4.09) | 0.19 |
| Smoking during pregnancy | 2.10 (0.12, 4.08) | −0.1 |
| ANT | ||
| HRT-SE | ||
| Minimally adjusted model | −8.50 (−20.05, 3.05) | |
| Maternal social class | −7.45 (−18.97, 4.08) | −12.41 |
| Maternal education | −7.79 (−19.29, 3.71) | 4.58 |
| Pre-pregnancy BMI | −7.98 (−19.50, 3.54) | 2.44 |
| Child’s birth weight | −8.02 (−19.55, 3.50) | 0.57 |
| Alcohol consumption | −8.01 (−19.51, 3.50) | −0.23 |
| Smoking during pregnancy | −7.99 (−19.51, 3.53) | −0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahaei, H.; Gignac, F.; Pinar, A.; Fernandez-Barrés, S.; Romaguera, D.; Vioque, J.; Santa-Marina, L.; Subiza-Pérez, M.; Llop, S.; Soler-Blasco, R.; et al. Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Multi-Centre Population-Based Birth Cohort Study in Spain. Nutrients 2022, 14, 518. https://doi.org/10.3390/nu14030518
Tahaei H, Gignac F, Pinar A, Fernandez-Barrés S, Romaguera D, Vioque J, Santa-Marina L, Subiza-Pérez M, Llop S, Soler-Blasco R, et al. Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Multi-Centre Population-Based Birth Cohort Study in Spain. Nutrients. 2022; 14(3):518. https://doi.org/10.3390/nu14030518
Chicago/Turabian StyleTahaei, Hana, Florence Gignac, Ariadna Pinar, Silvia Fernandez-Barrés, Dora Romaguera, Jesus Vioque, Loreto Santa-Marina, Mikel Subiza-Pérez, Sabrina Llop, Raquel Soler-Blasco, and et al. 2022. "Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Multi-Centre Population-Based Birth Cohort Study in Spain" Nutrients 14, no. 3: 518. https://doi.org/10.3390/nu14030518
APA StyleTahaei, H., Gignac, F., Pinar, A., Fernandez-Barrés, S., Romaguera, D., Vioque, J., Santa-Marina, L., Subiza-Pérez, M., Llop, S., Soler-Blasco, R., Arija, V., Salas-Salvadó, J., Tardón, A., Riaño-Galán, I., Sunyer, J., Guxens, M., & Julvez, J. (2022). Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Multi-Centre Population-Based Birth Cohort Study in Spain. Nutrients, 14(3), 518. https://doi.org/10.3390/nu14030518










