Physical Performance and Non-Esterified Fatty Acids in Men and Women after Transcatheter Aortic Valve Implantation (TAVI)
Abstract
:1. Introduction
2. Materials and Methods
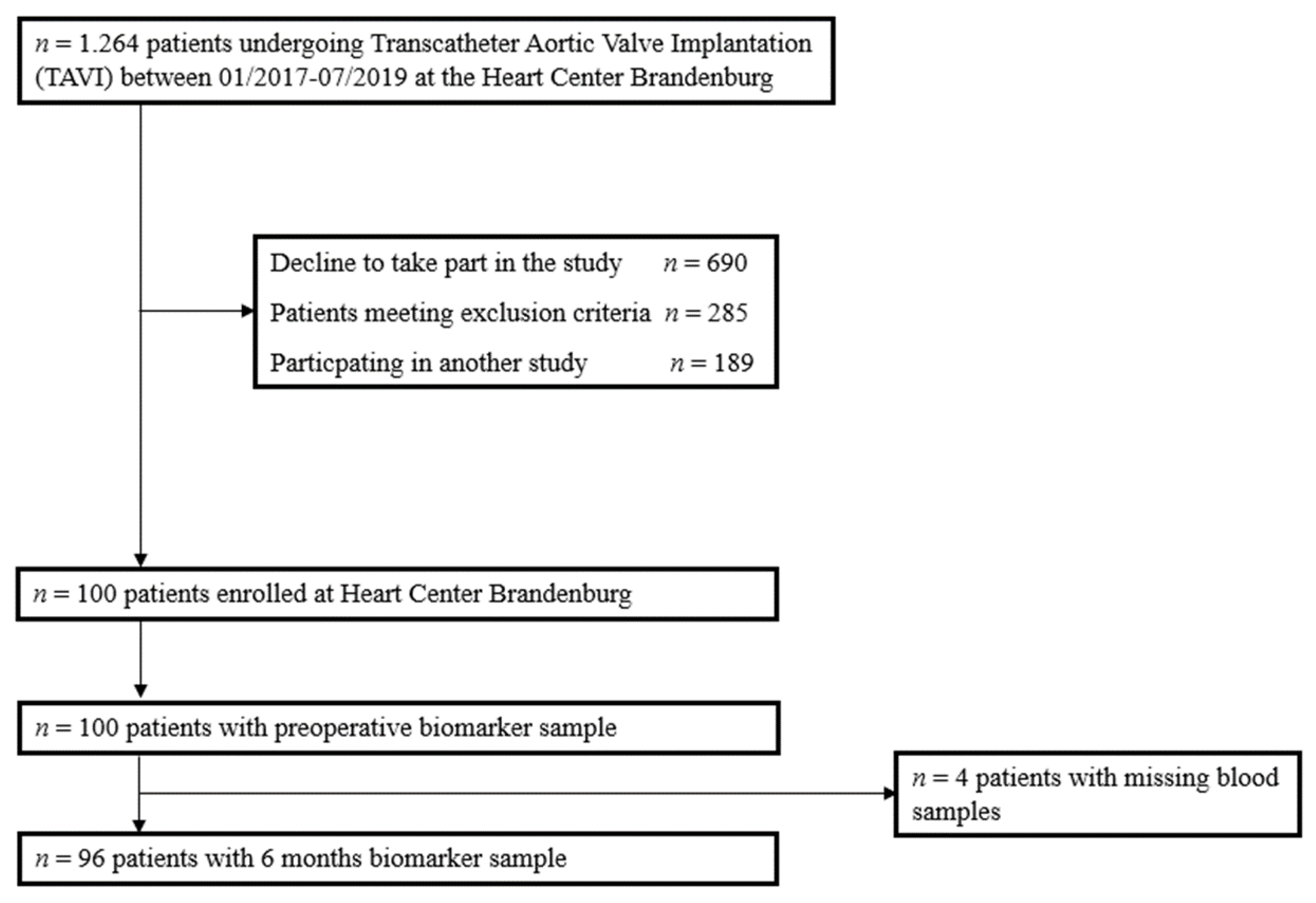
2.1. Study Design and Patients
2.2. Anthropometric Analysis
2.3. Physical Performance
2.4. Sample Collection
2.5. Data Collection
2.6. Transthoracic Echocardiography
2.7. TAVI Procedure
2.8. Statistics
3. Results
3.1. Patient Characteristics
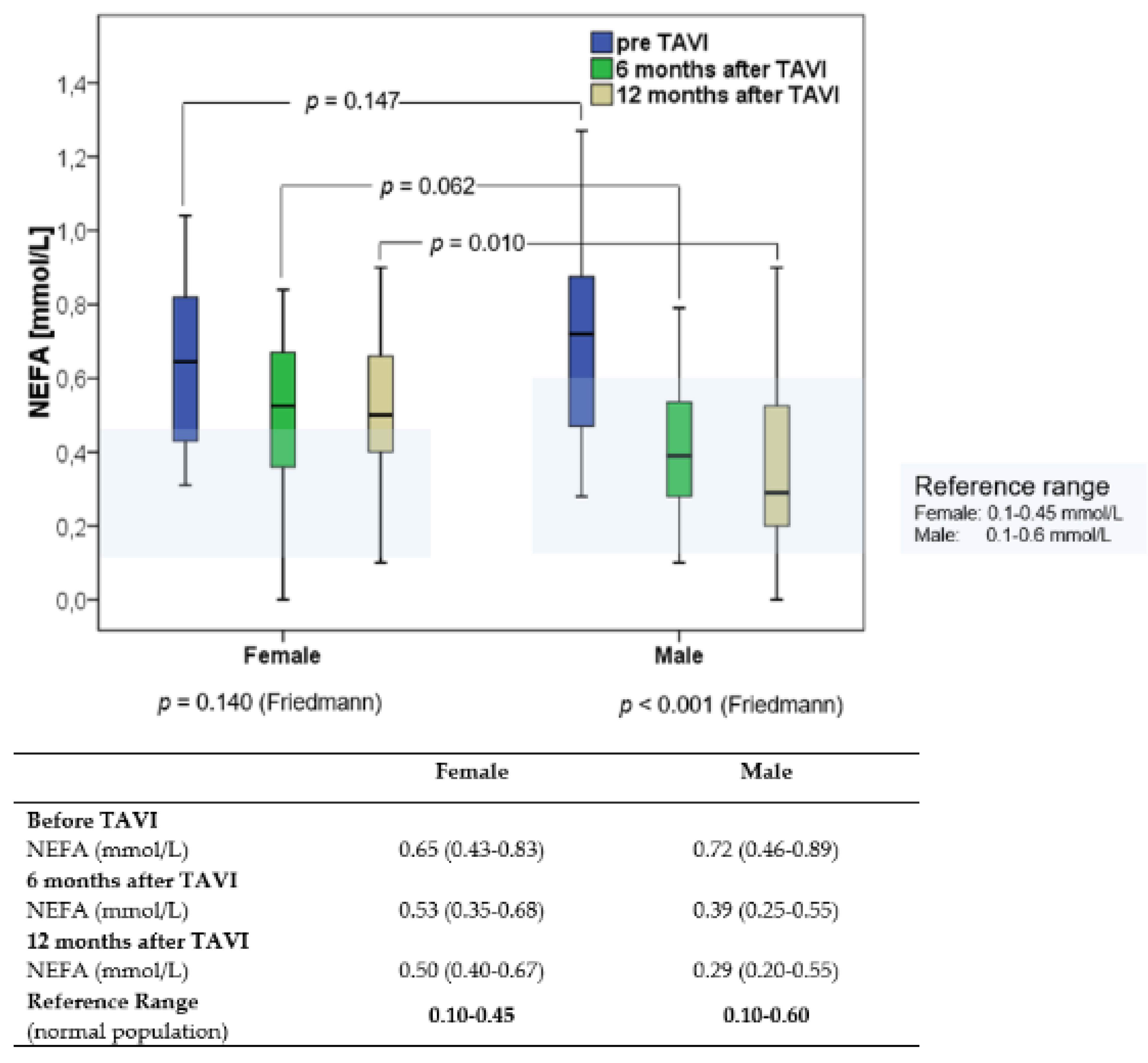
3.2. NEFA Concentrations before and after TAVI
3.3. Clinical Outcomes
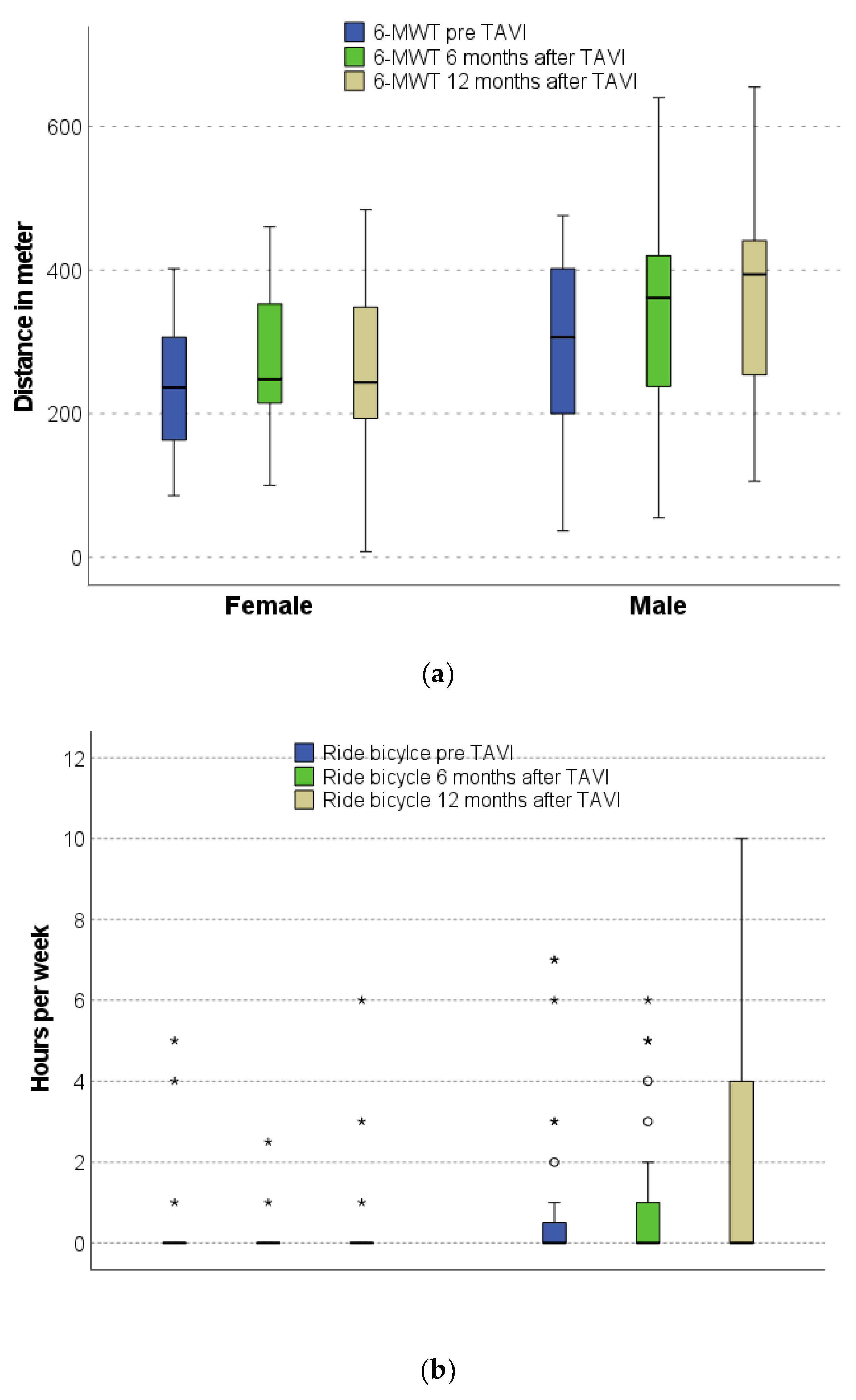
3.4. Physical Performance before and after TAVI
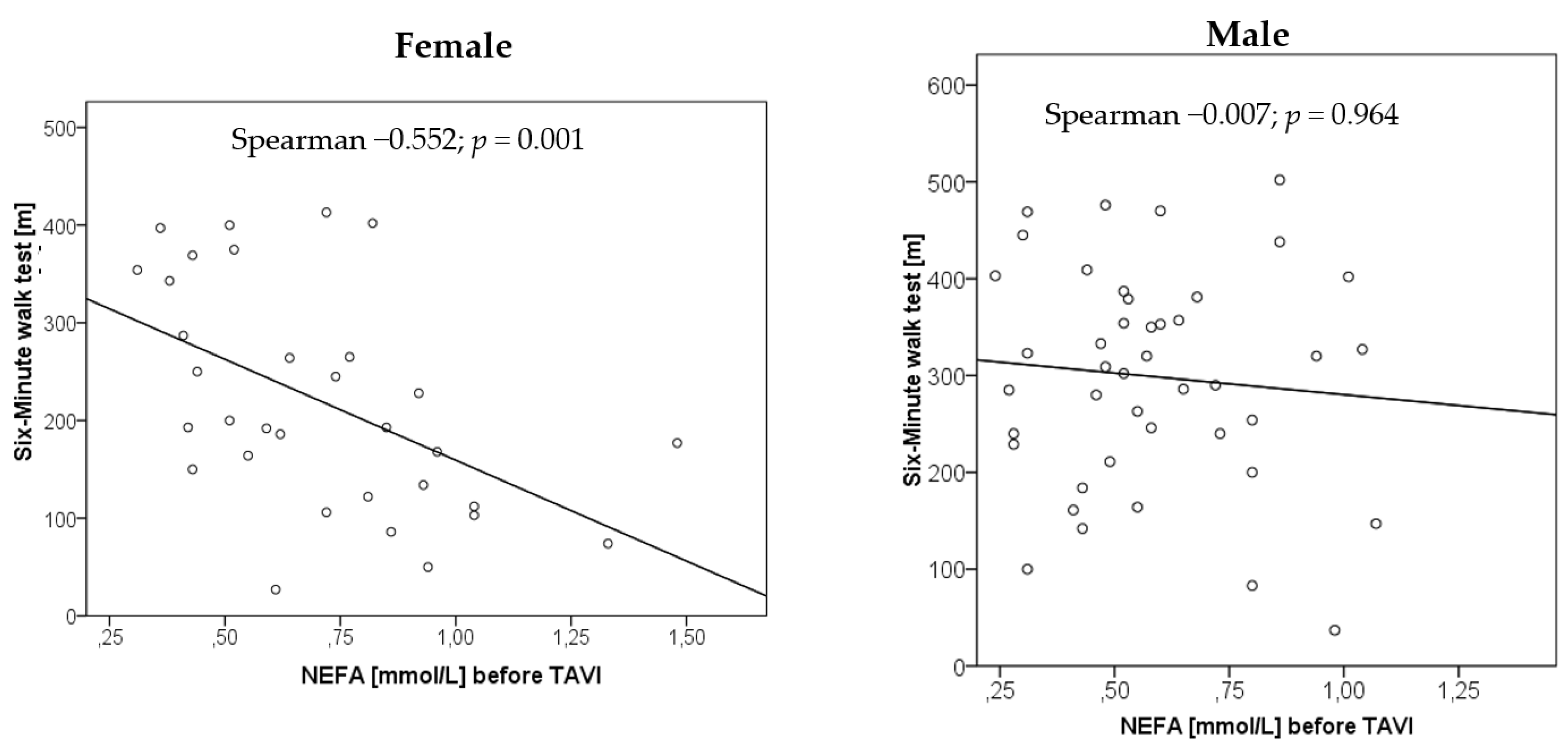
3.5. Relationship of NEFA Concentrations with Physical Performance
3.6. Relationship of NEFA Concentrations with Laboratory and Echocardiographic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bäck, M.; Gasser, T.C.; Michel, J.-B.; Caligiuri, G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc. Res. 2013, 99, 232–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plunde, O.; Bäck, M. Fatty acids and aortic valve stenosis. Kardiol. Polska 2021, 79, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Artiach, G.; Bäck, M. Omega-3 Polyunsaturated Fatty Acids and the Resolution of Inflammation: Novel Therapeutic Opportu-nities for Aortic Valve Stenosis? Front. Cell Dev. Biol. 2020, 8, 584128. [Google Scholar] [CrossRef]
- Van Driel, B.O.; Schuldt, M.; Algül, S.; Levin, E.; Güclü, A.; Germans, T.; Rossum, A.C.V.; Pei, J.; Harakalova, M.; Baas, A.; et al. Metabolomics in Severe Aortic Stenosis Reveals Intermediates of Nitric Oxide Synthesis as Most Distinctive Markers. Int. J. Mol. Sci. 2021, 22, 3569. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Luk, K.; Schulz, C.A.; Engert, J.C.; Do, R.; Hindy, G.; Rukh, G.; Dufresne, L.; Almgren, P.; Owens, D.S. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA 2014, 312, 1764–1771. [Google Scholar] [CrossRef] [Green Version]
- Denegri, A.; Romano, M.; Petronio, A.S.; Angelillis, M.; Giannini, C.; Fiorina, C.; Branca, L.; Barbanti, M.; Costa, G.; Brambilla, N.; et al. Gender Differences after Transcatheter Aortic Valve Replacement (TAVR): Insights from the Italian Clinical Service Project. J. Cardiovasc. Dev. Dis. 2021, 8, 114. [Google Scholar] [CrossRef]
- Simard, L.; Cote, N.; Dagenais, F.; Mathieu, P.; Couture, C.; Trahan, S.; Bosse, Y.; Mohammadi, S.; Page, S.; Joubert, P.; et al. Sex-Related Discordance Between Aortic Valve Calci-fication and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ. Res. 2017, 120, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, R.; Orii, M.; Fujiwara, J.; Yoshizawa, M.; Nakajima, Y.; Ishikawa, Y.; Kumagai, A.; Fusazaki, T.; Tashiro, A.; Kin, H.; et al. Sex-Related Differences in Cardiac Remodeling and Reverse Remodeling After Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis in a Japanese Population. Int. Hear. J. 2020, 61, 961–969. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Jensen, M.D. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [Green Version]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Jiang, W.; Wang, Y.; Wu, Y.; Chen, H.; Zhao, X. Plasma levels of free fatty acid differ in patients with left ventricular pre-served, mid-range, and reduced ejection fraction. BMC Cardiovasc. Disord. 2018, 18, 104. [Google Scholar] [CrossRef]
- Degoricija, V.; Trbušić, M.; Potočnjak, I.; Radulović, B.; Pregartner, G.; Berghold, A.; Scharnagl, H.; Stojakovic, T.; Tiran, B.; Frank, S. Serum concentrations of free fatty acids are associated with 3-month mortality in acute heart failure patients. Clin. Chem. Lab. Med. 2019, 57, 1799–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kreutzenberg, S.V.; Puato, M.; Kiwanuka, E.; Del Prato, S.; Pauletto, P.; Pasini, L.; Tiengo, A.; Avogaro, A. Elevated non-esterified fatty acids impair nitric oxide independent vasodilation, in humans: Evidence for a role of inwardly rectifying potassium channels. Atherosclerosis 2003, 169, 147–153. [Google Scholar] [CrossRef]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobczak, A.; Blindauer, C.; Stewart, A. Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef] [Green Version]
- Saeed, S.; Dweck, M.R.; Chambers, J. Sex differences in aortic stenosis: From pathophysiology to treatment. Expert Rev. Cardiovasc. Ther. 2020, 18, 65–76. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aufenanger, J.; Kattermann, R. Klinisch-chemische Meßgröße: Freie Fettsäuren (FFS), S. 319–320 in Greiling/Greßner: Lehrbuch der Klinischen Chemie und Pathobiochemie, 3rd ed.; Schattauer: Stuttgart, Germany, 1995. [Google Scholar]
- Magkos, F.; Patterson, B.W.; Mohammed, B.S.; Klein, S.; Mittendorfer, B. Women Produce Fewer but Triglyceride-Richer Very Low-Density Lipoproteins than Men. J. Clin. Endocrinol. Metab. 2007, 92, 1311–1318. [Google Scholar] [CrossRef] [Green Version]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djoussé, L.; Benkeser, D.; Arnold, A.; Kizer, J.R.; Zieman, S.J.; Lemaitre, R.N.; Tracy, R.P.; Gottdiener, J.S.; Mozaffarian, D.; Siscovick, D.S.; et al. Plasma free fatty acids and risk of heart failure: The Cardiovascular Health Study. Circ. Heart Fail. 2013, 6, 964–969. [Google Scholar] [CrossRef] [Green Version]
- Birkenfeld, A.L.; Boschmann, M.; Moro, C.; Adams, F.; Heusser, K.; Franke, G.; Berlan, M.; Luft, F.C.; Lafontan, M.; Jordan, J. Lipid mobilization with physiological atrial na-triuretic peptide concentrations in humans. J. Clin. Endocrinol. Metab. 2005, 90, 3622–3628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehlinger, D.E.; Weidmann, P.; Gnädinger, M.P.; Hasler, L.; Bachmann, C.; Shaw, S.; Hellmüller, B.; Lang, R.E. Increase in Circulating Insulin Induced by Atrial Natriuretic Peptide in Normal Humans. J. Cardiovasc. Pharmacol. 1986, 8, 1122–1129. [Google Scholar] [CrossRef]
- Nicholls, D.P.; Onuoha, G.N.; McDowell, G.; Elborn, J.; Riley, M.S.; Nugent, A.M.; Steele, I.C.; Shaw, C.; Buchanan, K.D. Neuroendocrine changes in chronic cardiac failure. Basic Res. Cardiol. 1996, 91, 13–20. [Google Scholar]
- Blaak, E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Henderson, G.C.; Fattor, J.A.; Horning, M.A.; Faghihnia, N.; Johnson, M.; Mau, T.L.; Luke-Zeitoun, M.; Brooks, G.A. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J. Physiol. 2007, 584, 963–981. [Google Scholar] [CrossRef] [PubMed]
- Lönnqvist, F.; Thörne, A.; Large, V.; Arner, P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arter. Thromb. Vasc. Biol. 1997, 17, 1472–1480. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Boschmann, M.; Moro, C.; Adams, F.; Heusser, K.; Tank, J.; Diedrich, A.; Schroeder, C.; Franke, G.; Berlan, M.; et al. ß-Adrenergic and Atrial Natriuretic Peptide Inter-actions on Human Cardiovascular and Metabolic Regulation. J. Clin. Endocrinol. Metab. 2006, 91, 5069–5075. [Google Scholar] [CrossRef] [Green Version]
- Szabó, T.; Postrach, E.; Mähler, A.; Kung, T.; Turhan, G.; Von Haehling, S.; Anker, S.D.; Boschmann, M.; Doehner, W. Increased catabolic activity in adipose tissue of patients with chronic heart failure. Eur. J. Hear. Fail. 2013, 15, 1131–1137. [Google Scholar] [CrossRef]
- Delarue, J.; Magnan, C. Free fatty acids and insulin resistance. Curr. Opin Clin. Nutr. Metab. Care 2007, 10, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Jawad Altisent, O.; Puri, R.; Regueiro, A.; Chamandi, C.; Rodriguez-Gabella, T.; Del Trigo, M.; Campelo-Parada, F.; Couture, T.; Marsal, J.R.; Côté, M.; et al. Predictors and Association with Clinical Outcomes of the Changes in Exercise Capacity After Transcatheter Aortic Valve Replacement. Circulation 2017, 136, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Miedema, M.D.; Maziarz, M.; Biggs, M.L.; Zieman, S.J.; Kizer, J.R.; Ix, J.H.; Mozaffarian, D.; Tracy, R.P.; Psaty, B.M.; Siscovick, D.S.; et al. Plasma-Free Fatty Acids, Fatty Acid–Binding Protein 4, and Mortality in Older Adults (from the Cardiovascular Health Study). Am. J. Cardiol. 2014, 114, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Scharnagl, H.; Tiran, B.; Wellnitz, B.; Seelhorst, U.; Boehm, B.O.; März, W. Elevated plasma free fatty acids predict sudden cardiac death: A 6.85-year follow-up of 3315 patients after coronary angiography. Eur. Hear. J. 2007, 28, 2763–2769. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.-L.; Cao, Y.-X.; Liu, H.-H.; Zhang, H.-W.; Guo, Y.-L.; Wu, N.-Q.; Zhu, C.-G.; Xu, R.-X.; Gao, Y.; Sun, J.; et al. Impact of free fatty acids on prognosis in coronary artery disease patients under different glucose metabolism status. Cardiovasc. Diabetol. 2019, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Demographics | ||||
|---|---|---|---|---|
| Overall Cohort n = 100 | Female n = 44 | Male n = 56 | p-Value | |
| Age (years) | 82.0 (78.0–85.0) | 82.5 (78.3–84.8) | 81.0 (77.3–85.0) | 0.808 |
| Blood pressure, systolic (mmHg) | 125 (111–135) | 125 (115–136) | 122 (110–135) | 0.394 |
| Blood pressure, diastolic (mmHg) | 70 (64–77) | 73 (66–86) | 70 (61–78) | 0.284 |
| BMI (kg/m2) | 27.7 (24.7–30.9) | 28.5 (23.7–33.5) | 27.2 (25.3–30.4) | 0.697 |
| BMI > 29, n (%) | 36 (36%) | 19 (43.2%) | 17 (30.4%) | 0.185 |
| BMI 24–29, n (%) | 47 (47%) | 14 (31.8%) | 33 (58.9%) | 0.007 |
| BMI < 24, n (%) | 17 (17%) | 11 (25.0%) | 6 (10.7%) | 0.059 |
| Skinfold thickness (mm) | 52 (38–66) | 54 (45–76) | 50 (35–64) | 0.270 |
| Waist size (cm) | 106 (99–113) | 106 (94–113) | 106 (100–114) | 0.312 |
| Total body fat (%) | 24.6 (19.2–29.3) | 30.4 (27.3–34.2) | 22.0 (17.6–25.2) | <0.001 |
| Level of functional exercise capacity | ||||
| Distance in 6-MWT (m) | 264 (173–356) | 197 (131–330) | 309 (229–381) | 0.007 |
| Borg scale | 13 (12–15) | 13 (12–15) | 13 (12–15) | 0.505 |
| Cardiac function | ||||
| LVEF (%) | 56.0 (48.0–60.0) | 55.5 (50.8–60.0) | 56.0 (45.0–60.0) | 0.569 |
| NT-proBNP (pg/mL) | 1.639 (734–4.841) | 1.867 (772–6.258) | 1.382 (583–3.910) | 0.266 |
| Haemoglobin (mmol/L) | 7.8 (7.1–8.6) | 7.4 (7.0–8.0) | 8.1 (7.2–8.9) | 0.007 |
| Haemoglobin < Sex-specific Cut-off *, n (%) | 23 (52.3%) | 33 (59.0%) | 0.506 | |
| Albumin (g/L) | 36.9 (34.7–39.4) | 36.9 (35.0–40.2) | 37.1 (34.1–39.1) | 0.635 |
| Heart failure | ||||
| HFrEF, n (%) | 13 (13%) | 5 (11.4%) | 8 (14.3%) | 0.150 |
| HFmrEF, n (%) | 13 (13%) | 3 (6.8%) | 10 (17.9%) | |
| HFpEF, n (%) | 72 (72%) | 34 (77.3%) | 38 (67.9%) | |
| NYHA class ~ | ||||
| NYHA II, n (%) | 15 (15%) | 3 (7.1%) | 12 (22.2%) | 0.205 |
| NYHA III, n (%) | 69 (69%) | 31 (73.8%) | 38 (70.4%) | |
| NYHA IV, n (%) | 11 (11%) | 7 (16.7%) | 4 (7.4%) | |
| Comorbidities | ||||
| Logistic EuroSCORE (%) | 12.8 (8.4–19.5) | 12.8 (9.8–19.0) | 12.4 (7.4–20.0) | 0.346 |
| Arterial Hypertension, n (%) | 94 (94.0%) | 41 (93.2%) | 53 (94.6%) | 0.760 |
| Chronic kidney disease #, n (%) | 68 (68.0%) | 34 (77.3%) | 34 (60.7%) | 0.078 |
| eGFR (mL/min/1.73 m2) | 54 (41–71) | 52 (39–67) | 55 (41–77) | 0.238 |
| Diabetes mellitus, n (%) (oral medication) | 24 (24.0%) | 8 (18.2%) | 16 (28.6%) | 0.227 |
| Insulin-dependent diabetes, n (%) | 22 (22.0%) | 8 (18.2%) | 14 (25%) | 0.414 |
| HbA1c (%) | 5.9 (5.6–6.3) | 5.9 (5.4–6.2) | 5.9 (5.6–6.4) | 0.272 |
| Chronic obstructive pulmonary disease, n (%) | 20 (20.0%) | 7 (15.9%) | 13 (23.2%) | 0.365 |
| Coronary heart disease, n (%) | 70 (70.0%) | 25 (56.8%) | 45 (80.4%) | 0.011 |
| Hyperlipidaemia, n (%) | 67 (67.0%) | 26 (59.1%) | 41 (73.2%) | 0.136 |
| Peripheral vascular disease, n (%) | 25 (25.0%) | 10 (22.7%) | 15 (26.8%) | 0.642 |
| Atrial fibrillation, n (%) | 55 (55.0%) | 23 (52.3%) | 32 (57.1%) | 0.688 |
| Coronary artery bypass grafting, n (%) | 14 (14.0%) | 2 (4.6%) | 12 (21.4%) | 0.016 |
| Previous myocardial infarction, n (%) | 17 (17.0%) | 7 (15.9%) | 10 (17.9%) | >0.99 |
| Previous stroke, n (%) | 15 (15.0%) | 5 (11.4%) | 10 (17.9%) | 0.412 |
| Malignant disease, n (%) | 21 (21.0%) | 12 (20.5%) | 9 (16.1%) | 0.172 |
| Medication | ||||
| ACE inhibitors, n (%) | 43 (43.4%) | 16 (37.2%) | 27 (48.2%) | 0.274 |
| AT1 blockers, n (%) | 32 (32.3%) | 14 (32.6%) | 18 (32.1%) | 0.965 |
| Aldosterone antagonists, n (%) | 20 (20.2%) | 9 (20.9%) | 11 (19.6%) | 0.874 |
| Betablocker, n (%) | 76 (76.8%) | 36 (83.7%) | 40 (71.4%) | 0.151 |
| Loop diuretics, n (%) | 63 (63.6%) | 29 (67.4%) | 34 (60.7%) | 0.490 |
| Thiazide, n (%) | 25 (25.3%) | 15 (34.9%) | 10 (17.9%) | 0.053 |
| Statins, n (%) | 73 (73.7%) | 32 (74.4%) | 41 (73.2%) | 0.893 |
| Ezetimibe, n (%) | 4 (4.0%) | 2 (4.7%) | 2 (3.6%) | 0.787 |
| DOACs, n (%) | 30 (30.3%) | 13 (30.2%) | 17 (30.4%) | >0.99 |
| Outcome | ||||
|---|---|---|---|---|
| Overall Cohort n = 100 | Female n = 44 | Male n = 56 | p-Value | |
| ∆ Body mass index (kg/m2) 6 months after TAVI | −0.31 (−0.72–0.69) | −0.32 (−0.75–0.42) | −0.30 (−0.63–0.84) | 0.383 |
| NT-proBNP (pg/mL) 6 months after TAVI | 1.053 (379–1.935) | 1.320 (518–2.367) | 878 (324–1.33) | 0.185 |
| Improvement in 6-MWT >10%, n (%) | 34 (55.7%) | 14 (60.9%) | 20 (52.6%) | 0.530 |
| Improvement in NYHA class, n (%) | 47 (66.2%) | 17 (63.0%) | 30 (68.2%) | 0.652 |
| Improvement in EF > 10%, n (%) | 24 (29.7%) | 9 (29.0%) | 15 (30.0%) | 0.926 |
| Postoperative complication | ||||
| Device implantation due to AV block III, n (%) | 15 (15.0%) | 3 (7.1%) | 12 (21.4%) | 0.051 |
| Left bundle branch block, n (%) | 15 (15.0%) | 5 (11.6%) | 10 (18.2%) | 0.572 |
| Paravalvular aortic regurgitation | ||||
| grade I–II grade II grade III | 10 (10.0%) 2 (2.0%) - | 4 (9.1%) 0 (0%) - | 6 (10.7%) 2 (3.6%) - | 0.788 0.311 |
| Acute kidney injury, n (%) | 11 (11.0%) | 5 (11.6%) | 6 (10.7%) | 0.886 |
| Bleeding, n (%) | 5 (5.0%) | 2 (4.8%) | 3 (5.5%) | >0.99 |
| Delir, n (%) | 4 (4.0%) | 1 (2.3%) | 3 (5.5%) | 0.631 |
| Stroke, n (%) | 2 (2.0%) | 1 (2.3%) | 1 (1.8%) | >0.99 |
| Length of stay in Intensive care unit (days) | 2.0 (1.5–14.0) | 2.0 (2.0–20.0) | 1.0 (5.0–6.0) | 0.554 |
| Length of stay in hospital (days) | 13.0 (9.0–17.0) | 13.0 (11.0–17.0) | 11.5 (9.0–17.0) | 0.180 |
| Rehospitalization within 30 days, n (%) | 10 (10.2%) | 3 (7.1%) | 7 (12.5%) | 0.509 |
| Worsening eGFR > 10% within 12 months | 32 (42.1%) | 15 (51.7%) | 17 (36.2%) | 0.182 |
| Mortality | ||||
| In-hospital mortality, n (%) | 5 (5.0%) | 3 (7.0%) | 2 (3.6%) | 0.650 |
| 6-month mortality, n (%) | 5 (5.0%) | 3 (7.0%) | 2 (3.6%) | 0.650 |
| 12-month mortality, n (%) | 6 (6.0%) | 3 (7.0%) | 3 (5.4%) | >0.99 |
| Female | Male | |||
|---|---|---|---|---|
| Spearman Rho | p-Value | Spearman Rho | p-Value | |
| Before TAVI | ||||
| NEFA with 6-MWT | −0.552 | 0.001 | −0.007 | 0.964 |
| NEFA with bicycle riding | 0.218 | 0.274 | 0.103 | 0.490 |
| 6 months after TAVI | ||||
| NEFA with 6-MWT | 0.278 | 0.169 | 0.077 | 0.660 |
| NEFA with bicycle riding | −0.223 | 0.306 | −0.039 | 0.839 |
| 12 months after TAVI | ||||
| NEFA with 6-MWT | −0.167 | 0.456 | −0.062 | 0.764 |
| NEFA with bicycle riding | 0.056 | 0.803 | −0.190 | 0.342 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Härdrich, M.; Haase-Fielitz, A.; Fielitz, J.; Boschmann, M.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Rudovich, N.; Weylandt, K.H.; Butter, C. Physical Performance and Non-Esterified Fatty Acids in Men and Women after Transcatheter Aortic Valve Implantation (TAVI). Nutrients 2022, 14, 203. https://doi.org/10.3390/nu14010203
Härdrich M, Haase-Fielitz A, Fielitz J, Boschmann M, Pivovarova-Ramich O, Pfeiffer AFH, Rudovich N, Weylandt KH, Butter C. Physical Performance and Non-Esterified Fatty Acids in Men and Women after Transcatheter Aortic Valve Implantation (TAVI). Nutrients. 2022; 14(1):203. https://doi.org/10.3390/nu14010203
Chicago/Turabian StyleHärdrich, Michaela, Anja Haase-Fielitz, Jens Fielitz, Michael Boschmann, Olga Pivovarova-Ramich, Andreas F. H. Pfeiffer, Natalia Rudovich, Karsten H. Weylandt, and Christian Butter. 2022. "Physical Performance and Non-Esterified Fatty Acids in Men and Women after Transcatheter Aortic Valve Implantation (TAVI)" Nutrients 14, no. 1: 203. https://doi.org/10.3390/nu14010203






