Vitamin D Supplementation in Exclusively Breastfed Infants Is Associated with Alterations in the Fecal Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Fecal Microbiota Analysis
2.4. Statistical Analysis
3. Results
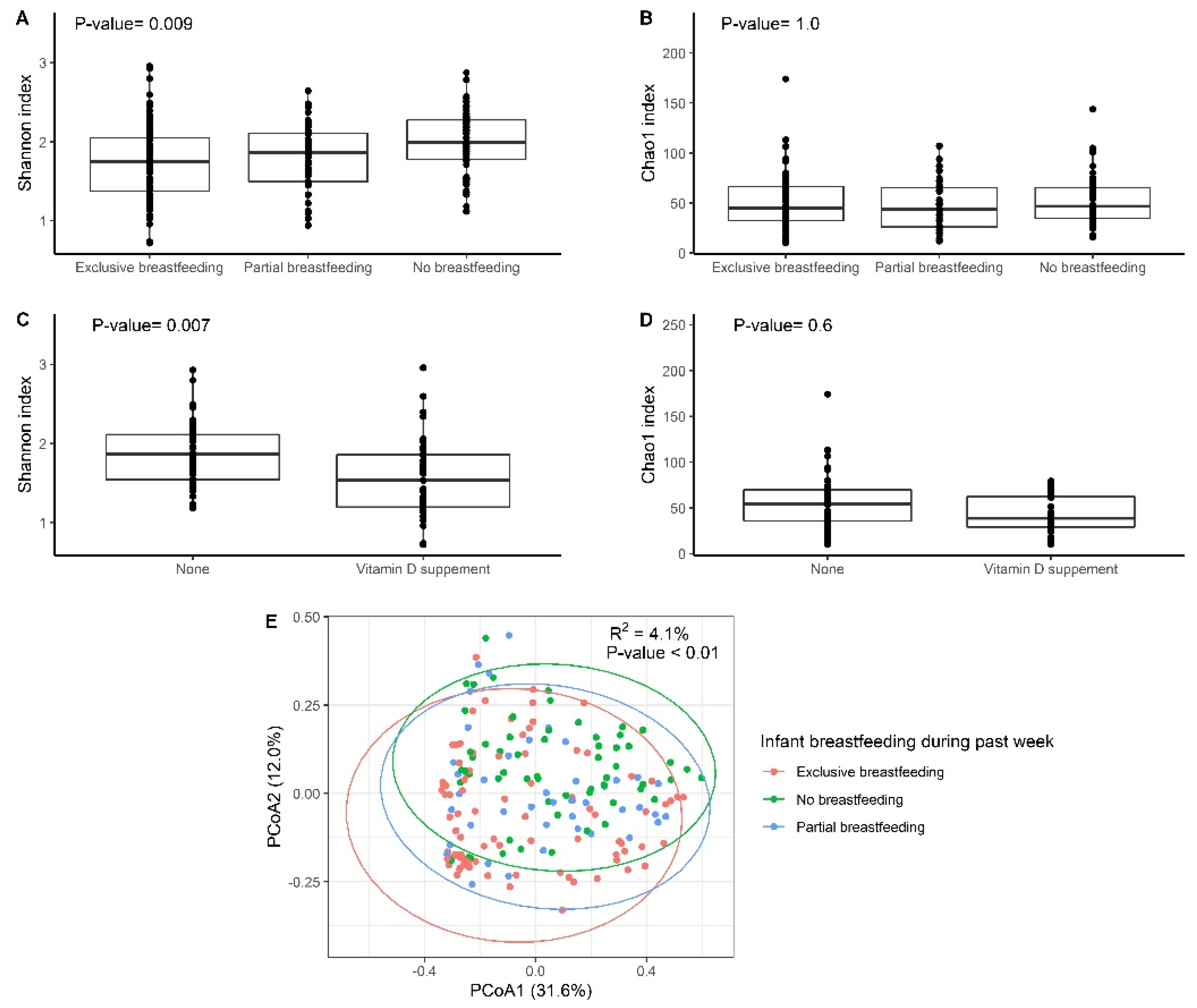
3.1. Participants and Feeding Practices
3.2. Gut Microbiota Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guinane, C.M.; Cotter, P.D. Role of the Gut Microbiota in Health and Chronic Gastrointestinal Disease: Understanding a Hidden Metabolic Organ. Therap. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early Infancy Microbial and Metabolic Alterations Affect Risk of Childhood Asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; et al. The Human Gut Microbiome in Early-Onset Type 1 Diabetes from the TEDDY Study. Nature 2018, 562, 589–594. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and Diabetes: An Evolving Relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The Gut Microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakayama, J. Development of the Gut Microbiota in Infancy and Its Impact on Health in Later Life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The Composition of the Gut Microbiota throughout Life, with an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, Birth Mode, and Diet Shape Microbiome Maturation during Early Life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Macías, E.; Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; González, S.; Martínez-Costa, C.; Collado, M.C. Maternal Diet Shapes the Breast Milk Microbiota Composition and Diversity: Impact of Mode of Delivery and Antibiotic Exposure. J. Nutr. 2021, 151, 330–340. [Google Scholar] [CrossRef]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-Analysis of Effects of Exclusive Breastfeeding on Infant Gut Microbiota across Populations. Nat. Commun. 2018, 9, 4169. [Google Scholar] [CrossRef]
- Fehr, K.; Moossavi, S.; Sbihi, H.; Boutin, R.C.T.; Bode, L.; Robertson, B.; Yonemitsu, C.; Field, C.J.; Becker, A.B.; Mandhane, P.J.; et al. Breastmilk Feeding Practices Are Associated with the Co-Occurrence of Bacteria in Mothers’ Milk and the Infant Gut: The CHILD Cohort Study. Cell Host Microbe 2020, 28, 285–297.e4. [Google Scholar] [CrossRef]
- Haddad, E.N.; Sugino, K.Y.; Kerver, J.M.; Paneth, N.; Comstock, S.S. The Infant Gut Microbiota at 12 Months of Age Is Associated with Human Milk Exposure but Not with Maternal Pre-Pregnancy Body Mass Index or Infant BMI-for-Age z-Scores. Curr. Res. Physiol. 2021, 4, 94–102. [Google Scholar] [CrossRef]
- Sugino, K.Y.; Ma, T.; Kerver, J.M.; Paneth, N.; Comstock, S.S. Human Milk Feeding Patterns at 6 Months of Age Are a Major Determinant of Fecal Bacterial Diversity in Infants. J. Hum. Lact 2020, 37, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human Milk Oligosaccharides: Shaping the Infant Gut Microbiota and Supporting Health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.A.E.; O’Neill, I.J.; Kujawska, M.; Gowrinadh Javvadi, S.; Wijeyesekera, A.; Flegg, Z.; Chalklen, L.; Hall, L.J. Breast Milk-Derived Human Milk Oligosaccharides Promote Bifidobacterium Interactions within a Single Ecosystem. ISME J. 2020, 14, 635–648. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal Development of the Gut Microbiome in Early Childhood from the TEDDY Study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef]
- CDC Vitamin D Is Needed to Support Healthy Bone Development. Available online: https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/diet-and-micronutrients/vitamin-d.html (accessed on 27 July 2021).
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Borges, M.C.; Martini, L.A.; Rogero, M.M. Current Perspectives on Vitamin D, Immune System, and Chronic Diseases. Nutrition 2011, 27, 399–404. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Till, C.; Green, R.; Flora, D.; Hornung, R.; Martinez-Mier, E.A.; Blazer, M.; Farmus, L.; Ayotte, P.; Muckle, G.; Lanphear, B. Fluoride Exposure from Infant Formula and Child IQ in a Canadian Birth Cohort. Environ. Int. 2020, 134, 105315. [Google Scholar] [CrossRef]
- Triantafyllidou, S.; Edwards, M. Lead (Pb) in Tap Water and in Blood: Implications for Lead Exposure in the United States. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1297–1352. [Google Scholar] [CrossRef]
- About|CHARM Study. Available online: https://www.epi.msu.edu/charmstudy/about (accessed on 30 September 2021).
- Paneth, N.; Monk, C. The importance of cohort research starting early in life to understanding child health. Curr. Opin. Pediatr. 2018, 30, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.Y.; Paneth, N.; Comstock, S.S. Michigan Cohorts to Determine Associations of Maternal Pre-Pregnancy Body Mass Index with Pregnancy and Infant Gastrointestinal Microbial Communities: Late Pregnancy and Early Infancy. PLoS ONE 2019, 14, e0213733. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. PLOS Computational Biology 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Thissen, D.; Steinberg, L.; Kuang, D. Quick and Easy Implementation of the Benjamini-Hochberg Procedure for Controlling the False Positive Rate in Multiple Comparisons. J. Educ. Behav. Stat. 2002, 27, 77–83. [Google Scholar] [CrossRef]
- Nnebe-Agumadu, U.H.; Racine, E.F.; Laditka, S.B.; Coffman, M.J. Associations between Perceived Value of Exclusive Breastfeeding among Pregnant Women in the United States and Exclusive Breastfeeding to Three and Six Months Postpartum: A Prospective Study. Int. Breastfeed. J. 2016, 11, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- CDC Results: Breastfeeding Rates. Available online: https://www.cdc.gov/breastfeeding/data/nis_data/results.html (accessed on 1 October 2021).
- Gartner, L.M.; Greer, F.R.; Section on Breastfeeding and Committee on Nutrition. American Academy of Pediatrics Prevention of Rickets and Vitamin D Deficiency: New Guidelines for Vitamin D Intake. Pediatrics 2003, 111, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Uday, S.; Kongjonaj, A.; Aguiar, M.; Tulchinsky, T.; Högler, W. Variations in Infant and Childhood Vitamin D Supplementation Programmes across Europe and Factors Influencing Adherence. Endocr. Connect. 2017, 6, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Field, C.J.; Weinberg, A.R.; Letourneau, N.; APrON Study Team. Both Mother and Infant Require a Vitamin D Supplement to Ensure That Infants’ Vitamin D Status Meets Current Guidelines. Nutrients 2018, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Flores, T.R.; Mielke, G.I.; Wendt, A.; Nunes, B.P.; Bertoldi, A.D. Prepregnancy Weight Excess and Cessation of Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2018, 72, 480–488. [Google Scholar] [CrossRef]
- Tao, X.-Y.; Huang, K.; Yan, S.-Q.; Zuo, A.-Z.; Tao, R.-W.; Cao, H.; Gu, C.-L.; Tao, F.-B. Pre-Pregnancy BMI, Gestational Weight Gain and Breast-Feeding: A Cohort Study in China. Public Health Nutr. 2017, 20, 1001–1008. [Google Scholar] [CrossRef]
- Thompson, L.A.; Zhang, S.; Black, E.; Das, R.; Ryngaert, M.; Sullivan, S.; Roth, J. The Association of Maternal Pre-Pregnancy Body Mass Index with Breastfeeding Initiation. Matern Child Health J. 2013, 17, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.H.; Donath, S. A Systematic Review of Maternal Obesity and Breastfeeding Intention, Initiation and Duration. BMC Pregnancy Childbirth 2007, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Bever Babendure, J.; Reifsnider, E.; Mendias, E.; Moramarco, M.W.; Davila, Y.R. Reduced Breastfeeding Rates among Obese Mothers: A Review of Contributing Factors, Clinical Considerations and Future Directions. Int. Breastfeed. J. 2015, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.J.; Pérez-escamilla, R. Identification of Risk Factors for Delayed Onset of Lactation. J. Am. Dietetic Assoc. 1999, 99, 450–454. [Google Scholar] [CrossRef]
- Lovelady, C.A. Is Maternal Obesity a Cause of Poor Lactation Performance? Nutr. Rev. 2005, 63, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.T.; Josefson, J.; Van Horn, L. Considerations for Preterm Human Milk Feedings When Caring for Mothers Who Are Overweight or Obese. Adv. Neonatal Care 2019, 19, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.H. An Endocrine Hypothesis to Explain Obesity-Related Lactation Insufficiency in Breastfeeding Mothers. J. Dairy Res. 2020, 87, 78–81. [Google Scholar] [CrossRef]
- Lei, W.-T.; Huang, K.-Y.; Jhong, J.-H.; Chen, C.-H.; Weng, S.-L. Metagenomic Analysis of the Gut Microbiome Composition Associated with Vitamin D Supplementation in Taiwanese Infants. Sci. Rep. 2021, 11, 2856. [Google Scholar] [CrossRef]
- Li, Y.C.; Chen, Y.; Du, J. Critical Roles of Intestinal Epithelial Vitamin D Receptor Signaling in Controlling Gut Mucosal Inflammation. J. Steroid Biochem. Mol. Biol. 2015, 148, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kanhere, M.; Chassaing, B.; Gewirtz, A.T.; Tangpricha, V. Role of Vitamin D on Gut Microbiota in Cystic Fibrosis. J. Steroid Biochem. Mol. Biol. 2018, 175, 82–87. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal Epithelial Vitamin D Receptor Signaling Inhibits Experimental Colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef]
- Prior, E.; Santhakumaran, S.; Gale, C.; Philipps, L.H.; Modi, N.; Hyde, M.J. Breastfeeding after Cesarean Delivery: A Systematic Review and Meta-Analysis of World Literature. Am. J. Clin. Nutr. 2012, 95, 1113–1135. [Google Scholar] [CrossRef]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast Milk Microbiota Is Shaped by Mode of Delivery and Intrapartum Antibiotic Exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Rautava, S.; Luoto, R.; Salminen, S.; Isolauri, E. Microbial Contact during Pregnancy, Intestinal Colonization and Human Disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 565–576. [Google Scholar] [CrossRef]
- Wachs, T.D.; Creed-Kanashiro, H.; Cueto, S.; Jacoby, E. Maternal Education and Intelligence Predict Offspring Diet and Nutritional Status. J. Nutr. 2005, 135, 2179–2186. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mancabelli, L.; Mangifesta, M.; Viappiani, A.; Lugli, G.A.; Ferrario, C.; Gioiosa, L.; Ferrarini, A.; et al. Deciphering Bifidobacterial-Mediated Metabolic Interactions and Their Impact on Gut Microbiota by a Multi-Omics Approach. ISME J. 2016, 10, 1656–1668. [Google Scholar] [CrossRef]
- Okada, Y.; Tsuzuki, Y.; Hokari, R.; Komoto, S.; Kurihara, C.; Kawaguchi, A.; Nagao, S.; Miura, S. Anti-Inflammatory Effects of the Genus Bifidobacterium on Macrophages by Modification of Phospho-IκB and SOCS Gene Expression. Int. J. Exp. Pathol. 2009, 90, 131–140. [Google Scholar] [CrossRef]
- Baumann-Dudenhoeffer, A.M.; D’Souza, A.W.; Tarr, P.I.; Warner, B.B.; Dantas, G. Infant Diet and Maternal Gestational Weight Gain Predict Early Metabolic Maturation of Gut Microbiomes. Nat. Med. 2018, 24, 1822–1829. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.-D.; Sun, X.-Q.; Ding, W.-J.; Wang, X.-Y.; Fan, J.-G. Gut Microbiota Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease. Hepatobiliary Pancreatic Diseases Int. 2017, 16, 375–381. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.D.; Ferreira, S.R.G. Gut Microbiota Interactions with the Immunomodulatory Role of Vitamin D in Normal Individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the Gut Microbiome: A Systematic Review of in Vivo Studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural History of the Infant Gut Microbiome and Impact of Antibiotic Treatments on Strain-Level Diversity and Stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, S.; Song, Y.; Feng, Y.; Lv, N.; Xue, Y.; Liu, F.; Wang, S.; Zhu, B.; Ma, J.; et al. The Perturbation of Infant Gut Microbiota Caused by Cesarean Delivery Is Partially Restored by Exclusive Breastfeeding. Front. Microbiol. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Exclusive Breastfeeding (N = 88) | Partial Breastfeeding (N = 43) | No Breastfeeding (N = 60) | p-Value |
|---|---|---|---|---|
| Infant age at sample collection (day), mean (SD) | 115.5 (17.3) | 135.9 (39.8) | 126.3 (31.9) | < 0.01 |
| Infant had any antibiotics since birth, n (%) | 14 (15.9) | 5 (11.6) | 8 (13.3) | 0.40 |
| Consumption of complementary food during past 24 h, n (%) | 0 (0.0) | 19 (44.2) | 21 (35.0) | < 0.013 |
| Infant probiotic supplement 2 during past 24 h, n (%) | 4 (4.5) | 1 (2.4) | 3 (3.3) | 0.90 |
| Infant Vitamin D supplement during past 24 h, n (%) | 35 (39.8) | 8 (18.6) | 1 (1.7) | < 0.01 |
| Delivery mode, n (%) | ||||
| Vaginal delivery | 66 (75) | 30 (69.8) | 36 (60) | 0.10 |
| C-section | 22 (25) | 13 (30.2) | 24 (40) | |
| Infant weight at delivery (gram), mean (SD) | 3461 (551) | 3336 (529) | 3269 (598) | 0.10 |
| Infant sex, n (%) | ||||
| Male | 43 (48.9) | 22 (51.2) | 30 (50) | 0.98 |
| Female | 45 (51.1) | 21 (48.8) | 30 (50) | |
| Maternal pre-pregnancy BMI, n (%) | ||||
| < 18.5 | 1 (1.1) | 0 (0.0) | 3 (5.0) | < 0.01 |
| 18.5–25 | 44 (50) | 17 (39.5) | 16 (26.7) | |
| > 25–30 | 24 (27.3) | 11 (25.6) | 12 (20.0) | |
| > 30 | 19 (21.6) | 15 (34.9) | 29 (48.3) | |
| Maternal education level, n (%) | ||||
| Did not finish high school | 0 (0.0) | 0 (0.0) | 6 (10.2) | < 0.01 |
| High school graduate or GED | 4 (4.6) | 4 (9.5) | 21 (35.6) | |
| Some college | 20 (23.0) | 12 (28.6) | 13 (22.0) | |
| College graduate or more | 63 (72.4) | 26 (61.9) | 19 (32.2) |
| Maternal Characteristics | Proportional Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Maternal age(year) | 1.02 | 0.96–1.09 | 0.48 |
| Maternal educational level | 2.66 | 1.72–4.21 | < 0.001 |
| Pre-pregnancy BMI (continuous) | 0.95 | 0.91–0.99 | 0.01 |
| Delivery mode (vaginal vs. C-section) | 0.56 | 0.29–1.05 | 0.07 |
| Infant age (day) | 0.99 | 0.98–1.0 | 0.07 |
| Variable | F Value | R2 | p-Value |
|---|---|---|---|
| Breastfeeding during past week | 4.0 | 4.10% | 0.001 * |
| Gestational age | 2.3 | 1.20% | 0.03 * |
| Infant sex | 1.1 | 0.50% | 0.36 |
| Delivery mode (vaginal vs. C-section) | 3.6 | 1.80% | 0.004 * |
| infant weight at delivery | 0.7 | 0.30% | 0.72 |
| Infant probiotic supplement during past 24 h | 1.0 | 0.50% | 0.38 |
| Infant had any antibiotics since birth | 0.9 | 1.00% | 0.47 |
| Maternal educational level | 2.7 | 1.30% | 0.02 * |
| Maternal pre-pregnancy BMI (continuous) | 0.37 | 0.20% | 0.96 |
| Taxonomy at Genus Level | Meta Data Value | Coefficient | N/N Not 0 | p-Value | q-Value 2 |
|---|---|---|---|---|---|
| Intestinibacter | Exclusive breastfeeding | −0.567 | 191/64 | 3.6 × 10−10 | 3.0 × 10−7 |
| Flavonifractor | Exclusive breastfeeding | −0.896 | 191/145 | 4.0 × 10−8 | 1.7 × 10−5 |
| Lachnoclostridium | Exclusive breastfeeding | −0.998 | 191/146 | 1.9 × 10−7 | 5.2 × 10−5 |
| Clostridium innocuum group | Exclusive breastfeeding | −0.393 | 191/44 | 4.9 × 10−6 | 6.8 × 10−4 |
| Lactobacillus | Exclusive breastfeeding | 0.680 | 191/115 | 4.3 × 10−6 | 6.8 × 10−4 |
| Lactococcus | Exclusive breastfeeding | −0.287 | 191/29 | 1.7 × 10−5 | 1.7 × 10−3 |
| Bifidobacterium | Exclusive breastfeeding | 0.535 | 191/186 | 2.4 × 10−4 | 0.018 |
| Eisenbergiella | Exclusive breastfeeding | −0.322 | 191/24 | 2.4 × 10−4 | 0.018 |
| Colidextribacter | Exclusive breastfeeding | −0.398 | 191/40 | 3.2 × 10−4 | 0.022 |
| Akkermansia | Exclusive breastfeeding | −0.550 | 191/124 | 1.3 × 10−3 | 0.066 |
| Uncultured Lachnospiraceae | Exclusive breastfeeding | −0.188 | 191/20 | 1.4 × 10−3 | 0.069 |
| Haemophilus | Exclusive breastfeeding | 0.463 | 191/141 | 1.7 × 10−3 | 0.073 |
| Staphylococcus | Exclusive breastfeeding | 0.293 | 191/56 | 1.8 × 10−3 | 0.073 |
| Incertae_Sedis | Exclusive breastfeeding | −0.358 | 191/99 | 1.6 × 10−3 | 0.073 |
| Flavonifractor | Partial breastfeeding | −0.909 | 191/145 | 7.2 × 10−7 | 1.5 × 10−4 |
| Haemophilus | Partial breastfeeding | 0.740 | 191/141 | 1.3 × 10−5 | 0.001 |
| Lachnoclostridium | Partial breastfeeding | −0.860 | 191/146 | 5.8 × 10−5 | 0.005 |
| Lactococcus | Partial breastfeeding | −0.258 | 191/29 | 5.6 × 10−4 | 0.031 |
| Alistipes | Pre-pregnancy BMI | 0.206 | 191/99 | 4.0 × 10−4 | 0.025 |
| Lachnospira | Age at sample collection | 0.171 | 191/84 | 5.7 × 10−4 | 0.032 |
| Breastfeeding Status | Taxonomy at Genus Level | Meta Data Value | Coefficient | N/N Not 0 | p-Value | q-Value 2 |
|---|---|---|---|---|---|---|
| Exclusively (N = 88) | Haemophilus | Vitamin D supplement (Yes) | −0.683 | 88/74 | 6.7 × 10−5 | 0.058 |
| Faecalitalea | Probiotic supplement (Yes) | 1.718 | 60/9 | 2.9 × 10−9 | 2.4 × 10−6 | |
| Not breastfeeding (N = 60) | Uncultured Lachnospiraceae | Probiotic supplement (Yes) | 1.269 | 60/10 | 5.1 × 10−5 | 0.021 |
| Alistipes | Pre-pregnancy BMI | 0.431 | 60/27 | 2.6 × 10−4 | 0.072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Bu, S.; Paneth, N.; Kerver, J.M.; Comstock, S.S. Vitamin D Supplementation in Exclusively Breastfed Infants Is Associated with Alterations in the Fecal Microbiome. Nutrients 2022, 14, 202. https://doi.org/10.3390/nu14010202
Ma T, Bu S, Paneth N, Kerver JM, Comstock SS. Vitamin D Supplementation in Exclusively Breastfed Infants Is Associated with Alterations in the Fecal Microbiome. Nutrients. 2022; 14(1):202. https://doi.org/10.3390/nu14010202
Chicago/Turabian StyleMa, Tengfei, Sihan Bu, Nigel Paneth, Jean M. Kerver, and Sarah S. Comstock. 2022. "Vitamin D Supplementation in Exclusively Breastfed Infants Is Associated with Alterations in the Fecal Microbiome" Nutrients 14, no. 1: 202. https://doi.org/10.3390/nu14010202
APA StyleMa, T., Bu, S., Paneth, N., Kerver, J. M., & Comstock, S. S. (2022). Vitamin D Supplementation in Exclusively Breastfed Infants Is Associated with Alterations in the Fecal Microbiome. Nutrients, 14(1), 202. https://doi.org/10.3390/nu14010202