The Role of Bioactive Compounds from Dietary Spices in the Management of Metabolic Syndrome: An Overview
Abstract
:1. Introduction
1.1. Metabolic Syndrome
1.2. Metabolic Syndrome Diagnostic Criteria
1.3. Metabolic Syndrome Risk Factors
2. Search Methodology
3. Herbal Approaches to Management in Metabolic Syndrome
3.1. Common Herbal Therapeutics, and Their Effect on Metabolic Syndrome Indicators
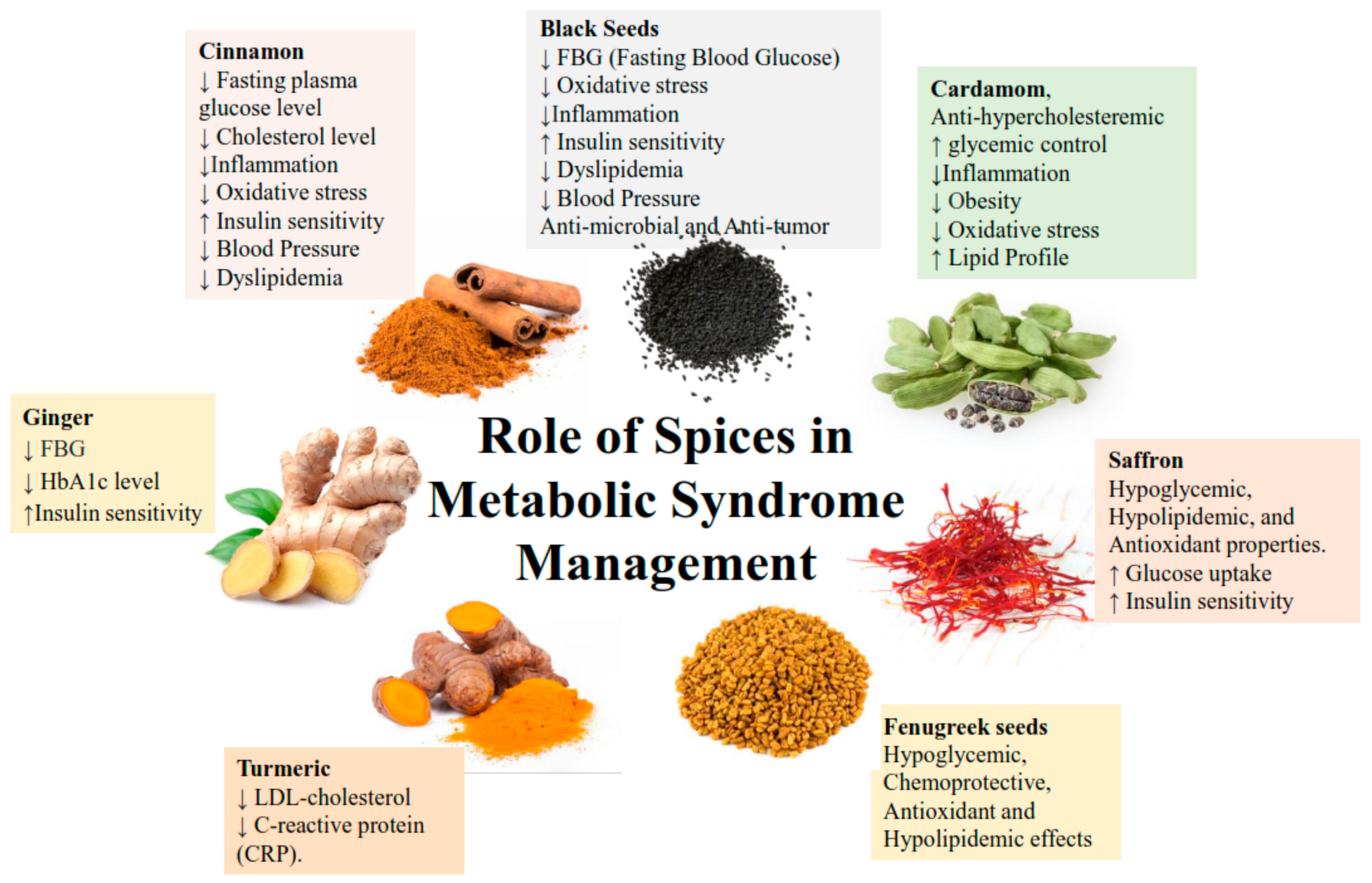
3.1.1. Ginger
3.1.2. Cinnamon
3.1.3. Black Seed
3.2. Other Herbal Interventions
3.2.1. Fenugreek
3.2.2. Saffron
3.2.3. Cardamon
3.2.4. Turmeric
4. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbier, D. Depression in the elderly. Clinical aspects. Presse Med. 2001, 30, 339–340. [Google Scholar]
- Joslin, E.P. The prevention of diabetes mellitus. JAMA 1921, 76, 79–84. [Google Scholar] [CrossRef]
- Haller, H. Epidermiology and associated risk factors of hyperlipoproteinemia. Z. Die Gesamte Inn. Med. Ihre Grenzgeb. 1977, 32, 124–128. [Google Scholar]
- Singer, P. Diagnosis of primary hyperlipoproteinemias. Z. Die Gesamte Inn. Med. Ihre Grenzgeb. 1977, 32, 129–133. [Google Scholar]
- Phillips, G.B. Sex hormones, risk factors and cardiovascular disease. Am. J. Med. 1978, 65, 7–11. [Google Scholar] [CrossRef]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Flemming, G.M.C.; Bussler, S.; Körner, A.; Kiess, W. Definition and early diagnosis of metabolic syndrome in children. J. Pediatr. Endocrinol. Metab. 2020, 33, 821–833. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a Who Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Macek, P.; Biskup, M.; Terek-Derszniak, M.; Krol, H.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. Optimal cut-off values for anthropometric measures of obesity in screening for cardiometabolic disorders in adults. Sci. Rep. 2020, 10, 11253. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection E. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486. [Google Scholar]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- Peng, W.; Liu, Y.; Malowany, M.; Chen, H.; Su, X.; Liu, Y. Metabolic syndrome and its relation to dietary patterns among a selected urbanised and semi-urbanised Tibetan population in transition from nomadic to settled living environment. Public Health Nutr. 2021, 24, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.-P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight Fact Sheet N 311; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Zhu, Q.; An, Y.A.; Kim, M.; Zhang, Z.; Zhao, S.; Zhu, Y.; Scherer, P.E. Suppressing adipocyte inflammation promotes insulin resistance in mice. Mol. Metab. 2020, 39, 101010. [Google Scholar] [CrossRef]
- Hotamisligil, G. The role of TNFα and TNF receptors in obesity and insulin resistance. J. Intern. Med. 1999, 245, 621–625. [Google Scholar] [CrossRef]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackland, D.T.; Weber, M.A. Global burden of cardiovascular disease and stroke: Hypertension at the core. Can. J. Cardiol. 2015, 31, 569–571. [Google Scholar] [CrossRef]
- Law, M.; Wald, N.; Morris, J. Lowering blood pressure to prevent myocardial infarction and stroke: A new preventive strategy. Health Technol. Assess. 2003, 7, 1–94. [Google Scholar] [CrossRef]
- Collaborative, P.; Neal, B.; MacMahon, S. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003, 163, 1069–1075. [Google Scholar]
- Jafar, T.H.; Stark, P.C.; Schmid, C.H.; Landa, M.; Maschio, G.; de Jong, P.E.; de Zeeuw, D.; Shahinfar, S.; Toto, R.; Levey, A.S. Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann. Intern. Med. 2003, 139, 244–252. [Google Scholar] [CrossRef]
- Karantas, I.D.; Okur, M.E.; Okur, N.Ü.; Siafaka, P.I. Dyslipidemia management in 2020: An update on diagnosis and therapeutic perspectives. Endocr. Metab. Immune Dis.-Drug Targets 2021, 21, 815–834. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Austin, M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A metaanalysis of population-based prospective studies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.P.; Doyle, J.T.; Gordon, T.; Hames, C.G.; Hjortland, M.C.; Hulley, S.B.; Kagan, A.; Zukel, W.J. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977, 55, 767–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, M.L.; Webb, D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J. Am. Coll. Nutr. 2008, 27, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Borg, R.; Kuenen, J.; Carstensen, B.; Zheng, H.; Nathan, D.; Heine, R.; Nerup, J.; Borch-Johnsen, K.; Witte, D.; Group, A.S. HbA1c and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: The A1C-Derived Average Glucose (ADAG) study. Diabetologia 2011, 54, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Association, A.D. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care 2004, 27, 2836–2842. [Google Scholar]
- He, D.; Xi, B.; Xue, J.; Huai, P.; Zhang, M.; Li, J. Association between Leisure Time Physical Activity and Metabolic Syndrome: A Meta-Analysis of Prospective Cohort Studies; Springer: New York, NY, USA, 2014. [Google Scholar]
- Rosmond, R.; Björntorp, P. The hypothalamic–pituitary–adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J. Intern. Med. 2000, 247, 188–197. [Google Scholar] [CrossRef]
- Fauci, A.S. Harrison’s Principles of Internal Medicine; McGraw-Hill Medical Publishing Division New York: New York, NY, USA, 2008; Volume 2. [Google Scholar]
- Seal, S.V.; Turner, J.D. The ‘Jekyll and Hyde’of Gluconeogenesis: Early Life Adversity, Later Life Stress, and Metabolic Disturbances. Int. J. Mol. Sci. 2021, 22, 3344. [Google Scholar] [CrossRef] [PubMed]
- Gohil, B.C.; Rosenblum, L.A.; Coplan, J.D.; Kral, J.G. Hypothalamic-pituitary-adrenal axis function and the metabolic syndrome X of obesity. CNS Spectr. 2001, 6, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.; Ingram, M.C.; Anderson, N.H.; Morrison, C.; Davies, E.; Connell, J.M. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension 1999, 33, 1364–1368. [Google Scholar] [CrossRef] [Green Version]
- Hucklebridge, F.; Clow, A.; Abeyguneratne, T.; Huezo-Diaz, P.; Evans, P. The awakening cortisol response and blood glucose levels. Life Sci. 1999, 64, 931–937. [Google Scholar] [CrossRef]
- Phillips, D.; Barker, D.; Fall, C.; Seckl, J.; Whorwood, C.; Wood, P.; Walker, B. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome? J. Clin. Endocrinol. Metab. 1998, 83, 757–760. [Google Scholar]
- Schwertner, H.A.; Troxler, R.G.; Uhl, G.S.; Jackson, W.G. Relationship between cortisol and cholesterol in men with coronary artery disease and type A behavior. Arterioscler. Thromb. Vasc. Biol. 1984, 4, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Björntorp, P. The regulation of adipose tissue distribution in humans. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1996, 20, 291–302. [Google Scholar]
- Smith, G.D.; Ben-Shlomo, Y.; Beswick, A.; Yarnell, J.; Lightman, S.; Elwood, P. Cortisol, testosterone, and coronary heart disease. Circulation 2005, 112, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, N.J.; Schmeltz, L.R. Metabolic syndrome management. Expert Opin. Pharmacother. 2007, 8, 2059–2075. [Google Scholar] [CrossRef]
- Grundy, S.M.; Hansen, B.; Smith, S.C.; Cleeman, J.I.; Kahn, R.A. Clinical management of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e19–e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, A.L.; Athinarayanan, S.J.; McCue, J.J.; Adams, R.N.; Keyes, M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D.; Hallberg, S.J. Type 2 Diabetes Prevention Focused on Normalization of Glycemia: A Two-Year Pilot Study. Nutrients 2021, 13, 749. [Google Scholar] [CrossRef]
- Cho, J.H.; Ko, J.; Lim, S.T. Relationship between metabolic syndrome and moderate-to-vigorous physical activity among adults 18 years old and over. PLoS ONE 2021, 16, e0258097. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, H.; Ye, J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr. Metab. Immune Disord.-Drug Targets 2008, 8, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M.A.; Winston, D. Herbal Therapy and Supplements: A Scientific and Traditional Approach; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Hasani-Ranjbar, S.; Nayebi, N.; Larijani, B.; Abdollahi, M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J. Gastroenterol. 2009, 15, 3073. [Google Scholar] [CrossRef]
- Phillips, A.W.; Osborne, J.A. Survey of alternative and nonprescription therapy use. Am. J. Health-Syst. Pharm. 2000, 57, 1361–1362. [Google Scholar] [CrossRef]
- Kaye, A.; Clarke, R.; Sabar, R.; Vig, S.; Dhawan, K.; Hofbauer, R.; Kaye, A. Herbal medicines: Current trends in anesthesiology practice—A hospital survey. J. Clin. Anesth. 2000, 12, 468–471. [Google Scholar] [CrossRef]
- Cappuccio, F.; Duneclift, S.; Atkinson, R.; Cook, D. Use of alternative medicines in a multi-ethnic population. Ethn. Dis. 2001, 11, 11–18. [Google Scholar] [PubMed]
- Yeh, G.Y.; Davis, R.B.; Phillips, R.S. Use of complementary therapies in patients with cardiovascular disease. Am. J. Cardiol. 2006, 98, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, V.; Ogru, E.; Heffernan, M.; Libinaki, R.; Lim, Y.; Ng, F. Studies on the use of “slimax”, a Chinese herbal mixture, in the treatment of human obesity. Pharm. Biol. 2000, 38, 30–35. [Google Scholar] [CrossRef]
- Boozer, C.; Nasser, J.; Heymsfield, S.; Wang, V.; Chen, G.; Solomon, J. An herbal supplement containing Ma Huang-Guarana for weight loss: A randomized, double-blind trial. Int. J. Obes. 2001, 25, 316. [Google Scholar] [CrossRef] [Green Version]
- Hackman, R.; Havel, P.; Schwartz, H.; Rutledge, J.; Watnik, M.; Noceti, E.; Stohs, S.; Stern, J.; Keen, C. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: A randomized controlled trial. Int. J. Obes. 2006, 30, 1545. [Google Scholar] [CrossRef] [Green Version]
- Preuss, H.; Bagchi, D.; Bagchi, M.; Rao, C.S.; Dey, D.; Satyanarayana, S. Effects of a natural extract of (–)-hydroxycitric acid (HCA-SX) and a combination of HCA-SX plus niacin-bound chromium and Gymnema sylvestre extract on weight loss. Diabetes Obes. Metab. 2004, 6, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; del Rio, M.; Ramazanov, T.; Klimenov, A.; Dzhamirze, S.; Kalyuzhin, O. Effects of Aralia mandshurica and Engelhardtia chrysolepis extracts on some parameters of lipid metabolism in women with nondiabetic obesity. Bull. Exp. Biol. Med. 2006, 141, 343–346. [Google Scholar] [CrossRef]
- Udani, J.; Hardy, M.; Madsen, D.C. Blocking carbohydrate absorption and weight loss: A clinical trial using Phase 2™ brand proprietary fractionated white bean extract. Altern. Med. Rev. 2004, 9, 63–69. [Google Scholar] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuksan, V.; Sung, M.-K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.-S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef]
- Dans, A.M.L.; Villarruz, M.V.C.; Jimeno, C.A.; Javelosa, M.A.U.; Chua, J.; Bautista, R.; Velez, G.G.B. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J. Clin. Epidemiol. 2007, 60, 554–559. [Google Scholar] [CrossRef]
- Mang, B.; Wolters, M.; Schmitt, B.; Kelb, K.; Lichtinghagen, R.; Stichtenoth, D.; Hahn, A. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. Eur. J. Clin. Investig. 2006, 36, 340–344. [Google Scholar] [CrossRef]
- Oben, J.E.; Ngondi, J.L.; Momo, C.N.; Agbor, G.A.; Sobgui, C.S.M. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: A double-blind placebo-controlled study. Lipids Health Dis. 2008, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Singhal, S.; Goyle, A.; Sharma, V. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree-bark powder: A randomised placebo-controlled trial. J. Assoc. Physicians India 2001, 49, 231–235. [Google Scholar] [PubMed]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.-A.; Najarzadeh, A.; Mozayan, M.R. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Islam, N.; Anila, N.; Gilani, A. Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome–A double blind randomized controlled trial–TAK-MetS trial. Complement. Ther. Med. 2015, 23, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hariri, M.; Abbasi, B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev. Diabet. Stud. 2014, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Safdar, M.; Khan, M.M.A.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef] [Green Version]
- Surh, Y.-J.; Park, K.-K.; Chun, K.-S.; Lee, L.; Lee, E.; Lee, S.S. Anti-tumor-promoting activities of selected pungent phenolic substances present in ginger. J. Environ. Pathol. Toxicol. Oncol. Organ Int. Soc. Environ. Toxicol. Cancer 1999, 18, 131–139. [Google Scholar]
- Marx, W.M.; Teleni, L.; McCarthy, A.L.; Vitetta, L.; McKavanagh, D.; Thomson, D.; Isenring, E. Ginger (Zingiber officinale) and chemotherapy-induced nausea and vomiting: A systematic literature review. Nutr. Rev. 2013, 71, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Feizi, A.; Hariri, M.; Darvishi, L.; Barani, A.; Taghiyar, M.; Shiranian, A.; Hajishafiee, M. Influence of ginger and cinnamon intake on inflammation and muscle soreness endued by exercise in Iranian female athletes. Int. J. Prev. Med. 2013, 4, S11. [Google Scholar]
- Shahin, S.M.; Jaleel, A.; Alyafei, M.A.M. The Essential Oil-Bearing Plants in the United Arab Emirates (UAE): An Overview. Molecules 2021, 26, 6486. [Google Scholar] [CrossRef]
- Odebunmi, E.; Oluwaniyi, O.; Bashiru, M. Comparative proximate analysis of some food condiments. J. Appl. Sci. Res. 2010, 6, 272–274. [Google Scholar]
- Prakash, J. Chemical composition and antioxidant properties of ginger root (Zingiber officinale). J. Med. Plants Res. 2010, 4, 2674–2679. [Google Scholar]
- Nwinuka, N.; Ibeh, G.; Ekeke, G. Proximate composition and levels of some toxicants in four commonly consumed spices. J. Appl. Sci. Environ. Manag. 2005, 9, 150–155. [Google Scholar]
- Hussain, J.; Bahader, A.; Ullah, F.; Rehman, N.; Khan, A.L.; Ullah, W.; Shinwari, Z.K. Proximate and nutrient analysis of the locally manufactured herbal medicines and its raw material. J. Am. Sci. 2009, 5, 91–96. [Google Scholar]
- Wei, C.-K.; Tsai, Y.-H.; Korinek, M.; Hung, P.-H.; El-Shazly, M.; Cheng, Y.-B.; Wu, Y.-C.; Hsieh, T.-J.; Chang, F.-R. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2017, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes. Planta Med. 2012, 78, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Jafarnejad, S.; Keshavarz, S.A.; Mahbubi, S.; Saremi, S.; Arab, A.; Abbasi, S.; Djafarian, K. Effect of ginger (Zingiber officinale) on blood glucose and lipid concentrations in diabetic and hyperlipidemic subjects: A meta-analysis of randomized controlled trials. J. Funct. Foods 2017, 29, 127–134. [Google Scholar] [CrossRef]
- Attari, V.E.; Mahluji, S.; Jafarabadi, M.A.; Ostadrahimi, A. Effects of Supplementation with Ginger (Zingiber officinale Roscoe) on Serum Glucose, Lipid Profile and Oxidative Stress in Obese Women: A Randomized, Placebo-Controlled Clinical Trial. Pharm. Sci. 2015, 21, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E.J. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Thomé, G.R.; Morsch, V.M.; Bottari, N.B.; Baldissarelli, J.; Oliveira, L.S.; Goularte, J.F.; Belló-Klein, A.; Oboh, G.; Schetinger, M.R.C. Dietary supplementation of ginger and turmeric rhizomes modulates platelets ectonucleotidase and adenosine deaminase activities in normotensive and hypertensive rats. Phytother. Res. 2016, 30, 1156–1163. [Google Scholar] [CrossRef]
- Andrade, C. Ginger for Migraine. J. Clin. Psych. 2021, 82, 21f14325. [Google Scholar] [CrossRef]
- Alizadeh-Navaei, R.; Roozbeh, F.; Saravi, M.; Pouramir, M.; Jalali, F.; Moghadamnia, A.A. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Altern. Med. Rev. 2008, 13, 358. [Google Scholar]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Mansour, M.S.; Ni, Y.-M.; Roberts, A.L.; Kelleman, M.; Roychoudhury, A.; St-Onge, M.-P. Ginger consumption enhances the thermic effect of food and promotes feelings of satiety without affecting metabolic and hormonal parameters in overweight men: A pilot study. Metab. Clin. Exp. 2012, 61, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Nayebifar, S.; Afzalpour, M.E.; Kazemi, T.; Eivary, S.H.A.; Mogharnasi, M. The effect of a 10-week high-intensity interval training and ginger consumption on inflammatory indices contributing to atherosclerosis in overweight women. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2016, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Safdar, M. Proximate composition and mineral analysis of cinnamon. Pak. J. Nutr. 2009, 8, 1456–1460. [Google Scholar] [CrossRef] [Green Version]
- Kawatra, P.; Rajagopalan, R. Cinnamon: Mystic powers of a minute ingredient. Pharmacogn. Res. 2015, 7, S1–S6. [Google Scholar] [CrossRef] [Green Version]
- Han, D.C.; Lee, M.-Y.; Shin, K.D.; Jeon, S.B.; Kim, J.M.; Son, K.-H.; Kim, H.-C.; Kim, H.-M.; Kwon, B.-M. 2′-benzoyloxycinnamaldehyde induces apoptosis in human carcinoma via reactive oxygen species. J. Biol. Chem. 2004, 279, 6911–6920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.H.; Kim, J.; Kim, J.-M.; Lee, S.-Y.; Shin, D.-S.; Son, K.-H.; Han, D.C.; Sung, Y.K.; Kwon, B.-M. Apoptosis induction of 2′-hydroxycinnamaldehyde as a proteasome inhibitor is associated with ER stress and mitochondrial perturbation in cancer cells. Biochem. Pharmacol. 2007, 74, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium and polyphenols from cinnamon improve insulin sensitivity: Plenary Lecture. Proc. Nutr. Soc. 2008, 67, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Feehan, J.; Prakash, M.D.; Stojanovska, L.; Flavel, M.R.; Kitchen, B.; Apostolopoulos, V. Immunomodulatory Properties of Polyphenol-Rich Sugarcane Extract on Human Monocytes. Biologics 2021, 1, 211–221. [Google Scholar] [CrossRef]
- Maleki, V.; Faghfouri, A.H.; Tabrizi, F.P.F.; Moludi, J.; Saleh-Ghadimi, S.; Jafari-Vayghan, H.; Qaisar, S.A. Mechanistic and therapeutic insight into the effects of cinnamon in polycystic ovary syndrome: A systematic review. J. Ovarian Res. 2021, 14, 130. [Google Scholar] [CrossRef]
- Allen, R.W.; Schwartzman, E.; Baker, W.L.; Coleman, C.I.; Phung, O.J. Cinnamon use in type 2 diabetes: An updated systematic review and meta-analysis. Ann. Fam. Med. 2013, 11, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, W.L.; Gutierrez-Williams, G.; White, C.M.; Kluger, J.; Coleman, C.I. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care 2008, 31, 41–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: A randomized, placebo-controlled, double-blind clinical trial. Diabet. Med. 2010, 27, 1159–1167. [Google Scholar] [CrossRef]
- Akilen, R.; Pimlott, Z.; Tsiami, A.; Robinson, N. Effect of short-term administration of cinnamon on blood pressure in patients with prediabetes and type 2 diabetes. Nutrition 2013, 29, 1192–1196. [Google Scholar] [CrossRef]
- Whitfield, P.; Parry-Strong, A.; Walsh, E.; Weatherall, M.; Krebs, J.D. The effect of a cinnamon-, chromium-and magnesium-formulated honey on glycaemic control, weight loss and lipid parameters in type 2 diabetes: An open-label cross-over randomised controlled trial. Eur. J. Nutr. 2016, 55, 1123–1131. [Google Scholar] [CrossRef]
- Magistrelli, A.; Chezem, J.C. Effect of ground cinnamon on postprandial blood glucose concentration in normal-weight and obese adults. J. Acad. Nutr. Diet. 2012, 112, 1806–1809. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95, 138–158. [Google Scholar] [CrossRef]
- Amin, B.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Heshmati, J.; Namazi, N.; Memarzadeh, M.-R.; Taghizadeh, M.; Kolahdooz, F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Food Res. Int. 2015, 70, 87–93. [Google Scholar] [CrossRef]
- Kaatabi, H.; Bamosa, A.O.; Badar, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Khadra, A.; Al-Almaie, S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial. PLoS ONE 2015, 10, e0113486. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, H.; Ahmad, A.; Masud, T.; Kaleem, M. Cardio-protective and anti-cancer therapeutic potential of Nigella sativa. Iran. J. Basic Med. Sci. 2014, 17, 967. [Google Scholar]
- Shishehbor, F.; Joola, P.; Malehi, A.S.; Jalalifar, M.A. The effect of black seed raisin on some cardiovascular risk factors, serum malondialdehyde, and total antioxidant capacity in hyperlipidemic patients: A randomized controlled trials. Ir. J. Med. Sci. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Datau, E.; Surachmanto, E.; Pandelaki, K.; Langi, J. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med. Indones. 2010, 42, 130–134. [Google Scholar]
- Shah, A.S.; Khan, G.M.; Badshah, A.; Shah, S.U.; Shah, K.U.; Mirza, S.A.; Khan, K.A. Nigella sativa provides protection against metabolic syndrome. Afr. J. Biotechnol. 2012, 11, 10919–10925. [Google Scholar]
- Dehkordi, F.R.; Kamkhah, A.F. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam. Clin. Pharmacol. 2008, 22, 447–452. [Google Scholar] [CrossRef]
- Heshmati, J.; Namazi, N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: A systematic review. Complement. Ther. Med. 2015, 23, 275–282. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Hamdan, N.S.; Mahmud, R.; Imam, M.U.; Saini, S.M.; Rashid, S.N.A.; Ghafar, S.A.A.; Ab Latiff, L.; Ismail, M. A randomised controlled trial on hypolipidemic effects of Nigella Sativa seeds powder in menopausal women. J. Transl. Med. 2014, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Najmi, A.; Haque, S.; Naseeruddin, M.; Khan, R. Effect of Nigella sativa oil on various clinical and biochemical parameters of metabolic syndrome. Int. J. Diabetes Dev. Ctries 2008, 16, 85–87. [Google Scholar]
- Shahzad, F.; Nasiruddin, M. Indigenous herbal product Nigella sativa proved effective as an anti-obesity therapy in metabolic syndrome. Asian J. Pharm. Clin. Res. 2011, 6, 61–64. [Google Scholar]
- Qidwai, W.; Ashfaq, T. Effect of dietary supplementation of black seed (N. Sativa L.) on lipid profile of patients suffering from diabetes. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2014, 13, 3–8. [Google Scholar] [CrossRef]
- Feyzi, S.; Varidi, M.; Zare, F.; Varidi, M.J. Fenugreek (Trigonella foenum graecum) seed protein isolate: Extraction optimization, amino acid composition, thermo and functional properties. J. Sci. Food Agric. 2015, 95, 3165–3176. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Raghuram, T.; Rao, N.S. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur. J. Clin. Nutr. 1990, 44, 301–306. [Google Scholar]
- Amin, A.; Alkaabi, A.; Al-Falasi, S.; Daoud, S.A. Chemopreventive activities of Trigonella foenum graecum (Fenugreek) against breast cancer. Cell Biol. Int. 2005, 29, 687–694. [Google Scholar] [CrossRef]
- Hettiarachchy, N.; Glenn, K.; Gnanasambandam, R.; Johnson, M. Natural antioxidant extract from fenugreek (Trigonella foenum graecum) for ground beef patties. J. Food Sci. 1996, 61, 516–519. [Google Scholar] [CrossRef]
- Stark, A.; Madar, Z. The effect of an ethanol extract derived from fenugreek (Trigonella foenum-graecum) on bile acid absorption and cholesterol levels in rats. Br. J. Nutr. 1993, 69, 277–287. [Google Scholar] [CrossRef]
- Jain, S.; Madhu, A. Regulation of trigonellin in Trigonella species by chemical mutagenic treatments. Indian Drugs 1988, 26, 14–16. [Google Scholar]
- Shang, M.; Cai, S.; Han, J.; Li, J.; Zhao, Y.; Zheng, J.; Namba, T.; Kadota, S.; Tezuka, Y.; Fan, W. Studies on flavonoids from Fenugreek (Trigonella foenum graecum L.). J. Chin. Mater. Med. 1998, 23, 614–616. [Google Scholar]
- Ahmadiani, A.; Javan, M.; Semnanian, S.; Barat, E.; Kamalinejad, M. Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract in the rat. J. Ethnopharmacol. 2001, 75, 283–286. [Google Scholar] [CrossRef]
- Knott, E.J.; Richard, A.J.; Mynatt, R.L.; Ribnicky, D.; Stephens, J.M.; Bruce-Keller, A. Fenugreek supplementation during high-fat feeding improves specific markers of metabolic health. Sci. Rep. 2017, 7, 12770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Bhandari, U.; Jamadagni, S. Fenugreek Seed Extract Inhibit Fat Accumulation and Ameliorates Dyslipidemia in High Fat Diet-Induced Obese Rats. BioMed Res. Int. 2014, 2014, 606021. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.D. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. Nutr. Res. 1986, 6, 1353–1364. [Google Scholar] [CrossRef]
- Kang, C.; Lee, H.; Jung, E.-S.; Seyedian, R.; Jo, M.; Kim, J.; Kim, J.-S.; Kim, E. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. 2012, 135, 2350–2358. [Google Scholar] [CrossRef]
- Jessie, S.W.; Krishnakantha, T. Inhibition of human platelet aggregation and membrane lipid peroxidation by food spice, saffron. Mol. Cell. Biochem. 2005, 278, 59–63. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Immunomodulatory and antioxidant effects of saffron aqueous extract (Crocus sativus L.) on streptozotocin-induced diabetes in rats. Indian Heart J. 2017, 69, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, F.; Sahebkar, A.; Aryaeian, N.; Pahlavani, N.; Fallah, S.; Moradi, N.; Abbasi, D.; Hosseini, A.F. Effects of Saffron Supplementation On Inflammation And Metabolic Responses In Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. Syndr. Obes. 2019, 12, 2107–2115. [Google Scholar] [CrossRef] [Green Version]
- Sohaei, S.; Hadi, A.; Karimi, E.; Arab, A. Saffron supplementation effects on glycemic indices: A systematic review and meta-analysis of randomized controlled clinical trials. Int. J. Food Prop. 2020, 23, 1386–1401. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.N.; Ulla, A.; Sumi, F.A.; Subhan, N.; Khan, T.; Sikder, B.; Hossain, H.; Reza, H.M.; Alam, M.A. Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis. 2017, 16, 151. [Google Scholar] [CrossRef] [Green Version]
- Nagashree, S.; Archana, K.K.; Srinivas, P.; Srinivasan, K.; Sowbhagya, H.B. Anti-hypercholesterolemic influence of the spice cardamom (Elettaria cardamomum) in experimental rats. J. Sci. Food Agric. 2017, 97, 3204–3210. [Google Scholar] [CrossRef]
- Pruthi, J.S. Major Spices of India. Crop Management and Post-Harvest Technology; Indian Council of Agricultural Research: Delhi, India, 1993. [Google Scholar]
- Bhaswant, M.; Poudyal, H.; Mathai, M.L.; Ward, L.C.; Mouatt, P.; Brown, L. Green and Black Cardamom in a Diet-Induced Rat Model of Metabolic Syndrome. Nutrients 2015, 7, 7691–7707. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, Y.; Siassi, F.; Rahimi, A.; Koohdani, F.; Doostan, F.; Qorbani, M.; Sotoudeh, G. The effect of cardamom supplementation on serum lipids, glycemic indices and blood pressure in overweight and obese pre-diabetic women: A randomized controlled trial. J. Diabetes Metab. Disord. 2017, 16, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef] [PubMed]
| Herbs | Target/Groups | Dose/Duration | Main Outcome | Study Reference |
|---|---|---|---|---|
| Slimax: extract of several plants: Hordeum vulgare, Polygonatum multiflorum, Dimocarpus longan, Ligusticum sinense, Lilium brownie, and Zingiber officinale | Healthy participants | 6 weeks | Significant decrease in body weight and body mass index (BMI). | [55] |
| Significant reduction in waist and hip circumference. | ||||
| Herbal supplement: (Ma Huang & Guarana) | Overweight participants | 72 mg of ephedra and 240 mg of caffeine for 8 weeks. | Significant decrease in body weight and total body fat. | [56] |
| -Control group (n = 24) | Significant reduction in hip and waist circumference. | |||
| -Intervention group (n = 24) | ||||
| A compound of Aralia mandshurica (A) and Engelhardtia chrysolepis (E) extracts called ARALOX | Obese non-diabetic women, n = 32 | 450 mg of Aralia mandshurica (A) and 450 mg of Engelhardtia chrysolepis (E) per day for 15 weeks. | Decrease in total body weight and fat weight. | [59] |
| -Control group: Diet + placebo, n = 16 | Reduction in plasma TGs. | |||
| -Intervention group: | ||||
| Diet + compound, n = 16 | ||||
| White bean extract (Phaseolus vulgaris) | Obese adults | 3000 mg per day of each for 8 weeks. | Weight reduction in the intervention group. | [60] |
| -Control group: placebo, n = 25 | Decrease in plasma TGs. | |||
| -Intervention group: white bean extract, n = 25 | ||||
| Turmeric (Curcuma longa L.) | Prediabetic adults | 750 mg per day of each for 9 months. | 16.4% of subjects in the placebo group were diagnosed with type 2 diabetes mellitus. | [61] |
| -Control group: placebo, n = 25 | None of the participants from the Curcuma longa-treated group were diagnosed with type 2 diabetes mellitus. | |||
| -Intervention group: Curcuma longa, n = 25 | ||||
| Korean red ginseng (KRG) (Panax ginseng) | Overweight participants | 6 g per day of each for 12 weeks. | No change in HbA1c in both groups. | [62] |
| n = 19, with well-controlled type 2 diabetes | Intervention group maintained good glycemic control and improved plasma glucose and plasma insulin regulation. | |||
| -Control group: placebo, n = 9 | ||||
| -Intervention group: KRG, n = 10 | ||||
| Bitter lemon (Momordica charantia) | Newly diagnosed with diabetes adults, | 3 g per day of each for 12 weeks. | There was no significant effect on mean FBG, total cholesterol, and weight in both groups. | [63] |
| -Control group: placebo, n = 20 | ||||
| -Intervention group: Momordica charantia, n = 20 | ||||
| Cinnamon (Cinnamomum) | Participants diagnosed diabetes mellitus type 2 | 3 g per day of each for 16 weeks. | The cinnamon extract has a moderate effect in reducing fasting plasma glucose concentrations in diabetic patients. | [64] |
| -Control group: placebo, n = 39 | ||||
| -Intervention group: cinnamon powder, n = 40 | ||||
| A combination of Cissus quadrangularis (CQ) and Irvingia gabonensis (IG) | Overweight and obese participants | Intervention group: 300 mg CQ + 500 mg IG = 800 mg of compound per day. | Significant reduction in Cholesterol and LDL of FBG levels. | [65] |
| -Control group: placebo, n = 36 | Control group: 800 of placebo per day. | Significant decrease in body weight, body fat percent, and waist size in both groups. | ||
| -Intervention group: compound of CQ and IG, n = 36 | Duration: 10 weeks | |||
| Terminalia arjuna tree-bark powder | coronary heart disease (CHD) patients | Group I: placebo capsules; | Significant antioxidant action in the vitamin E group and T. arjuna tree group. | [66] |
| Group I: control group, n = 35 | Group II: vitamin E capsules 400 units per day; | Significant hypo-cholesterolemic effect in the T. arjuna tree group. | ||
| Group II: vitamin E group, n = 35 | Group III received finely pulverized T. arjuna tree bark-powder (500 mg) per day | |||
| Group III: T. arjuna tree bark-powder group, n = 35 | for 30 days. | |||
| Ginger (Zingiber officinale) | Diabetic adults | 3 g of each per day | Reduction in FBS and HbA1c. | [67] |
| -Control group: placebo, n = 44 | for 8 weeks. | Improvement in insulin resistance. | ||
| -Intervention group: ginger powder, n = 44 | ||||
| Black seed (Nigella stevia) and turmeric (Curcuma longa L.) | Males with MetS n = 250 (randomly distributed | Black seeds (1.5 g/day) | Black seeds reduced lipids and FBG, while turmeric reduced LDL-cholesterol and C-reactive protein (CRP). | [68] |
| -Control group: | Turmeric (2.4 g/day) | |||
| Placebo group, n = 64 | combination (900 mg Black seeds and 1.5 g Turmeric/day) | |||
| -Treatment group: | placebo (2 g) | |||
| -Turmeric group, n = 62 | for 8 weeks. | |||
| -Black seed group, n = 62 | ||||
| -Combination group, n = 62 | ||||
| Cinnamon, cardamom, saffron (Crocus sativus) and ginger (Zingiber officinale) | Type 2 diabetes participants | For 8 weeks, | Significant beneficial effects on cholesterol, but not on measures of glycemic control, oxidative stress, and inflammation. | [69] |
| -Control group: | three glasses of black tea and either 3 g/day of cardamom, or cinnamon, or ginger, or 1 g saffron. Control group received three tea glasses without any treatment. | |||
| Placebo, n = 39 | ||||
| -Treatment groups: | ||||
| Cinnamon, n = 40 | ||||
| Cardamom, n = 42 | ||||
| Saffron, n = 42 | ||||
| Ginger, n = 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhatib, D.H.; Jaleel, A.; Tariq, M.N.M.; Feehan, J.; Apostolopoulos, V.; Cheikh Ismail, L.; Stojanovska, L.; Dhaheri, A.S.A. The Role of Bioactive Compounds from Dietary Spices in the Management of Metabolic Syndrome: An Overview. Nutrients 2022, 14, 175. https://doi.org/10.3390/nu14010175
Alkhatib DH, Jaleel A, Tariq MNM, Feehan J, Apostolopoulos V, Cheikh Ismail L, Stojanovska L, Dhaheri ASA. The Role of Bioactive Compounds from Dietary Spices in the Management of Metabolic Syndrome: An Overview. Nutrients. 2022; 14(1):175. https://doi.org/10.3390/nu14010175
Chicago/Turabian StyleAlkhatib, Dana Hasan, Abdul Jaleel, Maryam Naveed Muhammad Tariq, Jack Feehan, Vasso Apostolopoulos, Leila Cheikh Ismail, Lily Stojanovska, and Ayesha S. Al Dhaheri. 2022. "The Role of Bioactive Compounds from Dietary Spices in the Management of Metabolic Syndrome: An Overview" Nutrients 14, no. 1: 175. https://doi.org/10.3390/nu14010175









