Vitamin D Deficiency and Gestational Diabetes Mellitus in Relation to Body Mass Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.2.1. Assessment of Vitamin D Levels
2.2.2. Assessment of GDM
2.2.3. Assessment of BMI
2.2.4. Assessment of Covariates
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Assessment of the Association between VDD and GDM: Relationship with BMI
3.3. Assessment of Secondary Obstetric and Neonatal Events: Association with VDD and BMI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is Vitamin D Deficiency a Major Global Public Health? J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 138. [Google Scholar] [CrossRef] [Green Version]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K.; Hoffmann, D.K. Systematic Review a Systematic Review of Vitamin D Status in Populations Worldwide. Br. J. Nutr. 2021, 111, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; de Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada-Gómez, J.; Diaz-Curiel, M.; Sosa-Henriquez, M.; Malouf-Sierra, J.; Nogues-Solan, X.; Gomez-Alonso, C.; Rodriguez-Mañas, L.; Neyro-Bilbao, J.; Cortes, X.; Delgadillo, J. Low Calcium Intake and Inadequate Vitamin D Status in Postmenopausal Osteoporotic Women. J. Steroid Biochem. Mol. Biol. 2013, 136, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Mata-Granados, J.; Luque de Castro, M.; Quesada Gomez, J. Inappropriate Serum Levels of Retinol, Alpha-Tocopherol, 25 Hydroxyvitamin D3 and 24,25 Dihydroxyvitamin D3 Levels in Healthy Spanish Adults: Simultaneous Assessment by HPLC. Clin. Biochem. 2008, 41, 676–680. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.; Camargo, C.; Grant, C. Global Summary of Maternal and Newborn Vitamin D Status—A Systematic Review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D Supplementation for Women during Pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, Y.; Xue, H.; Xiong, J.; Cheng, G. Vitamin D and Gestational Diabetes Mellitus: A Systematic Review Based on Data Free of Hawthorne Effect. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 784–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urrutia, R.; Thorp, J. Vitamin D in Pregnancy: Current Concepts. Curr. Opin. Obstet. Gynecol. 2012, 24, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellamy, L.; Casas, J.; Hingorani, A.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet Lond. Engl. 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Nikooyeh, B.; Neyestani, T. Oxidative Stress, Type 2 Diabetes and Vitamin D: Past, Present and Future. Diabetes Metab. Res. Rev. 2016, 32, 260–267. [Google Scholar] [CrossRef]
- Wimalawansa, S. Associations of Vitamin D with Insulin Resistance, Obesity, Type 2 Diabetes, and Metabolic Syndrome. J. Steroid Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of Gestational Diabetes Mellitus and Its Association with Type 2 Diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Yang, Z.; Phung, H.; Freebairn, L.; Sexton, R.; Raulli, A.; Kelly, P. Contribution of Maternal Overweight and Obesity to the Occurrence of Adverse Pregnancy Outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmen, J.M.A.; Wielders, J.P.M.; Boer, A.K.; van den Ouweland, J.M.W.; Vader, H.L. The New Roche Vitamin D Total Assay: Fit for Its Purpose? Clin. Chem. Lab. Med. 2012, 50, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Leino, A.; Turpeinen, U.; Koskinen, P. Automated Measurement of 25-OH Vitamin D3 on the Roche Modular E170 Analyzer. Clin. Chem. 2008, 54, 2059–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, C.S.; Nexo, E.; Højskov, C.S.; Heickendorff, L. Analytical Validation of the Roche 25-OH Vitamin D Total Assay. Clin. Chem. Lab. Med. 2012, 50, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Iglesias Álvarez, E.M.; Granada Ybern, M.L.; Doladé Botías, M.; Barallat Martínez de Osaba, J.; Hidalgo Sáez, I.; Cruz Pastor Ferrer, M. Comparación de Las Concentraciones de Vitamina D Por 3 Métodos Comerciales. Rev. Lab. Clínico 2013, 6, 2–9. [Google Scholar] [CrossRef]
- VDSCP List of Certified Participants | CDC. Available online: https://www.cdc.gov/labstandards/vdscp_participants.html (accessed on 22 November 2021).
- Gómez de Tejada Romero, M.; Sosa Henríquez, M.; del Pino Montes, J.; Jodar Gimeno, E.; Quesada Gómez, J.; Cancelo Hidalgo, M.; Díaz Curiel, M.; Mesa Ramos, M.; Muñoz Torres, M.; Carpintero Benítez, P.; et al. Documento de Posición Sobre Las Necesidades y Niveles Óptimos de Vitamina D Sociedad Española de Investigación Ósea y Del Metabolismo Mineral (SEIOMM) y Sociedades Afines. Rev. Osteoporos. Y Metab. Miner. 2011, 3, 53–64. [Google Scholar]
- Carpenter, M.; Coustan, D. Criteria for Screening Tests for Gestational Diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Metzger, B.; Coustan, D. Summary and Recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998, 21 (Suppl. 2), B161–B167. [Google Scholar] [PubMed]
- Bloomgarden, Z. American Diabetes Association 60th Scientific Sessions, 2000: Diabetes and Pregnancy. Diabetes Care 2000, 23, 1699–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoy, R.; Lumbreras, B.; Bartha, J.L.; Ricart, W. Embarazo Nuevos Criterios Diagnósticos de Diabetes Mellitus Gestacional a Partir Del Estudio HAPO. ¿Son Válidos En Nuestro Medio? Av. Diabetol. 2010, 26, 139–142. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Consultation on Obesity (1999: Geneva, Switzerland). In Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2000; Volume 894. [Google Scholar]
- The Diabetes and Nutrition Study Group of the Spanish Diabetes Association. Diabetes Nutrition and Complications Trial. Diabetes Care 2004, 27, 984–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán, A.; Martín, P.; Runkle, I.; Pérez, N.; Abad, R.; Fernández, M.; del Valle, L.; Sanz, M.; Calle-Pascual, A. Benefits of Self-Monitoring Blood Glucose in the Management of New-Onset Type 2 Diabetes Mellitus: The St Carlos Study, a Prospective Randomized Clinic-Based Interventional Study with Parallel Groups. J. Diabetes 2010, 2, 203–211. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 November 2021).
- Pham, T.T.M.; Huang, Y.-L.; Chao, J.C.-J.; Chang, J.-S.; Chen, Y.-C.; Wang, F.-F.; Bai, C.-H. Plasma 25(OH)D Concentrations and Gestational Diabetes Mellitus among Pregnant Women in Taiwan. Nutrients 2021, 13, 2538. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Mo, M.; Xin, X.; Jiang, W.; Wu, J.; Huang, M.; Wang, S.; Muyiduli, X.; Si, S.; Shen, Y.; et al. The Interaction between Prepregnancy BMI and Gestational Vitamin D Deficiency on the Risk of Gestational Diabetes Mellitus Subtypes with Elevated Fasting Blood Glucose. Clin. Nutr. Edinb. Scotl. 2020, 39, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, G.; Sun, Y.; Du, Y.; Gao, R.; Snetselaar, L.G.; Santillan, M.K.; Bao, W. Association between Maternal Pre-Pregnancy Obesity and Preterm Birth According to Maternal Age and Race or Ethnicity: A Population-Based Study. Lancet Diabetes Endocrinol. 2019, 7, 707–714. [Google Scholar] [CrossRef]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between Perinatal Outcomes and Maternal Pre-Pregnancy Body Mass Index. Obes Rev. 2016, 17, 1091–1102. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [PubMed]
| Characteristics | Entire Sample | Non-GDM (n = 793, 89.5%) | GDM (n = 93, 10.5%) | p-Value |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age (years), mean (SD) | 32.0 (5.8) | 31.7 (5.7) | 34.2 (5.7) | <0.001 * |
| Questionnaire (St. Carlos Study), n (%) | ||||
| Physical activity | −0.7 (1.4) | −0.7 (1.4) | −0.8 (1.3) | 0.942 |
| Nutritional status | 3.9 (3.7) | 3.8 (3.7) | 4.0 (3.6) | 0.813 |
| Lifestyle | 3.1 (4.2) | 3.2 (3.9) | 3.2 (3.9) | 0.758 |
| Maternal smoking habit, n (%) | 91 (10.3) | 79 (10.0) | 12 (12.9) | 0.377 |
| Maternal hypothyroidism, n (%) | 48 (5.4) | 40 (5.0) | 8 (8.6) | 0.152 |
| Ethnicity, n (%) | 0.045 * | |||
| Caucasian | 766 (86.7) | 692 (87.6) | 74 (80.6) | |
| South American | 62 (7.0) | 54 (6.8) | 8 (8.6) | |
| Other | 55 (6.2) | 44 (5.6) | 11 (11.8) | |
| Pregnancy-related characteristics | ||||
| SAP, first trimester (mmHg), mean (SD) | 110.8 (11.7) | 110.2 (11.5) | 116.4 (12.0) | <0.001 * |
| DAP, first trimester (mmHg), mean (SD) | 68.8 (8.8) | 68.3 (8.6) | 73.0 (9.7) | <0.001 * |
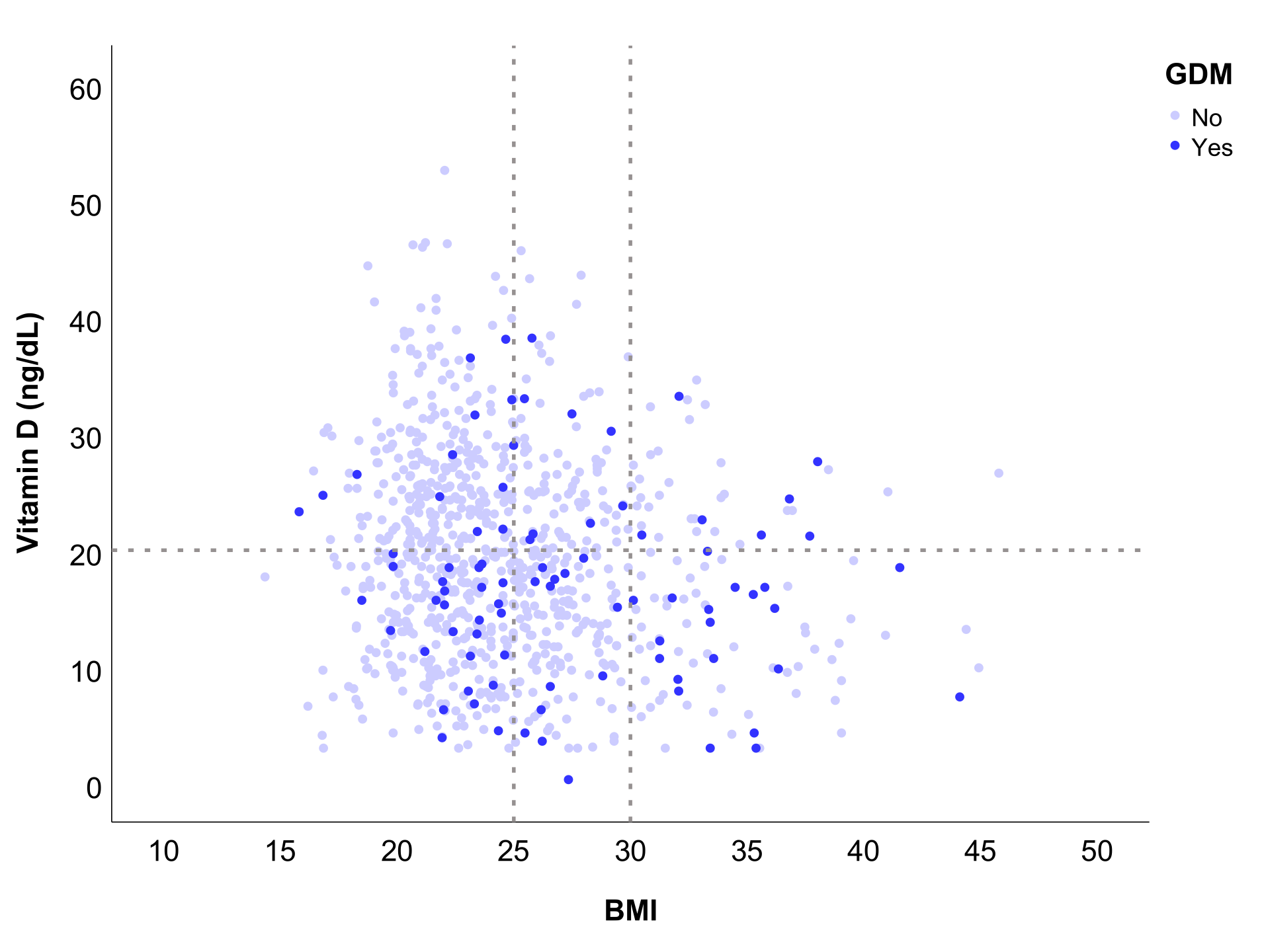
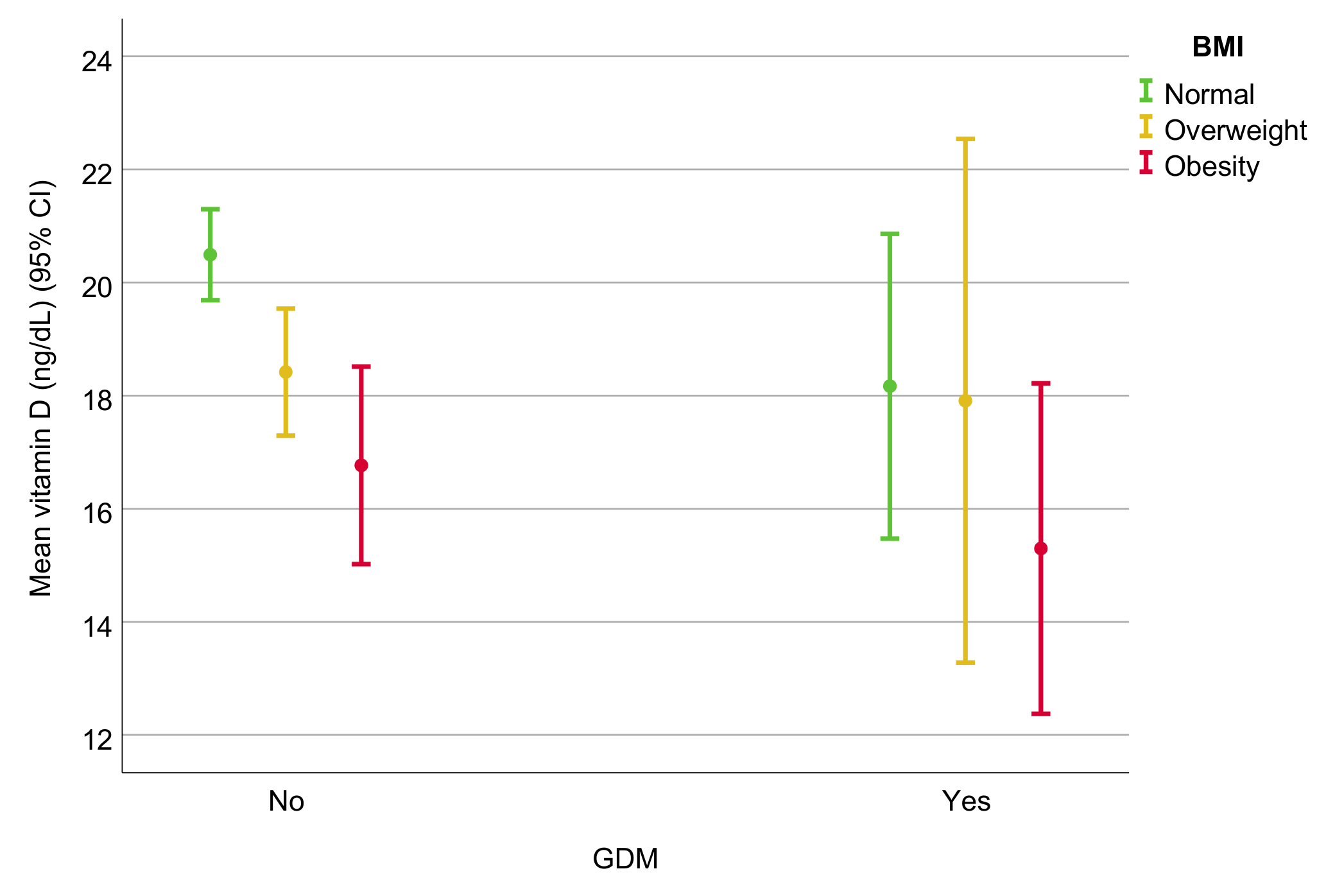
| VDD, n (%) | 489 (55.5) | 429 (54.1) | 60 (68.2) | 0.012 * |
| BMI, n (%) | 24.8 (4.8) | 24.5 (4.5) | 27.5 (5.9) | 0.021 * |
| BMI group, n (%) | <0.001 * | |||
| Normal (<25) | 532 (60.4) | 491 (62.3) | 41 (44.1) | |
| Overweight (25–30) | 234 (26.6) | 212 (26.9) | 22 (23.7) | |
| Obesity (>30) | 115 (13.0) | 85 (10.8) | 30 (32.2) | |
| Parity, n (%) | 0.464 | |||
| Primigravida | 446 (50.3) | 402 (50.7) | 44 (47.3) | |
| 2 pregnancies | 332 (37.5) | 298 (37.6) | 34 (36.6) | |
| ≥3 pregnancies | 108 (12.2) | 93 (11.7) | 15 (16.1) | |
| History of cesarean section, n (%) | 98 (11.1) | 85 (10.5) | 13 (14.0) | 0.345 |
| Gestational hypothyroidism, n (%) | 195 (22.0) | 175 (22.1) | 20 (21.5) | 0.901 |
| Blood test, mean (SD) | ||||
| Vitamin D (ng/dL) | 19.3 (8.9) | 19.5 (8.9) | 17.2 (8.6) | 0.021 * |
| C-reactive protein (mg/dL) | 5.7 (5.8) | 5.7 (6.0) | 6.2 (4.0) | 0.005 * |
| Ferritin (mg/dL) | 24.6 (25.5) | 24.3 (25.8) | 26.7 (21.7) | 0.249 |
| Cholesterol (mg/dL) | 224.3 (38.7) | 223.7 (38.8) | 229.5 (37.4) | 0.084 |
| HDL cholesterol (mg/dL) | 75.8 (15.3) | 76.3 (15.2) | 72.1 (15.7) | 0.034 * |
| LDL cholesterol (mg/dL) | 114.1 (32.5) | 113.5 (32.0) | 119.7 (36.1) | 0.197 |
| Triglycerides (mg/dL) | 177.2 (68.1) | 172.2 (62.4) | 220.5 (95.3) | <0.001 * |
| Fibrinogen (mg/dL) | 397.8 (56.3) | 394.5 (54.6) | 425.5 (62.3) | <0.001 * |
| Hemoglobin (g/dL) | 11.6 (0.9) | 11.6 (0.9) | 11.6 (0.8) | 0.747 |
| Hematocrit (%) | 34.2 (2.5) | 34.2 (2.5) | 34.2 (2.4) | 0.597 |
| Crude Model | Model 1 | Model 2 | Adjusted Model (AIC) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | 95%CI | p-Value | PR | 95%CI | p-Value | PR | 95%CI | p-Value | PR | 95%CI | p-Value | ||
| VDD: | No | 1 | 1 | 1 | 1 | ||||||||
| Yes | 1.718 | (1.119–2.637) | 0.013 * | 1.567 | (1.016–2.417) | 0.042 * | 1.660 | (1.042–2.645) | 0.033 * | 1.635 | (1.027–2.604) | 0.038 * | |
| BMI: | Normal | 1 | 1 | 1 | |||||||||
| Overweight | 1.220 | (0.744–2.001) | 0.431 | 1.137 | (0.680–1.899) | 0.625 | 0.724 | (0.404–1.299) | 0.279 | NS | |||
| Obesity | 3.385 | (2.212–5.181) | <0.001 * | 2.992 | (1.907–4.694) | <0.001 * | 1.395 | (0.788–2.469) | 0.253 | NS | |||
| Ethnicity: | Caucasian | 1 | - | ||||||||||
| South American | 1.336 | (0.675–2.642) | 0.406 | - | NS | NS | |||||||
| Other | 2.070 | (1.169–3.665) | 0.013 * | - | NS | NS | |||||||
| Parity: | Primigravida | 1 | - | ||||||||||
| 2 pregnancies | 1.038 | (0.679–1.587) | 0.863 | - | NS | NS | |||||||
| ≥3 pregnancies | 1.408 | (0.815–2.433) | 0.220 | - | NS | NS | |||||||
| Previous cesarean section | 1.305 | (0.755–2.256) | 0.340 | - | NS | NS | |||||||
| Gestational hypothyroidism | 0.971 | (0.608–1.551) | 0.901 | - | NS | NS | |||||||
| Age | 1.078 | (1.035–1.122) | <0.001 * | - | 1.062 | (1.020–1.104) | 0.003 * | 1.064 | (1.022–1.107) | 0.003 * | |||
| Triglycerides | 1.005 | (1.004–1.007) | <0.001 * | - | 1.003 | (1.001–1.005) | 0.002 * | 1.003 | (1.002–1.005) | <0.001 * | |||
| Fibrinogen | 1.008 | (1.005–1.011) | <0.001 * | - | 1.006 | (1.002–1.009) | 0.001 * | 1.005 | (1.002–1.009) | <0.001 * | |||
| SAP | 1.042 | (1.025–1.060) | <0.001 * | - | 1.027 | (1.009–1.045) | 0.003 * | 1.031 | (1.013–1.049) | <0.001 * | |||
| DAP | 1.055 | (1.032–1.079) | <0.001 * | - | NS | NS | |||||||
| CRP | 1.013 | (0.994–1.032) | 0.193 | - | NS | NS | |||||||
| Ferritin | 1.003 | (0.998–1.007) | 0.285 | - | NS | NS | |||||||
| Cholesterol | 1.003 | (0.999–1.008) | 0.160 | - | NS | NS | |||||||
| HDL | 0.984 | (0.970–0.997) | 0.019 * | - | NS | NS | |||||||
| LDL | 1.005 | (0.998–1.012) | 0.133 | - | NS | NS | |||||||
| Questionnaire: | Physical activity | 0.990 | (0.858–1.142) | 0.889 | - | NS | NS | ||||||
| Nutritional habit | 1.008 | (0.951–1.069) | 0.778 | - | NS | NS | |||||||
| Lifestyle | 1.005 | (0.958–1.055) | 0.827 | - | NS | NS | |||||||
| Hemoglobin | 1.046 | (0.837–1.308) | 0.692 | - | NS | NS | |||||||
| Hematocrit | 1.010 | (0.939–1.087) | 0.793 | - | NS | NS | |||||||
| Maternal smoking habit | 1.294 | (0.735–2.280) | 0.372 | - | NS | NS | |||||||
| Maternal hypothyroidism | 1.643 | (0.846–3.192) | 0.143 | - | NS | NS | |||||||
| VDD | p-Value | BMI Group | p-Value | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | Normal | Overweight | Obese | |||
| GDM | 28 (7.1) | 60 (12.3) | 0.012 * | 41 (7.7) | 22 (9.4) | 30 (26.1) | <0.001 * |
| Preterm birth | 23 (5.9) | 20 (4.1) | 0.224 | 23 (4.3) | 18 (7.7) | 2 (1.7) | 0.034 |
| Preeclampsia | 10 (2.6) | 13 (2.7) | 0.921 | 11 (2.1) | 6 (2.6) | 6 (5.2) | 0.158 |
| Cesarean section | 79 (20.3) | 90 (18.6) | 0.534 | 86 (16.3) | 52 (22.5) | 31 (27.4) | 0.009 * |
| Neonatal ICU admission | 79 (21.3) | 99 (21.2) | 0.961 | 106 (21.0) | 42 (18.8) | 32 (28.6) | 0.115 |
| Weight percentile | 0.054 | <0.001 * | |||||
| <10 | 35 (8.9) | 29 (5.9) | 44 (8.3) | 9 (3.8) | 10 (8.7) | ||
| 10–90 | 312 (79.6) | 377 (77.1) | 424 (79.7) | 188 (80.3) | 75 (65.2) | ||
| >90 | 30 (7.7) | 59 (12.1) | 39 (7.3) | 27 (11.5) | 25 (21.7) | ||
| Missing | 15 (3.8) | 24 (4.9) | 25 (4.7) | 10 (4.3) | 5 (4.3) | ||
| Height percentile | 0.068 | 0.078 | |||||
| <10 | 32 (8.2) | 21 (4.3) | 34 (6.4) | 10 (4.3) | 9 (7.8) | ||
| 10–90 | 272 (69.4) | 349 (71.4) | 386 (72.6) | 164 (70.1) | 69 (60.0) | ||
| >90 | 71 (18.1) | 88 (18.0) | 85 (16.0) | 44 (18.8) | 31 (27.0) | ||
| Missing | 17 (4.3) | 31 (6.3) | 27 (5.1) | 16 (6.8) | 6 (5.2) | ||
| Head circumference percentile | 0.465 | 0.039 | |||||
| <10 | 31 (7.9) | 43 (8.8) | 50 (9.4) | 12 (5.1) | 12 (10.4) | ||
| 10–90 | 322 (82.1) | 388 (79.3) | 431 (81.0) | 192 (82.1) | 84 (73.0) | ||
| >90 | 21 (5.4) | 24 (4.9) | 23 (4.3) | 12 (5.1) | 12 (10.4) | ||
| Missing | 18 (4.6) | 34 (7.0) | 28 (5.3) | 18 (7.7) | 7 (6.1) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agüero-Domenech, N.; Jover, S.; Sarrión, A.; Baranda, J.; Quesada-Rico, J.A.; Pereira-Expósito, A.; Gil-Guillén, V.; Cortés-Castell, E.; García-Teruel, M.J. Vitamin D Deficiency and Gestational Diabetes Mellitus in Relation to Body Mass Index. Nutrients 2022, 14, 102. https://doi.org/10.3390/nu14010102
Agüero-Domenech N, Jover S, Sarrión A, Baranda J, Quesada-Rico JA, Pereira-Expósito A, Gil-Guillén V, Cortés-Castell E, García-Teruel MJ. Vitamin D Deficiency and Gestational Diabetes Mellitus in Relation to Body Mass Index. Nutrients. 2022; 14(1):102. https://doi.org/10.3390/nu14010102
Chicago/Turabian StyleAgüero-Domenech, Nuria, Silvia Jover, Ana Sarrión, Javier Baranda, José A. Quesada-Rico, Avelino Pereira-Expósito, Vicente Gil-Guillén, Ernesto Cortés-Castell, and María J. García-Teruel. 2022. "Vitamin D Deficiency and Gestational Diabetes Mellitus in Relation to Body Mass Index" Nutrients 14, no. 1: 102. https://doi.org/10.3390/nu14010102