Associations of 25 Hydroxyvitamin D and High Sensitivity C-reactive Protein Levels in Early Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Populations
2.3. Measurements of Hs-CRP and 25(OH)D
2.4. Covariates
2.5. Statistical Analyses
3. Results
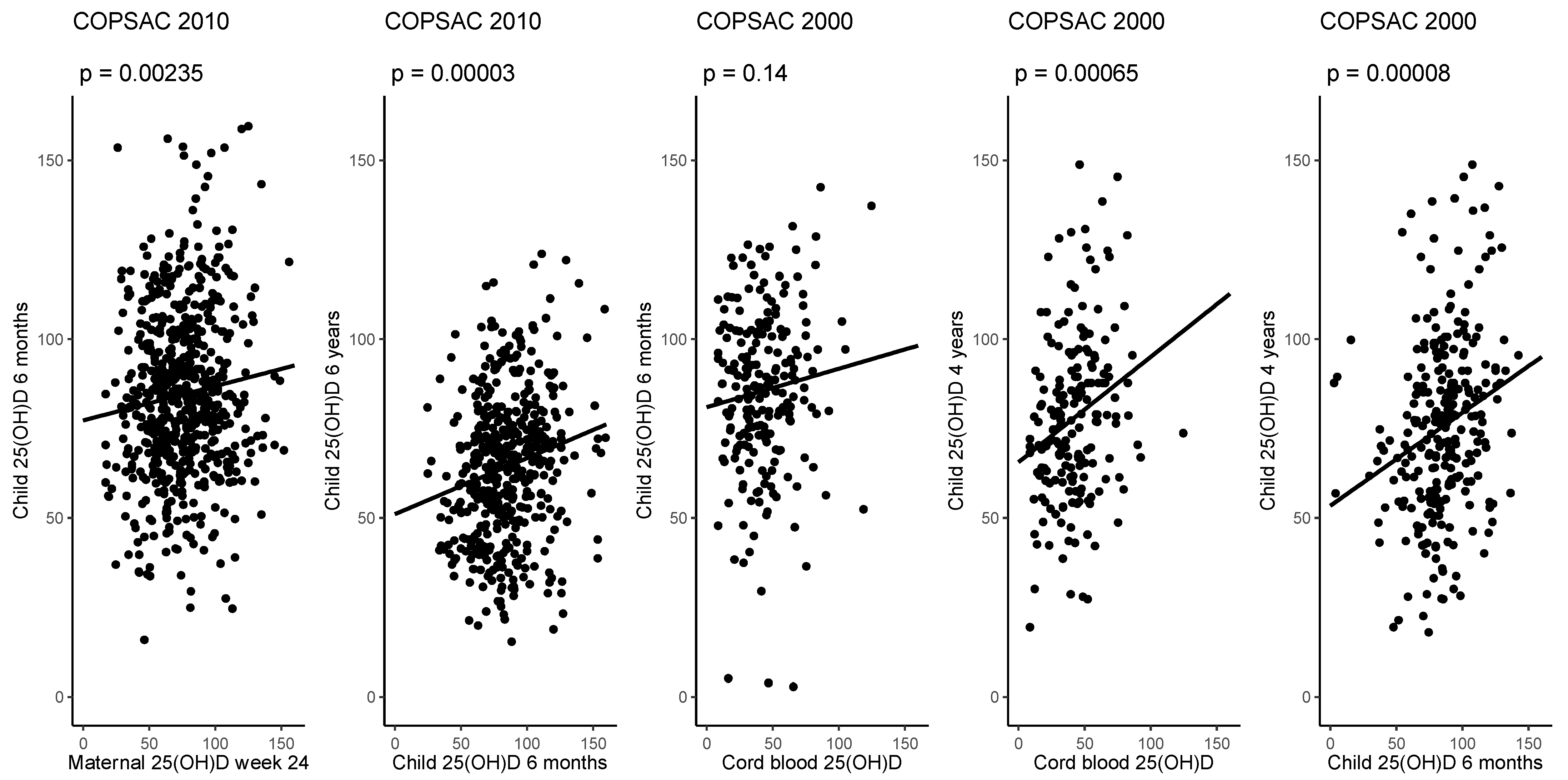
3.1. Associations of 25(OH)D from Pregnancy to Childhood and in Childhood
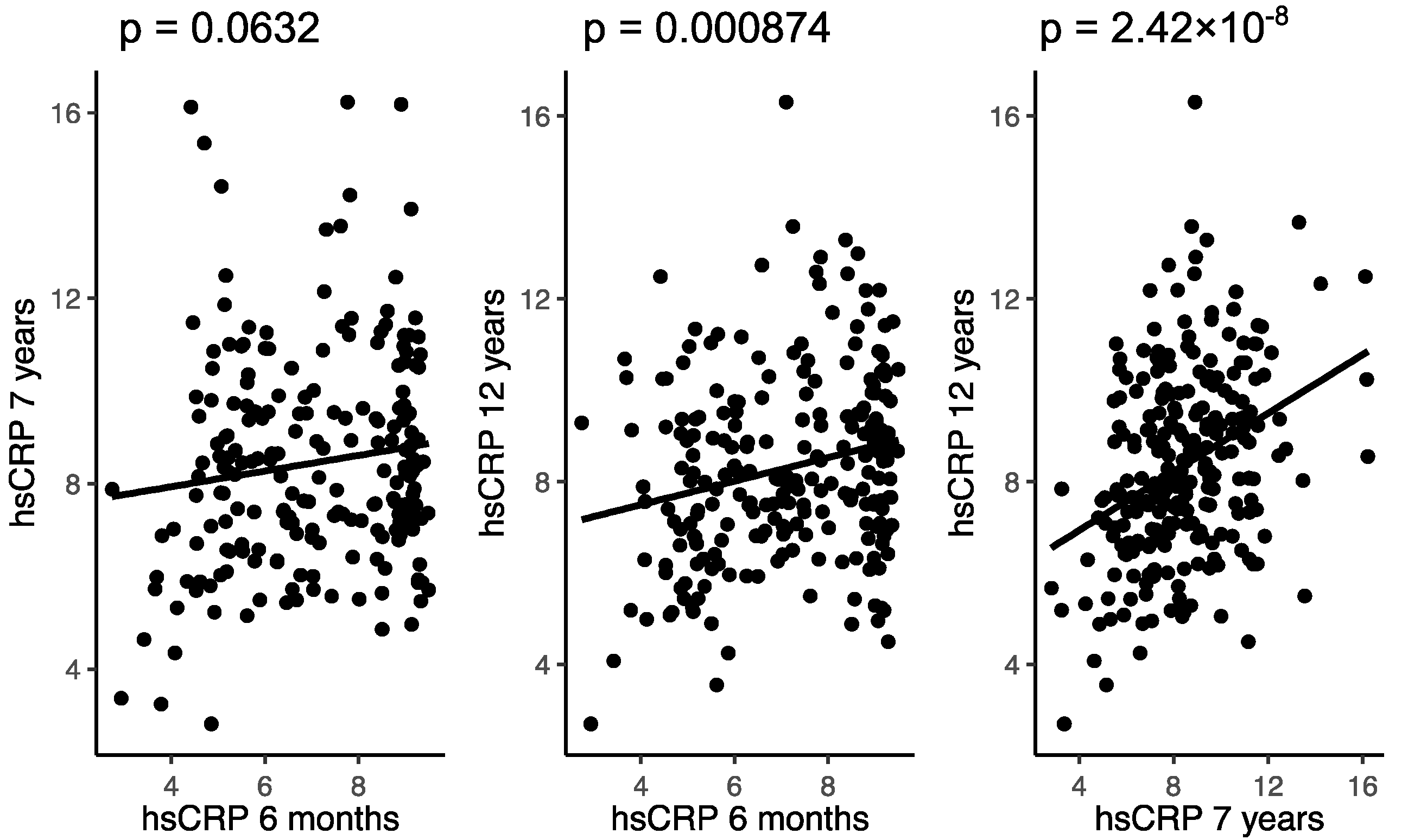
3.2. Associations of hs-CRP in Childhood
3.3. Association between Hs-CRP and 25(OH)D in Both Cohorts
4. Discussion
4.1. Primary Findings
4.2. Strengths and Limitations
4.3. Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hod, K.; Ringel-Kulka, T.; Martin, C.F.; Maharshak, N.; Ringel, Y. High-sensitive C-Reactive Protein as a Marker for Inflammation in Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2016, 50, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, F.A.H.; Izar, M.C.D.O. High-Sensitivity C-Reactive Protein and Cardiovascular Disease Across Countries and Ethnicities. Clinics 2016, 71, 235–242. [Google Scholar] [CrossRef]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, G.; Galeone, C.; Taverna, F.; Suatoni, P.; Morelli, D.; Pastorino, U. C-reactive protein level predicts mortality in COPD: A systematic review and meta-analysis. Eur. Respir. Rev. 2017, 26, 160070. [Google Scholar] [CrossRef]
- Chawes, B.; Stokholm, J.; Bønnelykke, K.; Brix, S.; Bisgaard, H. Neonates with reduced neonatal lung function have systemic low-grade inflammation. J. Allergy Clin. Immunol. 2015, 135, 1450–1456.e1. [Google Scholar] [CrossRef] [Green Version]
- Ko, A.R.; Kim, Y.H.; Sol, I.S.; Kim, M.J.; Yoon, S.H.; Kim, K.W.; Kim, K.-E. High-Sensitivity C-Reactive Protein Can Reflect Small Airway Obstruction in Childhood Asthma. Yonsei Med. J. 2016, 57, 690–697. [Google Scholar] [CrossRef]
- Chawes, B.L.; Stokholm, J.; Schoos, A.M.; Fink, N.R.; Brix, S.; Bisgaard, H. Allergic sensitization at school age is a systemic low-grade inflammatory disorder. Allergy 2017, 72, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Fink, N.R.; Chawes, B.L.; Thorsen, J.; Stokholm, J.; Krogfelt, K.A.; Schjørring, S.; Kragh, M.; Bønnelykke, K.; Brix, S.; Bisgaard, H. Neonates colonized with pathogenic bacteria in the airways have a low-grade systemic inflammation. Allergy 2018, 73, 2150–2159. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, T.; Obase, Y.; Kishikawa, R.; Iwanaga, T. Serum high-sensitivity C-reactive protein can be an airway inflammation predictor in bronchial asthma. Allergy Asthma Proc. 2015, 36, 167. [Google Scholar] [CrossRef]
- Deraz, T.; Kamel, T.B.; El-Kerdany, T.A.; El-Ghazoly, H.M. High-sensitivity C reactive protein as a biomarker for grading of childhood asthma in relation to clinical classification, induced sputum cellularity, and spirometry. Pediatr. Pulmonol. 2011, 47, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Fink, N.R.; Chawes, B.; Bønnelykke, K.; Thorsen, J.; Stokholm, J.; Rasmussen, M.A.; Brix, S.; Bisgaard, H. Levels of Systemic Low-grade Inflammation in Pregnant Mothers and Their Offspring are Correlated. Sci. Rep. 2019, 9, 3043. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Garland, J.; Thorsen, J.; Sevelsted, A.; Krakauer, M.; Vinding, R.K.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Effect of High-Dose vs Standard-Dose Vitamin D Supplementation in Pregnancy on Bone Mineralization in Offspring Until Age 6 Years. JAMA Pediatr. 2020, 174, 419. [Google Scholar] [CrossRef] [PubMed]
- Nørrisgaard, P.E.; Haubek, D.; Kühnisch, J.; Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Association of High-Dose Vitamin D Supplementation During Pregnancy with the Risk of Enamel Defects in Offspring. JAMA Pediatr. 2019, 173, 924–930. [Google Scholar] [CrossRef]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar]
- Schögler, A.; Muster, R.J.; Kieninger, E.; Casaulta, C.; Tapparel, C.; Jung, A.; Moeller, A.; Geiser, T.; Regamey, N.; Alves, M.P. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur. Respir. J. 2015, 47, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. 2004, 173, 6490. [Google Scholar] [CrossRef] [Green Version]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1689S–1696S. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Oddy, W.H.; Holt, P.; Ping-Delfos, W.C.S.; Mountain, J.; Lye, S.; Pennell, C.; Hart, P.H.; Walsh, J.P. Tracking of vitamin D status from childhood to early adulthood and its association with peak bone mass. Am. J. Clin. Nutr. 2017, 106, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Bisgaard, H.; Vissing, N.H.; Carson, C.G.; Bischoff, A.L.; Følsgaard, N.V.; Kreiner, E.; Chawes, B.; Stokholm, J.; Pedersen, S.B.; Bjarnadóttir, E.; et al. Deep phenotyping of the unselected COPSAC 2010 birth cohort study. Clin. Exp. Allergy 2013, 43, 1384–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisgaard, H. The Copenhagen Prospective Study on Asthma in Childhood (COPSAC): Design, rationale, and baseline data from a longitudinal birth cohort study. Ann. Allergy Asthma Immunol. 2004, 93, 381–389. [Google Scholar] [CrossRef]
- Schoos, A.-M.M.; Vinther, C.; Nørgaard, S.; Brustad, N.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Environmental and Genetic Determinants of Serum 25(OH)-Vitamin D Levels during Pregnancy and Early Childhood. Children 2019, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Bisgaard, H.; Stokholm, J.; Chawes, B.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.-M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish Oil–Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef]
- Højskov, C.S.; Heickendorff, L.; Møller, H.J. High-throughput liquid–liquid extraction and LCMSMS assay for determination of circulating 25(OH) vitamin D3 and D2 in the routine clinical laboratory. Clin. Chim. Acta 2010, 411, 114–116. [Google Scholar] [CrossRef]
- Ersfeld, D.L.; Rao, D.; Body, J.-J.; Sackrison, J.L.; Miller, A.B.; Parikh, N.; Eskridge, T.L.; Polinske, A.; Olson, G.T.; MacFarlane, G.D. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON® automated analyzer. Clin. Biochem. 2004, 37, 867–874. [Google Scholar] [CrossRef]
- Brustad, N.; Greve, J.H.; Mirzakhani, H.; Pedersen, C.T.; Eliasen, A.U.; Stokholm, J.; Lasky-Su, J.; Bønnelykke, K.; Litonjua, A.A.; Weiss, S.T.; et al. High-dose vitamin D during pregnancy and pathway gene polymorphisms in prevention of offspring persistent wheeze. Pediatr. Allergy Immunol. 2021, 32, 679–689. [Google Scholar] [CrossRef]
- Jorde, R.; Sneve, M.; Hutchinson, M.; Emaus, N.; Figenschau, Y.; Grimnes, G. Tracking of Serum 25-Hydroxyvitamin D Levels During 14 Years in a Population-based Study and During 12 Months in an Intervention Study. Am. J. Epidemiol. 2010, 171, 903–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poopedi, M.A.; Norris, S.A.; Micklesfield, L.K.; Pettifor, J.M. Does vitamin D status track through adolescence? Am. J. Clin. Nutr. 2015, 102, 1025–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, C.; Walker-Bone, K.; Arden, N.; Dennison, E. Novel insights into the pathogenesis of osteoporosis: The role of intrauterine programming. Rheumatology 2000, 39, 1312–1315. [Google Scholar] [CrossRef] [Green Version]
- Glynn, R.J.; MacFadyen, J.G.; Ridker, P.M. Tracking of High-Sensitivity C-Reactive Protein after an Initially Elevated Concentration: The JUPITER Study. Clin. Chem. 2009, 55, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Juonala, M.; Viikari, J.S.; Rönnemaa, T.; Taittonen, L.; Marniemi, J.; Raitakari, O.T. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1883–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Wan, Z.; Han, S.-F.; Li, B.-Y.; Zhang, Z.-L.; Qin, L.-Q. Effect of Vitamin D Supplementation on the Level of Circulating High-Sensitivity C-Reactive Protein: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2014, 6, 2206–2216. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, D.; Yin, K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| hs-CRP Estimate * | 95% CI | p Value | |
|---|---|---|---|
| Crude (n = 613) | −0.004 | −0.008; −0.0004 | 0.030 |
| Adjusted for environmental and demographic factors (n = 613) 1 | −0.002 | −0.006; 0.001 | 0.230 |
| Children with no infection (n = 405) 2 | −0.005 | −0.009; −0.001 | 0.027 |
| hs-CRP Estimate * | 95% CI | p Value | |
|---|---|---|---|
| Crude (n = 299) | 0.003 | −0.003; 0.009 | 0.401 |
| Adjusted for environmental and demographic factors (n = 299) 1 | 0.004 | −0.002; −0.010 | 0.157 |
| Children with no infection (n = 208) 2 | 0.003 | −0.004; −0.009 | 0.436 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brustad, N.; Fink, N.R.; Stokholm, J.; Bønnelykke, K.; Følsgaard, N.V.; Hougaard, D.; Brix, S.; Lasky-Su, J.; Weiss, S.T.; Chawes, B. Associations of 25 Hydroxyvitamin D and High Sensitivity C-reactive Protein Levels in Early Life. Nutrients 2022, 14, 15. https://doi.org/10.3390/nu14010015
Brustad N, Fink NR, Stokholm J, Bønnelykke K, Følsgaard NV, Hougaard D, Brix S, Lasky-Su J, Weiss ST, Chawes B. Associations of 25 Hydroxyvitamin D and High Sensitivity C-reactive Protein Levels in Early Life. Nutrients. 2022; 14(1):15. https://doi.org/10.3390/nu14010015
Chicago/Turabian StyleBrustad, Nicklas, Nadia R. Fink, Jakob Stokholm, Klaus Bønnelykke, Nilofar V. Følsgaard, David Hougaard, Susanne Brix, Jessica Lasky-Su, Scott T. Weiss, and Bo Chawes. 2022. "Associations of 25 Hydroxyvitamin D and High Sensitivity C-reactive Protein Levels in Early Life" Nutrients 14, no. 1: 15. https://doi.org/10.3390/nu14010015
APA StyleBrustad, N., Fink, N. R., Stokholm, J., Bønnelykke, K., Følsgaard, N. V., Hougaard, D., Brix, S., Lasky-Su, J., Weiss, S. T., & Chawes, B. (2022). Associations of 25 Hydroxyvitamin D and High Sensitivity C-reactive Protein Levels in Early Life. Nutrients, 14(1), 15. https://doi.org/10.3390/nu14010015








