Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants
Abstract
1. Introduction
1.1. Hidden Bioactivity of Natural Pigments
1.1.1. Nature Is Full of Splendid Color!
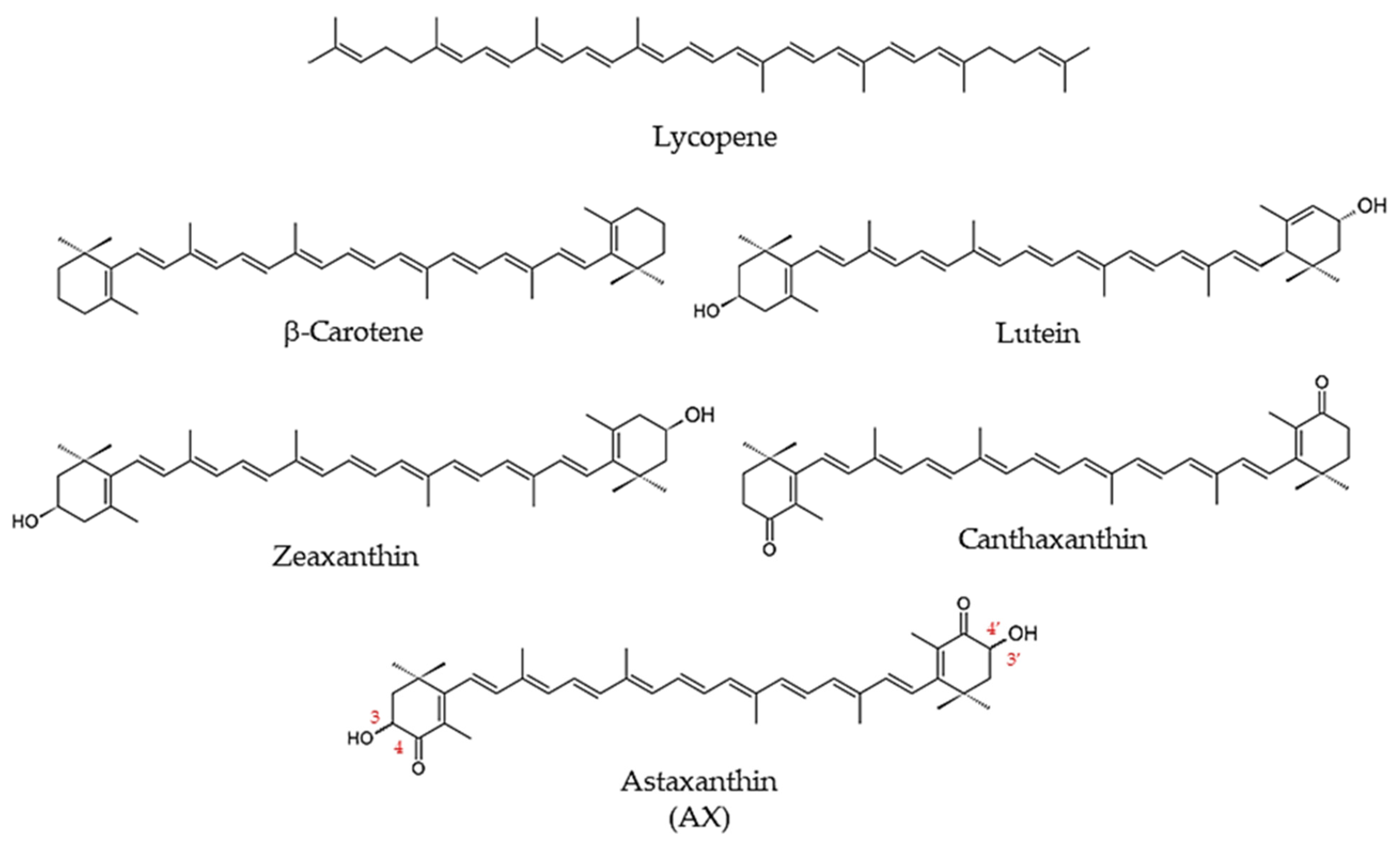
1.1.2. Carotenoids
1.1.3. What Is Astaxanthin?
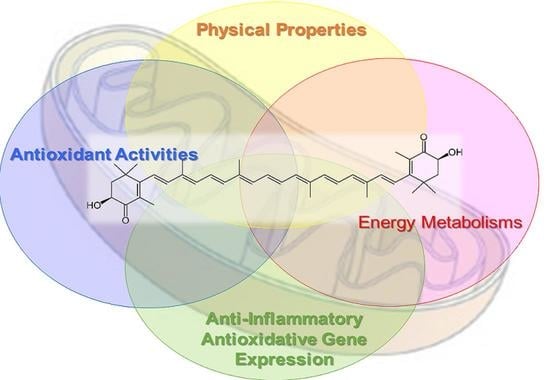
1.2. Biological Activity of Astaxanthin
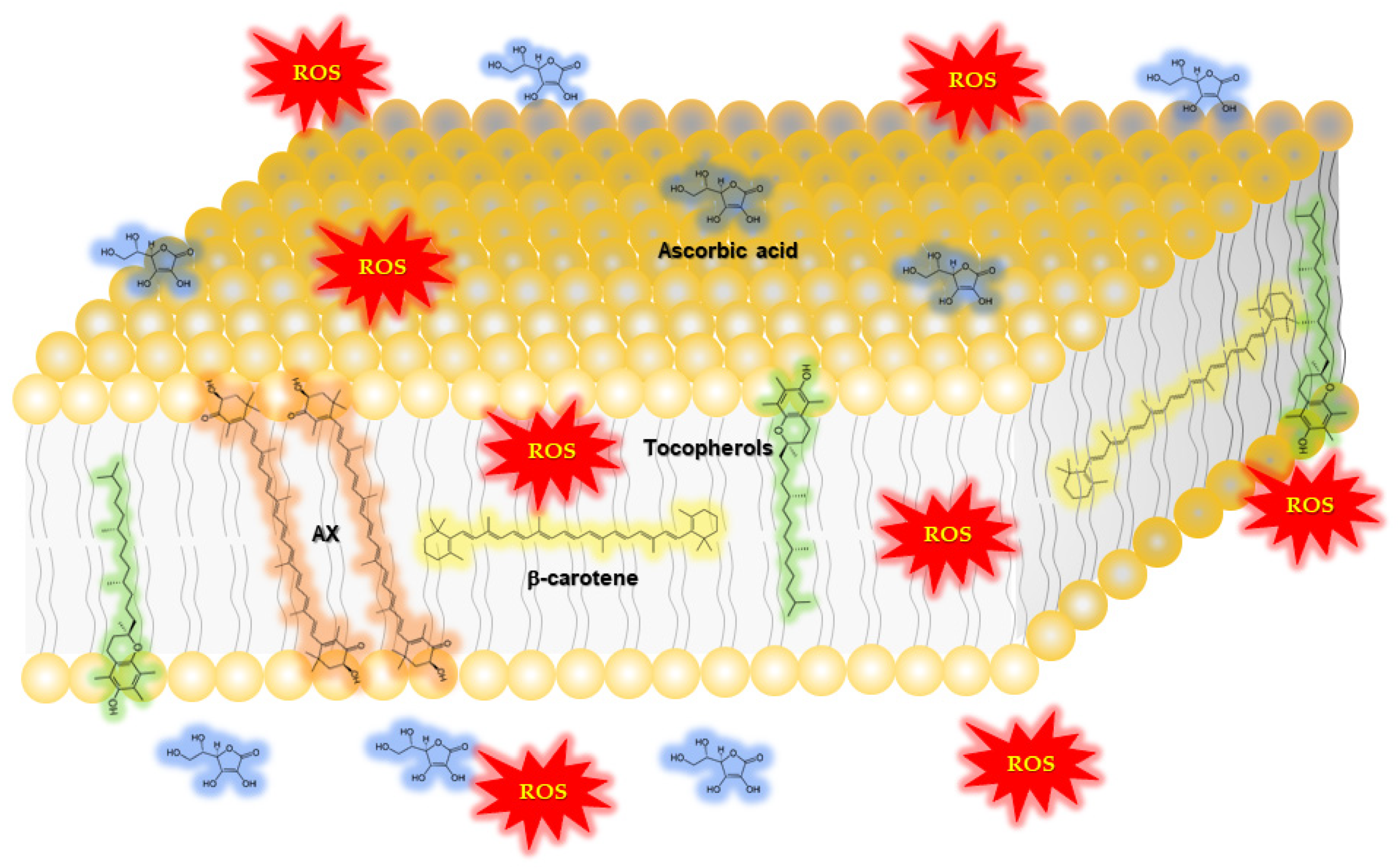
1.2.1. Function of Astaxanthin in Lipid Bilayers: Antioxidant Activity and Impact on Physical Properties
2. Mechanism by Which Astaxanthin Enhances Mitochondrial Energy Metabolism
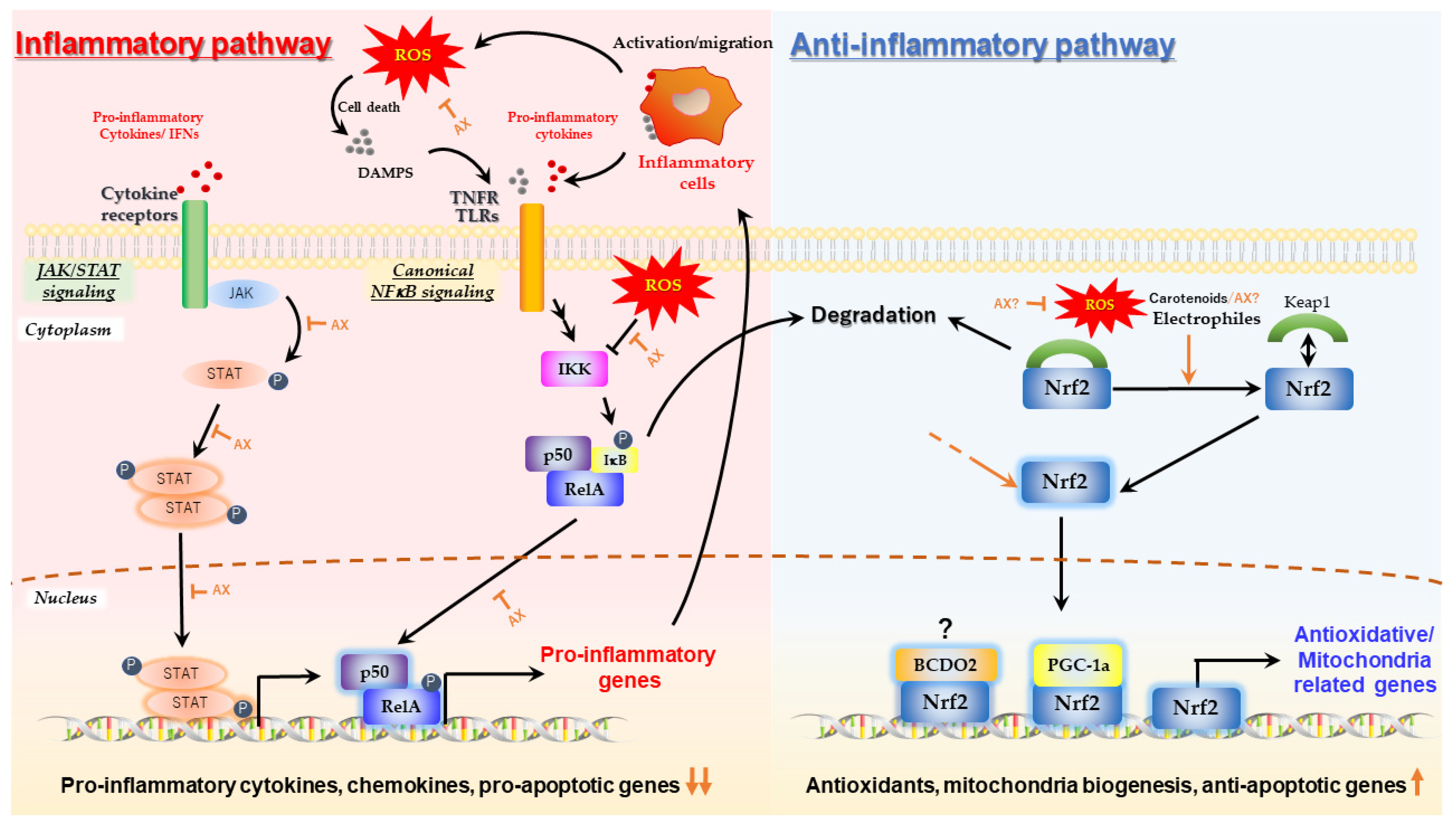
2.1. Protective Effect of Astaxanthin on Mitochondria; Astaxanthin as a Mitochondrial Antioxidant
2.2. Aggressive Enhancement of Mitochondrial Activity and Metabolism via Gene Expression by Astaxanthin
2.2.1. Nrf2 Pathway
2.2.2. Nuclear Receptors
2.2.3. AMPK/Sirtuins/PGC-1α Pathway
2.2.4. AX Contributes to Mitochondrial Quality Control
2.2.5. Is the AMPK-Activating Effect of AX Independent of Its Antioxidant Effect?
2.2.6. Direct Effects of Astaxanthin on Mitochondria: Actions beyond Its Antioxidant Activity
3. Prospect of Astaxanthin for Human Health Promotion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britton, G. Carotenoids. In Natural Food Colorants; Hendry, G.A.F., Houghton, J.D., Eds.; Springer: Boston, MA, USA, 1996; pp. 197–243. [Google Scholar]
- Britton, G.; Pfander, H.; Liaaen-Jensen, S. Carotenoids Volume 5: Nutrition and Health; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5. [Google Scholar]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Takemura, M.; Maoka, T. Carotenoid Biosynthesis in Animals: Case of Arthropods. In Carotenoids Biosynthetic Biofunctional Approaches; Springer: Singapore, 2021; Volume 1261, pp. 217–220. [Google Scholar] [CrossRef]
- Nishida, Y. Astaxanthin: Commercial production and its potential health-promoting effects. Oleoscience 2012, 12, 525–531. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Baskaran, V. Retinol-deficient rats can convert a pharmacological dose of astaxanthin to retinol: Antioxidant potential of astaxanthin, lutein, and β-carotene. Can. J. Physiol. Pharmacol. 2010, 88, 977–985. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Photo Protection of Haematococcus pluvialis Algae by Astaxanthin: Unique Properties of Astaxanthin Deduced by EPR, Optical and Electrochemical Studies. Antioxidants 2017, 6, 80. [Google Scholar] [CrossRef]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar]
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.-I.; Mukai, K. Kinetic Study of the Quenching Reaction of Singlet Oxygen by Carotenoids and Food Extracts in Solution. Development of a Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Agric. Food Chem. 2010, 58, 9967–9978. [Google Scholar] [CrossRef] [PubMed]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of Low-Density Lipoprotein Oxidation by Astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Kimura, F.; Satoh, A.; Miyazawa, T. Erythrocytes carotenoids after astaxanthin supplementation in middle-aged and senior Japanese subjects. J. Oleo Sci. 2011, 60, 495–499. [Google Scholar] [CrossRef]
- Matthews, S.J.; Ross, N.W.; Lall, S.P.; Gill, T.A. Astaxanthin binding protein in Atlantic salmon. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 144, 206–214. [Google Scholar] [CrossRef]
- Kawasaki, S.; Mizuguchi, K.; Sato, M.; Kono, T.; Shimizu, H. A Novel Astaxanthin-Binding Photooxidative Stress-Inducible Aqueous Carotenoprotein from a Eukaryotic Microalga Isolated from Asphalt in Midsummer. Plant Cell Physiol. 2013, 54, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Nur-E-Borhan, S.A.; Okada, S.; Watabe, S.; Yamaguchi, K. Carotenoproteins from the Exoskeleton and the Muscular Epithelium of the Black Tiger Prawn Penaeus monodon. Fish. Sci. 1995, 61, 337–343. [Google Scholar] [CrossRef][Green Version]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gabrielska, J.; Gruszecki, W.I. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: A 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta Biomembr. 1996, 1285, 167–174. [Google Scholar] [CrossRef]
- Socaciu, C.; Jessel, R.; Diehl, H.A. Carotenoid incorporation into microsomes: Yields, stability and membrane dynamics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 2799–2809. [Google Scholar] [CrossRef]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczyńska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and Orientation of Xanthophylls in a Lipid Bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- Kobayashi, M.; Katsuragi, T.; Tani, Y. Enlarged and Astaxanthin-Accumulating Cyst Cells of the Green Alga Haematococcus pluvialis. J. Biosci. Bioeng. 2001, 92, 565–568. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Sakuma, K.; Kuchide, M.; Tokuda, H.; Maoka, T.; Toyokuni, S.; Oka, S.; Yasuhara, M.; Yoshikawa, T. Astaxanthin Limits Exercise-Induced Skeletal and Cardiac Muscle Damage in Mice. Antioxid. Redox Signal. 2003, 5, 139–144. [Google Scholar] [CrossRef]
- McAllister, M.J.; Mettler, J.A.; Patek, K.; Butawan, M.; Bloomer, R.J. Astaxanthin supplementation increases glutathione concentrations but does not impact fat oxidation during exercise in active young men. Int. J. Sport Nutr. Exerc. Metab. 2021, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Petyaev, I.M.; Klochkov, V.A.; Chalyk, N.E.; Pristensky, D.V.; Chernyshova, M.P.; Kyle, N.H.; Bashmakov, Y.K. Markers of Hypoxia and Oxidative Stress in Aging Volunteers Ingesting Lycosomal Formulation of Dark Chocolate Containing Astaxanthin. J. Nutr. Health Aging 2018, 22, 1092–1098. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M. The effect of astaxanthin on vascular endothelial growth factor (VEGF) levels and peroxidation reactions in the aqueous humor. J. Clin. Biochem. Nutr. 2016, 59, 10–15. [Google Scholar] [CrossRef]
- Baralic, I.; Andjelkovic, M.; Djordjevic, B.; Dikic, N.; Radivojevic, N.; Suzin-Zivkovic, V.; Radojevic-Skodric, S.; Pejic, S. Effect of Astaxanthin Supplementation on Salivary IgA, Oxidative Stress, and Inflammation in Young Soccer Players. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of Astaxanthin Supplementation on Paraoxonase 1 Activities and Oxidative Stress Status in Young Soccer Players. Phytother. Res. 2012, 27, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M.; Obara, Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013, 53, 1–7. [Google Scholar] [CrossRef]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of Astaxanthin on Oxidative Stress in Overweight and Obese Adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Hashimoto, H.A.K.; Okamoto, Y.; Takahashi, J.; Chikuda, M.; Obara, Y. Effect of astaxanthin consumption on hydroper-oxides in the aqueous. Jpn. J. Clin. Ophthalmol. 2011, 65, 465–470. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.; Shin, W.G. Protective Effects of Haematococcus Astaxanthin on Oxidative Stress in Healthy Smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhao, P.; Li, B.; Zhang, J.; Huang, C. Antioxidant effects and impact on human health of astaxanthin. Chin. J. Food Hyg. 2011, 23, 313–316. [Google Scholar]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Iwabayashi, M.; Fujioka, N.; Nomoto, K.; Miyazaki, R.; Takahashi, H.; Hibino, S.; Takahashi, Y.; Nishikawa, K.; Nishida, M.; Yonei, Y. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti-Aging Med. 2009, 6, 15–21. [Google Scholar] [CrossRef]
- Yamada, T.; Ryo, K.; Tai, Y.; Tamaki, Y.; Inoue, H.; Mishima, K.; Tsubota, K.; Saito, I. Evaluation of Therapeutic Effects of Astaxanthin on Impairments in Salivary Secretion. J. Clin. Biochem. Nutr. 2010, 47, 130–137. [Google Scholar] [CrossRef][Green Version]
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Geraghty, D.P.; Sharman, J.E.; Coombes, J.S. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008, 9, 17. [Google Scholar] [CrossRef]
- Karppi, J.; Rissanen, T.H.; Nyyssönen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Chyun, J.H. The Effects of Astaxanthin Supplements on Lipid Peroxidation and Antioxidant Status in Postmeno-pausal Women. Nutr. Sci. 2004, 7, 41–46. [Google Scholar]
- Widomska, J.; Zareba, M.; Subczynski, W.K. Can Xanthophyll-Membrane Interactions Explain Their Selective Presence in the Retina and Brain? Foods 2016, 5, 7. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts defined: A report on the Keystone symposium on lipid rafts and cell function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef]
- Sakai, S.; Sugawara, T.; Matsubara, K.; Hirata, T. Inhibitory Effect of Carotenoids on the Degranulation of Mast Cells via Suppression of Antigen-induced Aggregation of High Affinity IgE Receptors. J. Biol. Chem. 2009, 284, 28172–28179. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Hirata, T.; Sugawara, T. Inhibitory Effect of Carotenoids on Ligand-induced Lipid Raft Translocation of Immunoreceptors. J. Oleo Sci. 2019, 68, 149–158. [Google Scholar] [CrossRef]
- Palozza, P.; Barone, E.; Mancuso, C.; Picci, N. The protective role of carotenoids against 7-keto-cholesterol formation in solution. Mol. Cell. Biochem. 2007, 309, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, M.; Nishida, Y.; Ishibashi, H.; Wada, T.; Fujisaka, S.; Takikawa, A.; Urakaze, M.; Sasaoka, T.; Usui, I.; Tobe, K. Impact of Divergent Effects of Astaxanthin on Insulin Signaling in L6 Cells. Endocrinology 2013, 154, 2600–2612. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Suzuki, Y.; Ohgami, K.; Shiratori, K.; Jin, X.-H.; Ilieva, I.; Koyama, Y.; Yazawa, K.; Yoshida, K.; Kase, S.; Ohno, S. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-κB signaling pathway. Exp. Eye Res. 2006, 82, 275–281. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2009, 49, 119–126. [Google Scholar] [CrossRef]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; De Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin Treatment Reduced Oxidative Induced Pro-Inflammatory Cytokines Secretion in U937: SHP-1 as a Novel Biological Target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef]
- Terazawa, S.; Nakajima, H.; Shingo, M.; Niwano, T.; Imokawa, G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion-independent manner. Exp. Dermatol. 2012, 21, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014, 23, 178–183. [Google Scholar] [CrossRef]
- Farruggia, C.; Yang, Y.; Kim, B.; Pham, T.; Bae, M.; Park, Y.-K.; Lee, J.-Y. Astaxanthin Plays Anti-inflammatory and Antioxidant Effects by Inhibiting NFkB Nuclear Translocation and NOX2 Expression in Macrophages. FASEB J. 2015, 29, 603–608. [Google Scholar] [CrossRef]
- Hara, K.; Hamada, C.; Wakabayashi, K.; Kanda, R.; Kaneko, K.; Horikoshi, S.; Tomino, Y.; Suzuki, Y. Scavenging of reactive oxygen species by astaxanthin inhibits epithelial–mesenchymal transition in high glucose-stimulated mesothelial cells. PLoS ONE 2017, 12, e0184332. [Google Scholar] [CrossRef]
- Li, D.; Tong, W.; Liu, D.; Zou, Y.; Zhang, C.; Xu, W. Astaxanthin mitigates cobalt cytotoxicity in the MG-63 cells by modulating the oxidative stress. BMC Pharmacol. Toxicol. 2017, 18, 58. [Google Scholar] [CrossRef]
- Sakai, S.; Nishida, A.; Ohno, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; Andoh, A. Astaxanthin, a xanthophyll carotenoid, prevents development of dextran sulphate sodium-induced murine colitis. J. Clin. Biochem. Nutr. 2019, 64, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Lim, J.W.; Kim, H. Astaxanthin Inhibits Interleukin-6 Expression in Cerulein/Resistin-Stimulated Pancreatic Acinar Cells. Mediat. Inflamm. 2021, 2021, 5587297. [Google Scholar] [CrossRef]
- Manabe, E.; Handa, O.; Naito, Y.; Mizushima, K.; Akagiri, S.; Adachi, S.; Takagi, T.; Kokura, S.; Maoka, T.; Yoshikawa, T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell. Biochem. 2007, 103, 1925–1937. [Google Scholar] [CrossRef]
- Kowshik, J.; Baba, A.B.; Giri, H.; Reddy, G.D.; Dixit, M.; Nagini, S. Astaxanthin Inhibits JAK/STAT-3 Signaling to Abrogate Cell Proliferation, Invasion and Angiogenesis in a Hamster Model of Oral Cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Mitochondrial Dysfunction Indirectly Elevates ROS Production by the Endoplasmic Reticulum. Cell Metab. 2013, 18, 145–146. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2015, 23, 303–314. [Google Scholar] [CrossRef]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–897. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miyaji, N.; Yang, M.; Mills, E.M.; Taniyama, S.; Uchida, T.; Nikawa, T.; Li, J.; Shi, J.; Tachibana, K.; et al. Astaxanthin Prevents Atrophy in Slow Muscle Fibers by Inhibiting Mitochondrial Reactive Oxygen Species via a Mitochondria-Mediated Apoptosis Pathway. Nutrients 2021, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Krestinin, R.; Baburina, Y.; Odinokova, I.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Krestinina, O. Isopro-terenol-Induced Permeability Transition Pore-Related Dysfunction of Heart Mitochondria Is Attenuated by Astaxanthin. Biomedicines 2020, 8, 437. [Google Scholar]
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Oxidative Stress-Induced Mitochondrial Dysfunction. Oxid. Med. Cell. Longev. 2020, 2020, 4629189. [Google Scholar] [CrossRef]
- Sztretye, M.; Singlár, Z.; Szabó, L.; Angyal, Á.; Balogh, N.; Vakilzadeh, F.; Szentesi, P.; Dienes, B.; Csernoch, L. Improved Tetanic Force and Mitochondrial Calcium Homeostasis by Astaxanthin Treatment in Mouse Skeletal Muscle. Antioxidants 2020, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Lobos, P.; Ponce, A.; Cataldo, K.; Meza, D.; Farías, P.; Estay, C.; Oyarzun-Ampuero, F.; Herrera-Molina, R.; Paula-Lima, A.; et al. Astaxanthin Counteracts Excitotoxicity and Reduces the Ensuing Increases in Calcium Levels and Mitochondrial Reactive Oxygen Species Generation. Mar. Drugs 2020, 18, 335. [Google Scholar] [CrossRef] [PubMed]
- Altunrende, M.E.; Gezen-Ak, D.; Atasoy, I.L.; Candas, E.; Dursun, E. The Role of Astaxanthin on Transcriptional Regulation of NMDA Receptors Voltage Sensitive Calcium Channels and Calcium Binding Proteins in Primary Cortical Neurons. Arch. Neuropsychiatry 2018, 55, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Lu, C.W.; Wang, S.J. Astaxanthin Inhibits Glutamate Release in Rat Cerebral Cortex Nerve Terminals via Suppression of Voltage-Dependent Ca2+ Entry and Mitogen-Activated Protein Kinase Signaling Pathway. J. Agric. Food Chem. 2010, 58, 8271–8278. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, Y.; Li, S. Astaxanthin attenuates glutamate-induced apoptosis via inhibition of calcium influx and endoplasmic reticulum stress. Eur. J. Pharmacol. 2017, 806, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.H.; Aoi, W.; Takami, M.; Terajima, H.; Tanimura, Y.; Naito, Y.; Itoh, Y.; Yoshikawa, T. The astaxanthin-induced improvement in lipid metabolism during exercise is mediated by a PGC-1α increase in skeletal muscle. J. Clin. Biochem. Nutr. 2014, 54, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin ameliorates features of metabolic syndrome in SHR/NDmcr-cp. Life Sci. 2007, 80, 522–529. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of Astaxanthin Supplementation on Exercise-Induced Fatigue in Mice. Biol. Pharm. Bull. 2006, 29, 2106–2110. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachex Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Uchiyama, K.; Mizushima, K.; Kuroda, M.; Akagiri, S.; Takagi, T.; Handa, O.; Kokura, S.; Yoshida, N.; Ichikawa, H.; et al. Microarray profiling of gene expression patterns in glomerular cells of astaxanthin-treated diabetic mice: A nutrigenomic approach. Int. J. Mol. Med. 2006, 18, 685–695. [Google Scholar] [CrossRef]
- Miotto, P.M.; LeBlanc, P.J.; Holloway, G.P. High-Fat Diet Causes Mitochondrial Dysfunction as a Result of Impaired ADP Sensitivity. Diabetes 2018, 67, 2199–2205. [Google Scholar] [CrossRef]
- Shi, S.Y.; Lu, S.-Y.; Sivasubramaniyam, T.; Revelo, X.; Cai, E.P.; Luk, C.T.; Schroer, S.A.; Patel, P.; Kim, R.; Bombardier, E.; et al. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 2015, 6, 7415. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar]
- Lian, F.; Wang, X. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer 2008, 123, 1262–1268. [Google Scholar] [CrossRef]
- Bhattarai, G.; So, H.-S.; Kieu, T.T.T.; Kook, S.-H.; Lee, J.-C.; Jeon, Y.-M. Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System. Nutrients 2021, 13, 3575. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Chu, C.; Liu, S. Astaxanthin protects retinal ganglion cells from acute glaucoma via the Nrf2/HO-1 pathway. J. Chem. Neuroanat. 2020, 110, 101876. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Dergunov, S.; Zhang, W.; Gentleman, S.; Redmond, T.; Pinkhassik, E.; Poliakov, E. Xanthophylls Modulate Palmitoylation of Mammalian β-Carotene Oxygenase 2. Antioxidants 2021, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, L.; Lyu, Y.; Chowanadisai, W.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; He, H.; Wang, W.; Medeiros, D.M.; et al. Ablation of β,β-carotene-9′,10′-oxygenase 2 remodels the hypothalamic metabolome leading to metabolic disorders in mice. J. Nutr. Biochem. 2017, 46, 74–82. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Wong, S.Y.; Lu, P.; Hartson, S.D.; Medeiros, D.M.; Wang, W.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; et al. Deficiency of β-carotene oxygenase 2 induces mitochondrial fragmentation and activates the STING-IRF3 pathway in the mouse hypothalamus. J. Nutr. Biochem. 2020, 88, 108542. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lu, P.; Guo, X.; Song, K.; Lyu, Y.; Bothwell, J.; Wu, J.; Hawkins, O.; Clarke, S.L.; Lucas, E.A.; et al. β-carotene oxygenase 2 deficiency-triggered mitochondrial oxidative stress promotes low-grade inflammation and metabolic dysfunction. Free. Radic. Biol. Med. 2021, 164, 271–284. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef]
- DeFronzo, R.A. The Triumvirate: -Cell, Muscle, Liver: A Collusion Responsible for NIDDM. Diabetes 1988, 37, 667–687. [Google Scholar] [CrossRef]
- Yoshihara, T.; Sugiura, T.; Shibaguchi, T.; Naito, H. Role of astaxanthin supplementation in prevention of disuse muscle atrophy: A review. J. Phys. Fit. Sports Med. 2019, 8, 61–71. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Narkar, V.A.; Fan, W.; Downes, M.; Yu, R.T.; Jonker, J.W.; Alaynick, W.A.; Banayo, E.; Karunasiri, M.S.; Lorca, S.; Evans, R.M. Exercise and PGC-1α-Independent Synchronization of Type I Muscle Metabolism and Vasculature by ERRγ. Cell Metab. 2011, 13, 283–293. [Google Scholar] [CrossRef]
- Cho, Y.; Hazen, B.C.; Russell, A.; Kralli, A. Peroxisome Proliferator-activated Receptor γ Coactivator 1 (PGC-1)- and Estrogen-related Receptor (ERR)-induced Regulator in Muscle 1 (PERM1) Is a Tissue-specific Regulator of Oxidative Capacity in Skeletal Muscle Cells. J. Biol. Chem. 2013, 288, 25207–25218. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Eichner, L.J.; Shaw, R.J.; Auwerx, J. Transcriptional Coregulators: Fine-Tuning Metabolism. Cell Metab. 2014, 20, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Wei, Y.-H. Roles of Mitochondrial Sirtuins in Mitochondrial Function, Redox Homeostasis, Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 5266. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.-N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-I. Astaxanthin as a Peroxisome Proliferator-Activated Receptor (PPAR) Modulator: Its Therapeutic Implications. Mar. Drugs 2019, 17, 242. [Google Scholar] [CrossRef]
- Inoue, M.; Tanabe, H.; Matsumoto, A.; Takagi, M.; Umegaki, K.; Amagaya, S.; Takahashi, J. Astaxanthin functions differently as a selective peroxisome proliferator-activated receptor γ modulator in adipocytes and macrophages. Biochem. Pharmacol. 2012, 84, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Ayaori, M.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Sasaki, M.; Komatsu, T.; Yogo, M.; et al. Astaxanthin Enhances ATP-Binding Cassette Transporter A1/G1 Expressions and Cholesterol Efflux from Macrophages. J. Nutr. Sci. Vitaminol. 2012, 58, 96–104. [Google Scholar] [CrossRef]
- Harrison, E.H.; Quadro, L. Apocarotenoids: Emerging Roles in Mammals. Annu. Rev. Nutr. 2018, 38, 153–172. [Google Scholar] [CrossRef]
- Teicher, V.B.; Kucharski, N.; Martin, H.-D.; van der Saag, P.; Sies, H.; Stahl, W. Biological Activities of Apo-canthaxanthinoic Acids Related to Gap Junctional Communication. Arch. Biochem. Biophys. 1999, 365, 150–155. [Google Scholar] [CrossRef]
- Hix, L.M.; Frey, D.A.; McLaws, M.D.; Østerlie, M.; Lockwood, S.F.; Bertram, J.S. Inhibition of chemically-induced neoplastic transformation by a novel tetrasodium diphosphate astaxanthin derivative. Carcinogenesis 2005, 26, 1634–1641. [Google Scholar] [CrossRef]
- Hix, L.M.; Lockwood, S.F.; Bertram, J.S. Upregulation of connexin 43 protein expression and increased gap junctional communication by water soluble disodium disuccinate astaxanthin derivatives. Cancer Lett. 2004, 211, 25–37. [Google Scholar] [CrossRef]
- Daubrawa, F.; Sies, H.; Stahl, W. Astaxanthin Diminishes Gap Junctional Intercellular Communication in Primary Human Fibroblasts. J. Nutr. 2005, 135, 2507–2511. [Google Scholar] [CrossRef]
- Kistler, A.; Liechti, H.; Pichard, L.; Wolz, E.; Oesterhelt, G.; Hayes, A.; Maurel, P. Metabolism and CYP-inducer properties of astaxanthin in man and primary human hepatocytes. Arch. Toxicol. 2001, 75, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T. A Marine Carotenoid, Fucoxanthin, Induces Regulatory T Cells and Inhibits Th17 Cell Differentiationin Vitro. Biosci. Biotechnol. Biochem. 2011, 75, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2010, 105, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2015, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Canto, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef]
- Kanazashi, M.; Tanaka, M.; Murakami, S.; Kondo, H.; Nagatomo, F.; Ishihara, A.; Roy, R.R.; Fujino, H. Amelioration of capillary regression and atrophy of the soleus muscle in hindlimb-unloaded rats by astaxanthin supplementation and intermittent loading. Exp. Physiol. 2014, 99, 1065–1077. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Wang, Y.; Wang, M.; Li, H.; Lin, S.; Mao, C.; Wang, B.; Song, X.; Lv, C. Astaxanthin prevents pulmonary fibrosis by promoting myofibroblast apoptosis dependent on Drp1-mediated mitochondrial fission. J. Cell. Mol. Med. 2015, 19, 2215–2231. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Moore, T.; Zhou, Z.; Cohn, W.; Norheim, F.; Lin, A.J.; Kalajian, N.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol. Metab. 2018, 21, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, T.; Deuster, P. Astaxanthin Protects Against Heat-induced Mitochondrial Alterations in Mouse Hypothalamus. Neuroscience 2021, 476, 12–20. [Google Scholar] [CrossRef]
- Lee, H.; Lim, J.W.; Kim, H. Effect of Astaxanthin on Activation of Autophagy and Inhibition of Apoptosis in Helicobacter pylori-Infected Gastric Epithelial Cell Line AGS. Nutrients 2020, 12, 1750. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yan, W.-Y.; Lei, Y.-H.; Wan, Z.; Hou, Y.-Y.; Sun, L.-K.; Zhou, J.-P. SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 313. [Google Scholar] [CrossRef]
- Hu, F.B.; Sigal, R.J.; Rich-Edwards, J.W.; Colditz, G.; Solomon, C.G.; Willett, W.C.; Speizer, F.E.; Manson, J.E. Walking Compared with Vigorous Physical Activity and Risk of Type 2 Diabetes in Women. JAMA 1999, 282, 1433–1439. [Google Scholar] [CrossRef]
- Helmrich, S.P.; Ragland, D.; Leung, R.W.; Paffenbarger, R.S. Physical Activity and Reduced Occurrence of Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1991, 325, 147–152. [Google Scholar] [CrossRef]
- Okada, K.; Furusyo, N.; Sawayama, Y.; Kanamoto, Y.; Murata, M.; Hayashi, J. Prevalence and risk factors for diabetes: A ten year follow-up study of the Yaeyama district of Okinawa. Fukuoka Igaku Zasshi 2010, 101, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of Diet and Exercise in Preventing NIDDM in People with Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Auciello, F.R.; Ross, F.A.; Ikematsu, N.; Hardie, D.G. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Lett. 2014, 588, 3361–3366. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.-P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St.-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Hwang, A.B.; Ryu, E.-A.; Artan, M.; Chang, H.-W.; Kabir, M.H.; Nam, H.-J.; Lee, D.; Yang, J.-S.; Kim, S.; Mair, W.B.; et al. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2014, 111, E4458–E4467. [Google Scholar] [CrossRef]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef] [PubMed]
- Misu, H.; Takayama, H.; Saito, Y.; Mita, Y.; Kikuchi, A.; Ishii, K.-A.; Chikamoto, K.; Kanamori, T.; Tajima, N.; Lan, F.; et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017, 23, 508–516. [Google Scholar] [CrossRef]
- Joseph, B.K.; Liu, H.-Y.; Francisco, J.; Pandya, D.; Donigan, M.; Gallo-Ebert, C.; Giordano, C.; Bata, A.; Nickels, J.T., Jr. Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J. Biol. Chem. 2015, 290, 10588–10598. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, M.N.Y.; Ishibashi, H.; Koshimizu, Y.; Fujisala, S.; Usui, I.; Tobe, K. Astaxanthin, a Strong Antioxidant, Possesses Various Effects on Insulin Signaling In Vitro. In Proceedings of the ADA, 71st Scientific Sessions (2011), San Diego, CA, USA; 2011. [Google Scholar]
- Okamoto, S.; Asgar, N.F.; Yokota, S.; Saito, K.; Minokoshi, Y. Role of the α2 subunit of AMP-activated protein kinase and its nuclear localization in mitochondria and energy metabolism-related gene expressions in C2C12 cells. Metabolism 2018, 90, 52–68. [Google Scholar] [CrossRef]
- Ost, M.; Werner, F.; Dokas, J.; Klaus, S.; Voigt, A. Activation of AMPKα2 Is Not Crucial for Mitochondrial Uncoupling-Induced Metabolic Effects but Required to Maintain Skeletal Muscle Integrity. PLoS ONE 2014, 9, e94689. [Google Scholar] [CrossRef]
- Hu, X.; Xu, X.; Lu, Z.; Zhang, P.; Fassett, J.; Xin, Y.; Hall, J.L.; Viollet, B.; Bache, R.J.; Huang, Y.; et al. AMP Activated Protein Kinase- 2 Regulates Expression of Estrogen-Related Receptor-α, a Metabolic Transcription Factor Related to Heart Failure Development. Hypertension 2011, 58, 696–703. [Google Scholar] [CrossRef]
- Huss, J.M.; Garbacz, W.G.; Xie, W. Constitutive activities of estrogen-related receptors: Transcriptional regulation of metabolism by the ERR pathways in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1912–1927. [Google Scholar] [CrossRef]
- LaBarge, S.; McDonald, M.; Smith-Powell, L.; Auwerx, J.; Huss, J.M. Estrogen-related receptor-α (ERRα) deficiency in skeletal muscle impairs regeneration in response to injury. FASEB J. 2013, 28, 1082–1097. [Google Scholar] [CrossRef]
- Matsakas, A.; Yadav, V.; Lorca, S.; Evans, R.M.; Narkar, V.A. Revascularization of Ischemic Skeletal Muscle by Estrogen-Related Receptor-γ. Circ. Res. 2012, 110, 1087–1096. [Google Scholar] [CrossRef]
- Brandauer, J.; Vienberg, S.G.; Andersen, M.A.; Ringholm, S.; Risis, S.; Larsen, P.S.; Kristensen, J.M.; Frøsig, C.; Leick, L.; Fentz, J.; et al. AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle. J. Physiol. 2013, 591, 5207–5220. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Uchiyama, A. Clinical efficacy of astaxanthin-containing Haematococcus pluvialis extract for the volunteers at risk of metabolic syndrome. J. Clin. Biochem. Nutr. 2008, 43, 38–43. [Google Scholar]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin im-proves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T.; Kadowaki, T. Adiponectin/AdipoR Research and Its Implications for Lifestyle-Related Diseases. Front. Cardiovasc. Med. 2019, 6, 116. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kawaguchi, Y.; Fujimoto, T.; Kanayama, N.; Magari, M.; Tokumitsu, H. Differential AMP-activated Protein Kinase (AMPK) Recognition Mechanism of Ca2+/Calmodulin-dependent Protein Kinase Kinase Isoforms. J. Biol. Chem. 2016, 291, 13802–13808. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Kauppila, T.E.S.; Kauppila, J.H.K.; Larsson, N.-G. Mammalian Mitochondria and Aging: An Update. Cell Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Creo, A.L.; Cortes, T.M.; Dasari, S.; Nair, K.S. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J. Clin. Investig. 2018, 128, 3671–3681. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D. Aging and Insulin Resistance: Just Say iNOS. Diabetes 2013, 62, 346–348. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.-I. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Ropelle, E.R.; Pauli, J.R.; Cintra, D.E.; da Silva, A.S.; De Souza, C.T.; Guadagnini, D.; Carvalho, B.M.; Caricilli, A.M.; Katashima, C.K.; Carvalho-Filho, M.A.; et al. Targeted Disruption of Inducible Nitric Oxide Synthase Protects Against Aging, S-Nitrosation, and Insulin Resistance in Muscle of Male Mice. Diabetes 2012, 62, 466–470. [Google Scholar] [CrossRef]
- Oh, Y.S.; Seo, E.-H.; Lee, Y.-S.; Cho, S.C.; Jung, H.S.; Park, S.C.; Jun, H.-S. Increase of Calcium Sensing Receptor Expression Is Related to Compensatory Insulin Secretion during Aging in Mice. PLoS ONE 2016, 11, e0159689. [Google Scholar] [CrossRef] [PubMed]
- Templeman, N.M.; Flibotte, S.; Chik, J.H.; Sinha, S.; Lim, G.; Foster, L.J.; Nislow, C.; Johnson, J.D. Reduced Circulating Insulin Enhances Insulin Sensitivity in Old Mice and Extends Lifespan. Cell Rep. 2017, 20, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.H.; Dalton, A.; Calzini, L.; Tuluca, A.; Hoyte, D.; Ives, S.J. The impact of age and sex on body composition and glucose sensitivity in C57BL/6J mice. Physiol. Rep. 2019, 7, e13995. [Google Scholar] [CrossRef] [PubMed]
- Laboratory, T.J. White Paper/Eight Considerations for Designing Studies with Aged Mice; The Jackson Laboratory: Bar Harbor, ME, USA, 2020; Volume WP009, Available online: https://resources.jax.org/white-papers/whitepaper-aged-b6-study-considerations (accessed on 21 November 2021).
- Siersbæk, M.S.; Ditzel, N.; Hejbøl, E.K.; Præstholm, S.M.; Markussen, L.K.; Avolio, F.; Li, L.; Lehtonen, L.; Hansen, A.K.; Schrøder, H.D.; et al. C57BL/6J substrain differences in response to high-fat diet intervention. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Flurkey, K.; McUrrer, J.; Harrison, D. Mouse Models in Aging Research. In The Mouse in Biomedical Research; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; Academic Press: Burlington, UK, 2007; pp. 637–672. [Google Scholar]
- Chow, H.-M.; Shi, M.; Cheng, A.; Gao, Y.; Chen, G.; Song, X.; So, R.W.L.; Zhang, J.; Herrup, K. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat. Neurosci. 2019, 22, 1806–1819. [Google Scholar] [CrossRef]
- Sekikawa, T.; Kizawa, Y.; Li, Y.; Takara, T. Cognitive function improvement with astaxanthin and tocotrienol intake: A randomized, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2020, 67, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of Composite Supplement Containing Astaxanthin and Sesamin on Cognitive Functions in People with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. 2018, 62, 1767–1775. [Google Scholar] [CrossRef]
- Huang, C.; Wen, C.; Yang, M.; Li, A.; Fan, C.; Gan, D.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Astaxanthin Improved the Cognitive Deficits in APP/PS1 Transgenic Mice Via Selective Activation of mTOR. J. Neuroimmune Pharmacol. 2020, 16, 609–619. [Google Scholar] [CrossRef]
- Hongo, N.; Takamura, Y.; Nishimaru, H.; Matsumoto, J.; Tobe, K.; Saito, T.; Saido, T.C.; Nishijo, H. Astaxanthin Ameliorated Parvalbumin-Positive Neuron Deficits and Alzheimer’s Disease-Related Pathological Progression in the Hippocampus of AppNL-G-F/NL-G-F Mice. Front. Pharmacol. 2020, 11, 307. [Google Scholar] [CrossRef]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomized, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.O.; Panda, B.P.; Parvez, S.; Kaundal, M.; Hussain, S.; Akhtar, M.; Najmi, A.K. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer’s disease. Biomed. Pharmacother. 2018, 110, 47–58. [Google Scholar] [CrossRef]
- Liu, S.Z.; Valencia, A.P.; VanDoren, M.P.; Shankland, E.G.; Roshanravan, B.; Conley, K.E.; Marcinek, D.J. Astaxanthin supplementation enhances metabolic adaptation with aerobic training in the elderly. Physiol. Rep. 2021, 9, e14887. [Google Scholar] [CrossRef]
- Liu, S.Z.; Ali, A.S.; Campbell, M.D.; Kilroy, K.; Shankland, E.G.; Roshanravan, B.; Marcinek, D.J.; Conley, K.E. Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. J. Cachexia Sarcopenia Muscle 2018, 9, 826–833. [Google Scholar] [CrossRef]
- Fujino, H.; Kondo, H.; Kanazashi, M.; Nakanishi, R.; Tanaka, M.; Ishihara, A. Dietary Astaxanthin Supplementation Improves Walking Performance and Blood Lactate Level After Walking Test in Community-dwelling Elderly Subjects: 453 Board #290 June 1, 9:30 AM—11:00 AM. Med. Sci. Sports Exerc. 2016, 48, 129. [Google Scholar]
- Vial, G.; Chauvin, M.-A.; Bendridi, N.; Durand, A.; Meugnier, E.; Madec, A.-M.; Bernoud-Hubac, N.; de Barros, J.-P.P.; Fontaine, É.; Acquaviva, C.; et al. Imeglimin Normalizes Glucose Tolerance and Insulin Sensitivity and Improves Mitochondrial Function in Liver of a High-Fat, High-Sucrose Diet Mice Model. Diabetes 2014, 64, 2254–2264. [Google Scholar] [CrossRef]
- Brown, D.R.; Warner, A.R.; Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. The effect of astaxanthin supplementation on performance and fat oxidation during a 40 km cycling time trial. J. Sci. Med. Sport 2020, 24, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.H.; Hantla, D.; Capelli, B.; Ding, L.; Li, Y.; Artaria, C. Effect of Astaxanthin Supplementation on Cardiorespiratory Function in Runners. EC Nutr. 2016, 11, 253–259. [Google Scholar] [CrossRef]
- Klinkenberg, L.J.J.; Res, P.T.; Haenen, G.; Bast, A.; van Loon, L.J.; Van Dieijen-Visser, M.P.; Meex, S.J. Effect of Antioxidant Supplementation on Exercise-Induced Cardiac Troponin Release in Cyclists: A Randomized Trial. PLoS ONE 2013, 8, e79280. [Google Scholar] [CrossRef] [PubMed]
- Res, P.T.; Cermak, N.M.; Stinkens, R.; Tollakson, T.J.; Haenen, G.; Bast, A.; van Loon, L.J. Astaxanthin Supplementation Does Not Augment Fat Use or Improve Endurance Performance. Med. Sci. Sports Exerc. 2013, 45, 1158–1165. [Google Scholar] [CrossRef]
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012, 52. [Google Scholar]
- Earnest, C.; Lupo, M.; White, K.; Church, T. Effect of Astaxanthin on Cycling Time Trial Performance. Int. J. Sports Med. 2011, 32, 882–888. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Fry, A.; Schilling, B.; Chiu, L.; Hori, N.; Weiss, L. Astaxanthin Supplementation Does Not Attenuate Muscle Injury Following Eccentric Exercise in Resistance-Trained Men. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 401–412. [Google Scholar] [CrossRef]
- Sawaki, K.; Yoshigi, H.; Aoki, K.; Koikawa, N.; Azumane, A.; Kaneko, K.; Yamaguchi, M. Sports Performance Benefits from Taking Natural Astaxanthin Characterized by Visual Acuity and Muscle Fatigue Improvement in Humans. J. Clin. Ther. Med. 2002, 18, 1085–1100. [Google Scholar]
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Kuwahata, M.; Higashi, A. Astaxanthin-, β-Carotene-, and Resveratrol-Rich Foods Support Resistance Training-Induced Adaptation. Antioxidants 2021, 10, 113. [Google Scholar] [CrossRef]
- Fleischmann, C.; Horowitz, M.; Yanovich, R.; Raz, H.; Heled, Y. Asthaxanthin Improves Aerobic Exercise Recovery Without Affecting Heat Tolerance in Humans. Front. Sports Act. Living 2019, 1, 17. [Google Scholar] [CrossRef]
- Takami, M.; Aoi, W.; Terajima, H.; Tanimura, Y.; Wada, S.; Higashi, A. Effect of dietary antioxidant-rich foods combined with aerobic training on energy metabolism in healthy young men. J. Clin. Biochem. Nutr. 2019, 64, 79–85. [Google Scholar] [CrossRef]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Hongo, N. Daily Fatigue—reducing Effect of Astaxanthin―A Randomized, Placebo—controlled, Double—blind, Par-allel—group Study. Jpn. Pharmacol. Ther. 2017, 45, 61–72. [Google Scholar]
- Malmstena, C.L.; Lignell, A. Dietary Supplementation with Astaxanthin-Rich Algal Meal Improves Strength Endurance—A Double Blind Placebo Controlled Study on Male Students. Carotenoid Sci. 2008, 13, 20–22. [Google Scholar]
- Tajima, T.; Nagata, A. Effects of astaxanthin ingestion on exercise-induced physiological changes. Health Behav. Sci. 2004, 3, 5–10. [Google Scholar] [CrossRef]
- Shokri-Mashhadi, N.; Tahmasebi, M.; Mohammadi-Asl, J.; Zakerkish, M.; Mohammadshahi, M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14022. [Google Scholar] [CrossRef]
- Kato, T.; Kasai, T.; Sato, A.; Ishiwata, S.; Yatsu, S.; Matsumoto, H.; Shitara, J.; Murata, A.; Shimizu, M.; Suda, S.; et al. Effects of 3-Month Astaxanthin Supplementation on Cardiac Function in Heart Failure Patients with Left Ventricular Systolic Dysfunction—A Pilot Study. Nutrients 2020, 12, 1896. [Google Scholar] [CrossRef]
- Chan, K.-C.; Chen, S.-C.; Chen, P.-C. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J. Funct. Foods 2018, 53, 22–27. [Google Scholar] [CrossRef]
- Canas, J.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Yamaga, M.; Furuichi, Y.; Yokote, K. Astaxanthin Improves Nonalcoholic Fatty Liver Disease in Werner Syndrome with Diabetes Mellitus. J. Am. Geriatr. Soc. 2015, 63, 1271–1273. [Google Scholar] [CrossRef]
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of a New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Fukamauchi, M. Food functionality of astaxanthin-10: Synergistic effects of astaxanthin intake and aerobic exercise. Food Style 2007, 11, 22–24. [Google Scholar]
- Baburina, Y.; Krestinin, R.; Odinokova, I.; Sotnikova, L.; Kruglov, A.; Krestinina, O. Astaxanthin Inhibits Mitochondrial Permeability Transition Pore Opening in Rat Heart Mitochondria. Antioxidants 2019, 8, 576. [Google Scholar] [CrossRef]
- Scialo’, F.; Sriram, A.; Fernández-Ayala, D.J.M.; Gubina, N.; Lõhmus, M.; Nelson, G.; Logan, A.; Cooper, H.M.; Navas, P.; Enríquez, J.A.; et al. Mitochondrial ROS Produced via Reverse Electron Transport Extend Animal Lifespan. Cell Metab. 2016, 23, 725–734. [Google Scholar] [CrossRef]
- Dogan, S.A.; Cerutti, R.; Benincá, C.; Calvo, G.T.B.; Jacobs, H.T.; Zeviani, M.; Szibor, M.; Viscomi, C. Perturbed Redox Signaling Exacerbates a Mitochondrial Myopathy. Cell Metab. 2018, 28, 764–775.e5. [Google Scholar] [CrossRef]
- Fontaine, E. Metformin and respiratory chain complex I: The last piece of the puzzle? Biochem. J. 2014, 463, e3–e5. [Google Scholar] [CrossRef] [PubMed]
- Bridges, H.R.; Jones, A.J.Y.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014, 462, 475–487. [Google Scholar] [CrossRef]
- García-Ruiz, I.; Solís-Muñoz, P.; Fernandez-Moreira, D.; Muñoz-Yagüe, T.; Solis-Herruzo, J.A. Pioglitazone leads to an inactivation and disassembly of complex I of the mitochondrial respiratory chain. BMC Biol. 2013, 11, 88. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Wiley, S.; Rogers, G.W.; Andreyev, A.Y.; Petrosyan, S.; Loviscach, M.; Wall, E.A.; Yadava, N.; Heuck, A.; Ferrick, D.A.; et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl. Acad. Sci. USA 2013, 110, 5422–5427. [Google Scholar] [CrossRef]
- Sanz, M.-N.; Sánchez-Martín, C.; Detaille, D.; Vial, G.; Rigoulet, M.; El-Mir, M.-Y.; Rodríguez-Villanueva, G. Acute Mitochondrial Actions of Glitazones on the Liver: A Crucial Parameter for their Antidiabetic Properties. Cell. Physiol. Biochem. 2011, 28, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Konrad, D.; Rudich, A.; Bilan, P.J.; Patel, N.; Richardson, C.; Witters, L.A.; Klip, A. Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 2005, 48, 954–966. [Google Scholar] [CrossRef]
- Schaar, C.E.; Dues, D.J.; Spielbauer, K.K.; Machiela, E.; Cooper, J.F.; Senchuk, M.; Hekimi, S.; Van Raamsdonk, J.M. Mitochondrial and Cytoplasmic ROS Have Opposing Effects on Lifespan. PLoS Genet. 2015, 11, e1004972. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.; Yang, W.; Hekimi, S. The Intrinsic Apoptosis Pathway Mediates the Pro-Longevity Response to Mitochondrial ROS in C. elegans. Cell 2014, 157, 897–909. [Google Scholar] [CrossRef]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef]
- Yazaki, K.; Yoshikoshi, C.; Oshiro, S.; Yanase, S. Supplemental Cellular Protection by a Carotenoid Extends Lifespan via Ins/IGF-1 Signaling inCaenorhabditis elegans. Oxid. Med. Cell. Longev. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Sergi, C.; Shen, F.; Liu, S.-M. Insulin/IGF-1R, SIRT1, and FOXOs Pathways—An Intriguing Interaction Platform for Bone and Osteosarcoma. Front. Endocrinol. 2019, 10, 93. [Google Scholar] [CrossRef]
- Amengual, J.; Lobo, G.P.; Golczak, M.; Li, H.N.M.; Klimova, T.; Hoppel, C.L.; Wyss, A.; Palczewski, K.; von Lintig, J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2010, 25, 948–959. [Google Scholar] [CrossRef]
- Lobo, G.P.; Isken, A.; Hoff, S.; Babino, D.; von Lintig, J. BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 2012, 139, 2966–2977. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vachali, P.P.; Gorusupudi, A.; Shen, Z.; Sharifzadeh, H.; Besch, B.M.; Nelson, K.; Horvath, M.M.; Frederick, J.M.; Baehr, W.; et al. Inactivity of human, -carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc. Natl. Acad. Sci. USA 2014, 111, 10173–10178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H. Multiple Mechanisms of Anti-Cancer Effects Exerted by Astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Thomas-Ahner, J.; Moran, N.E.; Cooperstone, J.L.; Erdman, J.W.; Young, G.S.; Clinton, S.K. β-Carotene 9′,10′ Oxygenase Modulates the Anticancer Activity of Dietary Tomato or Lycopene on Prostate Carcinogenesis in the TRAMP Model. Cancer Prev. Res. 2016, 10, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Moran, N.E.; Cichon, M.J.; Riedl, K.M.; Schwartz, S.J.; Erdman, J.W.; Pearl, D.K.; Thomas-Ahner, J.M.; Clinton, S.K.; Young, G.S.; et al. β-Carotene-9’,10’-oxygenase status modulates the impact of dietary tomato and lycopene on hepatic nuclear receptor-, stress-, and metabolism-related gene expression in mice. J. Nutr. 2014, 144, 431–439. [Google Scholar] [CrossRef]
- Ip, B.C.; Liu, C.; Ausman, L.M.; von Lintig, J.; Wang, X.-D. Lycopene Attenuated Hepatic Tumorigenesis via Differential Mechanisms Depending on Carotenoid Cleavage Enzyme in Mice. Cancer Prev. Res. 2014, 7, 1219–1227. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Hartson, S.D.; Davis, M.A.; He, H.; Medeiros, D.M.; Wang, W.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; et al. Lack of β, β-carotene-9′, 10′-oxygenase 2 leads to hepatic mitochondrial dysfunction and cellular oxidative stress in mice. Mol. Nutr. Food Res. 2016, 61, 1600576. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Marisiddaiah, R.; Zaripheh, S.; Wiener, D.; Rubin, L.P. Mitochondrial β-Carotene 9′,10′ Oxygenase Modulates Prostate Cancer Growth via NF-κB Inhibition: A Lycopene-Independent Function. Mol. Cancer Res. 2016, 14, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Dohl, J.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Astaxanthin but not quercetin preserves mitochondrial integrity and function, ameliorates oxidative stress, and reduces heat-induced skeletal muscle injury. J. Cell. Physiol. 2019, 234, 13292–13302. [Google Scholar] [CrossRef] [PubMed]
- Koshinaka, K.; Honda, A.; Masuda, H.; Sato, A. Effect of Quercetin Treatment on Mitochondrial Biogenesis and Exercise-Induced AMP-Activated Protein Kinase Activation in Rat Skeletal Muscle. Nutrients 2020, 12, 729. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, W.; Zhao, B.; Xie, J.; Sun, Q.; Shi, X.; Yan, B.; Tian, G.; Liang, X. Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway in vivo and in vitro. Front. Neurosci. 2021, 15, 636172. [Google Scholar] [CrossRef]
- Rayamajhi, N.; Kim, S.-K.; Go, H.; Joe, Y.; Callaway, Z.; Kang, J.-G.; Ryter, S.W.; Chung, H.T. Quercetin Induces Mitochondrial Biogenesis through Activation of HO-1 in HepG2 Cells. Oxid. Med. Cell. Longev. 2013, 2013, 154279. [Google Scholar] [CrossRef]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of Astaxanthin inHaematococcusAlgal Extract: The Effects of Timing of Diet and Smoking Habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.; Ytrestøyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef]
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]


| Author/Year/Reference | Study Design | Subjects | Dose | Duration | Outcome |
|---|---|---|---|---|---|
| McAllister M.J. et al., 2021 [32] | Randomized, double-blind, placebo-controlled, crossover study | 14 healthy subjects | 0, 6 mg/day | 4 weeks | Glutathione was ∼7% higher following AX compared with placebo (p < 0.05). No effect on plasma hydrogen peroxide or malondialdehyde (MDA; p > 0.05). Advanced oxidation protein products (AOPP) reduced by ∼28% (N.S.; p = 0.45). |
| Petyaev I.M., et al., 2018 [33] | Randomized, blinded, four-arm, prospective study | 32 subjects with oxidative stress, 8 subjects taking AX only | 0, 7 mg/day * | 4 weeks | Reduced serum oxidized LDL by 55.4% after 4 weeks (p < 0.05). Reduced MDA by 52.7% after 4 weeks (p < 0.05). |
| Chalyk, N. et al., 2017 [34] | Open-label, prospective study | 31 subjects; 18 obese, 8 overweight, 5 healthy weight | 4 mg/day | 92 days | Plasma MDA decreased with AX by 11.2% on day 15 and by 21.7% on day 29 (N.S.) |
| Hashimoto H. et al., 2016 [35] | Open-label, prospective study | 35 subjects during cataract surgery | 6 mg/day | 2 weeks | Superoxide anion scavenging activity (U/mL) 18.2 ± 4.1 at 0 weeks reduced to 19.9 ± 3.6 after 2 weeks of supplementation compared with baseline, p < 0.05. Total hydroperoxides (U CARR) from 1.16 ± 0.18 at 0 weeks reduced to 1.04 ± 0.31 after 2 weeks of supplementation compared with baseline, p < 0.05 |
| Baralic, I. et al., 2015 [36] | Randomized, double-blind, placebo-controlled, prospective study | 40 healthy subjects (soccer players) | 0, 4 mg/day | 90 days | Improved prooxidant-antioxidant balance (PAB; p < 0.05) |
| Baralic I. et al., 2013 [37] | Randomized, double-blind, prospective study | 40 healthy subjects (soccer players) | 0, 4 mg/day | 90 days | Protected thiol groups against oxidative modification (increase in -SH groups, p < 0.05; improved PON1 activity towards paraoxon and diazoxon, p < 0.05 and p < 0.01, respectively) |
| Hashimoto, H. et al., 2013 [38] | Open-label, prospective study | 35 cataract patients | 6 mg/day | 2 weeks | Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05), increased superoxide scavenging activity (p< 0.05) |
| Choi H.D. et al., 2011 [39] | Randomized, two-arm, prospective study | 23 obese and overweight subjects | 5 and 20 mg/day | 3 weeks | 5 mg/day: MDA decreased by 34.6%, isoprostane (ISP) decreased by 64.9%, superoxide dismutase (SOD) increased by 193%, and total antioxidant capacity (TAC) increased by 121% after 3 weeks compared with baseline (p < 0.01). 20 mg/day: MDA decreased by 35.2%, ISP decreased by 64.7%, SOD increased by 194%, and TAC increased by 125% after 3 weeks compared with baseline (p < 0.01). |
| Choi, H.D. et al., 2011 [40] | Randomized, double-blind, placebo-controlled, prospective study | 27 overweight subjects | 0, 20 mg/day | 12 weeks | MDA reduced by 17.3% and 29% after 8 and 12 weeks compared with placebo (p < 0.01), isoprostane (ISP) reduced by 40.2% and 52.9% after 8 and 12 weeks compared with placebo (p < 0.01), superoxide dismutase (SOD) increased by 124.8% after 12 weeks compared with placebo (p < 0.01), and total antioxidant capacity (TAC) increased by 130.1% after 12 weeks compared with placebo (p < 0.05) (See Table 3 for other outcomes.) |
| Hashimoto H. et al., 2011 [41] | Open-label, prospective study | 35 cataract patients | 6 mg/day | 2 weeks | Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05) |
| Kim, J.H. et al., 2011 [42] | Randomized, Repeated, measured, prospective study | 39 heavy smokers, 39 non-smokers | 0, 5, 20, or 40 mg/day | 3 weeks | 5 mg/day: MDA and ISP significantly lower after 2 and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05) 20 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). 40 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 2 and 3 weeks compared with baseline in smokers (p < 0.05) |
| Nakagawa K. et al., 2011 [43] | Randomized, double-blind, placebo-controlled, prospective study | 30 healthy subjects | 0, 6, 12 mg/day | 12 weeks | 6 mg/day: reduction in total phospholipid hydroperoxides (PLOOH) after 12 weeks compared with baseline (p < 0.01) and compared with placebo (p < 0.05). Reduced phosphatidyl-ethanolamine hydroperoxide (PEOOH) after 12 weeks compared with baseline (p < 0.05) and compared with placebo (p < 0.05). 12 mg/day: 48% reduction in total PLOOH after 12 weeks compared with baseline (p < 0.01) and 35% less total PLOOH at 12 weeks compared with the control group (p < 0.05). The 12 mg/day group had 46% less phosphatidylcholine hydroperoxide (PCOOH) at 12 weeks compared with baseline (p < 0.01). |
| Peng L. et al., 2011 [44] | Randomized, placebo-controlled study | 115 healthy subjects | 0, 40 mg/day | 90 days | Comparing with the control group, MDA contents in the test group decreased significantly (p < 0.01), and SOD and GSH-Px activities increased significantly (p < 0.01). |
| Park J.S. et al., 2010 [45] | Randomized, double-blind, placebo-controlled, prospective study | 42 healthy subjects | 2 or 8 mg/day | 8 weeks | 2 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05). 8 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05) |
| Iwabayashi M. et al., 2009 [46] | Open-label, prospective study | 35 healthy subjects (with high oxidative stress) | 12 mg/day | 8 weeks | Increased blood biological antioxidant potential (BAP; +4.6%, p < 0.05) |
| Yamada T. et al., 2010 [47] | Open-label,prospective study | 6 healthy subjects and 6 Sjoegren’s syndrome subjects | 12 mg/day | 2 weeks | Reduced protein oxidation (−10%, p < 0.05) |
| Fassett, R.G. et al., 2008 [48] | Randomized, double-blind, placebo-controlled, prospective study | 58 renal transplant recipients | 0, 12 mg/day | 12 months | Total plasma F2-isoprostanes reduced by 23.0% in placebo and 29.7% in AX groups (N.S.) |
| Karppi, J. et al., 2007 [49] | Randomized, double-blind, placebo-controlled, prospective study | 39 healthy subjects | 0, 8 mg/day | 3 months | Decreased oxidation of fatty acids in healthy men (p < 0.05) |
| Kim Y.K. et al., 2004 [50] | Open-label, prospective study | 15 healthy postmenopausal women | 0, 2, 8 mg/day | 8 weeks | Decreased plasma TBARS levels: 2 mg group from 1.42 ± 0.18 to 1.13 ± 0.18 nM/mg (p < 0.05). 8 mg AX group from 1.62 ± 0.14 nM/mg to 1.13 ± 0.12 nM/mg after 8 weeks (p < 0.05). Increased TAS from 0.85 ± 0.42 mM/L to 1.90 ± 0.58 mM/L in the 8 mg group. Urinary 8-isoprostanes excretion did not decrease significantly. (See Table 3 for other outcomes.) |
| Author/Year/Reference | Study Design | Subjects | Dose | Duration | Outcome |
|---|---|---|---|---|---|
| <Subjects: healthy athletes, high daily physical activity> | |||||
| Brown, R.D. et al., 2021 [193] | Randomized, double-blind, placebo-controlled, crossover study | 12 recreationally trained male cyclists 27.5 ± 5.7 years, VO2peak: 56.5 ± 5.5 mL⋅kg−1⋅min−1, Wmax: 346.8 ± 38.4 W | 0, 12 mg/day | 7 days | Completion time of the 40-km cycling time trial improved by 1.2 ± 1.7% with AX supplementation, from 70.76 ± 3.93 min in the placebo condition to 69.90 ± 3.78 min in the AX condition (mean improvement time = 51 ± 71 s, p = 0.029, g = 0.21). Whole body fat oxidation rate was also greater in the AX group between 39–40 km (+0.09 ± 0.13 g⋅min−1, p = 0.044, g = 0.52) and respiratory exchange ratio was lower (−0.03 ± 0.04, p = 0.024, g = 0.60). |
| Talbott I. et al., 2018 [194] | Randomized, double-blind, placebo-controlled, prospective study | 28 recreational runners (42 ± 8 years) | 0, 12 mg/day | 8 weeks | Reduced average heart rate at submaximal endurance intensities (aerobic threshold, AeT and anaerobic threshold, AT), but not at higher “peak” intensities. |
| Klinkenberg L.J. et al., 2013 [195] | Randomized, double-blind, placebo-controlled prospective study | 32 well-trained male cyclists 25 ± 5 years, V˙O2peak = 60 ± 5 mL·kg−1·min−1, Wmax = 5.4 ± 0.5 W·kg−1 | 0, 20 mg/day * | 4 weeks | N.S; effect on exercise-induced cardiac troponin T release (p = 0.24), changes in antioxidant capacity markers (trolox equivalent antioxidant capacity, uric acid, and malondialdehyde). Markers of inflammation (high-sensitivity C-reactive protein) and exercise-induced skeletal muscle damage (creatine kinase). |
| Res T. et al., 2013 [196] | Randomized, double-blind, placebo-controlled, prospective study | 32 trained male cyclists or triathletes 25 ± 1 years, V˙O2peak = 60 ± 1 mL·kg−1·min−1, Wmax = 395 ± 7 W | 0, 20 mg/day | 4 weeks | N.S; total plasma antioxidant capacity (p = 0.90) or attenuated malondialdehyde levels (p = 0.63). Whole-body fat oxidation rates during submaximal exercise (from 0.71 +/− 0.04 to 0.68 ± 0.03 g⋅min−1 and from 0.66 ± 0.04 to 0.61 ± 0.05 g⋅min−1 in the placebo and AX groups, respectively; p = 0.73), time trial performance (from 236 ± 9 to 239 ± 7 and from 238 ± 6 to 244 ± 6 W in the placebo and AX groups, respectively; p = 0.63). |
| Djordjevic B. et al., 2011 [197] | Randomized, Double-blind, placebo-controlled, prospective study | 32 male elite soccer players | 0, 4 mg/day | 90 days | Changes in elevated O2-¯ concentrations after soccer exercise were statistically significant only in the placebo group (exercise × supplementation effect, p < 0.05); TAS values decreased significantly only in the placebo group after exercise (p < 0.01). After intervention, total SH group content increased (21% and 9%, respectively), and the effect of AX was marginally significant (p = 0.08). Basal SOD activity was significantly reduced in both the placebo and AX groups at the end of the study (main training effect, p < 0.01). Post-exercise CK and AST levels were significantly lower in the AX group than in the placebo group (p < 0.05) |
| Earnest C.P. et al., 2011 [198] | Randomized, double-blind, placebo-controlled, prospective study | 14 amateur endurance-trained subjects 18–39 years, V˙O2peak = 52.84 ± 3.5 mL·kg−1·min−1, Wmax = 330 ± 26 W | 0, 4 mg/day | 28 days | Improved performance in the 20-km cycling time trial in the AX group (n = 7, −121 s; 95% CI, −185, −53), but not in the placebo group (n = 7, −19 s; 95% CI, −84, 45). AX group significantly increased power output (20 W; 95% CI, 1, 38), whereas the placebo group did not (1.6 W; 95% CI, −17, 20). N.S; carbohydrate, fat oxidation and blood indices indicative of fuel mobilization. |
| Bloomer, R.J. et al., 2005 [199] | Randomized, placebo-controlled, prospective study | 20 resistance trained male subjects (25.1 ± 1.6 years) | 0, 4 mg/day * | 3 months | N.S; Muscle soreness, creatine kinase (CK), and muscle performance were measured before and through 96-h post-eccentric exercise |
| Sawaki K. et al., 2002 [200] | Randomized double-blind placebo-controlled, prospective study | 16 healthy adult male subjects | 0, 6 mg/day | 4 weeks | In the AX group, the serum lactate concentration after 2 min of activity (1200 m run) was significantly lower than that in the control group. |
| <Subjects: healthy subjects> | |||||
| Kawamura A. et al., 2021 [201] | Randomized controlled open-label, prospective study | 26 healthy male subjects | N/A (1 mg AX/100 g salmon) * | 10 weeks | The skeletal muscle mass was higher after training than before training in both control and intervention groups (p < 0.05). Increased maximal voluntary contraction after training in the intervention group (p < 0.05), but not significantly increased in the control group. (See Table 3 for other outcomes.) |
| Fleischmann C. et al., 2019 [202] | Randomized, double-blind, placebo-controlled, prospective study | 22 healthy subjects | 0, 12 mg/day | 30 days | Decreased raise in blood lactate caused by the VO2 Max test; AX group (9.4 ± 3.1 and 13.0 ± 3.1 mmole⋅L−1 in the AX and placebo groups, respectively p < 0.02). Change in oxygen uptake during recovery (−2.02 ± 0.64 and 0.83 ± 0.79% of VO2 Max in the AX and placebo group, respectively, p = 0.001). N.S; anaerobic threshold or VO2 Max. physiological or biochemical differences in the heat tolerance test (HTT) (2 h walk at 40 °C, 40% relative humidity. |
| Takami M. et al., 2019 [203] | Open-label, prospective study | 20 healthy young male subjects | c.a, 4.5 mg/day * from salmon | 4 weeks | Increased maximum workload by training in both groups (p = 0.009), and increased oxygen consumption during exercise in the antioxidant group only (p = 0.014). There were positive correlations between maximum workload and fat (r = 0.575, p = 0.042) and carbohydrate oxidations (r = 0.520, p = 0.059) in the antioxidant group. (See Table 3 for other outcomes.) |
| Imai A. et al., 2018 [204] | Randomized, double-blind, placebo-controlled, crossover study | 42 healthy subjects | 0, 6 mg/day * | 4 weeks | Elevated PCOOH levels during mental and physical tasks were attenuated by AX supplementation. Improved recovery from mental fatigue compared with the placebo. No differences were found between AX and the placebo in other secondary outcomes, such as subjective feelings, work efficiency, and autonomic activity. |
| Hongo N. et al., 2017 [205] | Randomized, double-blind placebo-controlled, prospective study | 39 healthy subjects | 0, 12 mg/day * | 12 weeks | Intent-to-treat (ITT) analysis; fatigue after physical and mental stress was significantly lower in the AX group than in the placebo at week 8; the change in POMS Friendliness was significantly higher in the AX group than in the control group at week 8; the rate of change in BAP values at week 12 was not significantly different between the AX and control groups. The rate of change in BAP values at week 12 was not significantly different between the AX group and the control. |
| Malmstena C.L.L. et al., 2008 [206] | Randomized, double-blind, placebo-controlled, prospective study | 40 young healthy subjects (17–19 years) | 0, 4 mg/day | 3 months | Increased average number of knee bending (squats) increased by 27.05 (from 49.32 to 76.37, AX group) vs. 9.0 (from 46.06 to 55.06, placebo subjects), p = 0.047. |
| Tajima T. et al., 2004 [207] | Randomized, double-blind, placebo-controlled, crossover study | 18 healthy subjects (35.7 ± 4 years) | 0, 5 mg/day | 2 weeks | Increased in CVRR and HF/TF (Heart rate variability) were significant during exercise at 70% maximum heart rate (HRmax) intensity (p < 0.05). Also, after the AX supplementation, decreased minute ventilation (VE) during exercise at 70% HRmax (p < 0.05). Decreased LDL cholesterol (chol) (p < 0.05) and respiratory quotient after exercise. |
| <Subjects: elderly subjects> | |||||
| Liu S.Z. et al., 2021 [189] | Randomized, double-blind, placebo-controlled, prospective study | 42 elderly subjects (65–82 years) | 0, 12 mg/day * | 12 weeks | In endurance training (ET), specific muscular endurance was improved only in the AX group (Pre 353 ± 26 vs. Post 472 ± 41) and submaximal graded exercise test duration was improved in both groups (placebo 40.8 ± 9.1% vs. AX 41.1 ± 6.3%). The increase in fat oxidation at low intensity after ET was greater in AX (placebo 0. 23 ± 0.15 g vs. AX 0.76 ± 0.18 g), and was associated with reduced carbohydrate oxidation and improved exercise efficiency in men, but not in women. |
| Liu S.Z. et al., 2018 [190] | Randomized double-blind, placebo-controlled, prospective study | 42 elderly subjects (65–82 years) | 0, 12 mg/day * | 12 weeks | Administration of AX increased maximal voluntary force (MVC) by 14.4% (± 6.2%, p < 0.02), tibialis anterior muscle size (cross-sectional area, CSA) by 2.7% (± 1.0%, p < 0.01), and specific impulse increased by 11.6% (MVC/CSA, ± 6.0%, p = 0.05), respectively, whereas placebo treatment did not alter these characteristics (MVC, 2.9% ± 5.6%; CSA, 0.6% ± 1.2%; MVC/CSA, 2.4 ± 5.7%; all p > 0.6). |
| Fujino H. et al., 2016 [191] | Randomized, double-blind, placebo-controlled, prospective study | 29 community-dwelling healthy elderly subjects (80.9 ± 1.5 years.) | 0, 12 mg/twice a day * | 3 months | Decrease in d-ROM values with AX group (p < 0.01), but not the placebo group; the AX group had a therapeutic effect on 6-min walking distance compared with the placebo group (p < 0.05). AX group had an increase in distance and number of steps in the 6-min walking test compared with the placebo group. Furthermore, the rate of increase in blood lactate levels after walking was lower in the AX group than in the placebo group (p < 0.01). |
| Author/Year/Reference | Study Design | Subjects | Dose | Duration | Outcome |
|---|---|---|---|---|---|
| Shokri-Mashhadi, N. et al., 2021 [208] | Randomized, double-blind, placebo-controlled, prospective study | 44 patients with type 2 diabetes | 0, 8 mg/day | 8 weeks | Decrease plasma levels of MDA and IL-6 (p < 0.05) and decrease the expression level of miR-146a, associated with inflammatory markers (fold change: −1/388) (p < 0.05). |
| Kawamura A. et al., 2021 [201] | Randomized controlled Open-label, prospective study | 26 healthy male subjects | N/A (1 mg AX/100 g salmon) * | 10 weeks | Higher resting oxygen consumption after training in the intervention group only (p < 0.05). Serum carbonylated protein level as an oxidative stress marker tended to be lower immediately after exercise than before exercise in the intervention group only (p = 0.056). (See Table 2. for other outcomes.) |
| Kato T. et al., 2020 [209] | Open-label, prospective study | 16 patients with systolic heart failure | 12 mg/day * | 3 months | Increased left ventricular ejection fraction (LVEF) from 34.1 ± 8.6% to 38.0 ± 10.0% (p = 0.031) and 6-min walk distance increased from 393.4 ± 95.9 m to 432.8 ± 93.3 m (p = 0.023). Significant relationships were observed between percent changes in dROM level and those in LVEF. |
| Chan K. et al., 2019 [210] | Randomized controlled Open-label, prospective study | 54 patients with type 2 diabetes | 0, 6, 12 mg/day | 8 weeks | Increased plasma AX levels and decreased fasting plasma glucose and HbA1c levels. In 12 mg AX group, reduced in plasma triglyceride, total chol and LDL levels. Lowered changes in plasma IL-6 and TNF-α levels and plasma vWF level and higher changes in AT-III level. In 12 mg AX group, decreased changes in plasma FVII and PAI-1 levels. |
| Takami M. et al., 2019 [211] | Open-label, prospective study | 20 healthy young male subjects | c.a, 4.5 mg/day * from salmon | 4 weeks | Higher carbohydrate oxidation during rest in the post-training than that in the pre-training only in the antioxidant group. More decreased levels of serum insulin and HOMA-IR after training were observed in the antioxidant group than in the control group. (See Table 2. for other outcomes.) |
| Mashhadi N.S. et al., 2018 [163] | Randomized, double-blind, placebo-controlled, prospective study | 44 participants with type 2 diabetes | 0, 8 mg/day | 8 weeks | Increased the serum adiponectin concentration, reduced visceral body fat mass (p < 0.01), serum triglyceride and VLDL chol concentrations, systolic blood pressure, fructosamine concentration (p < 0.05) and marginally reduced the plasma glucose concentration (p = 0.057). |
| Canas J. A. et al., 2017 [211] | Randomized, double-blind, placebo-controlled, prospective study | 20 children with simple obesity (BMI > 90%) | 500 μg/day * (MCS) | 6 months | Mixed-carotenoid supplementation (MCS) increased β-carotene, total adiponectin, and high-molecular-weight adiponectin in plasma compared with placebo; MCS decreased BMI z-score, waist-to-height ratio, and subcutaneous adipose tissue compared with placebo. AX was used as a part of MCS. |
| Takemoto M. et al., 2015 [212] | Case report | 1 Werner syndrome patient | 12 mg/day * | 6 months | Improved blood transaminase concentrations before AX intervention and 3 and 6 months after initiation were: AST 40 IU/L, 41 IU/L, and 20 IU/L; ALT 69 IU/L, 62 IU/L, and 34 IU/L; GGT 38 IU/L, 41 IU/L, and 35 IU/L; and cholinesterase 360 IU/L, 366 IU/L, and 331 IU/L, respectively. Liver-to-spleen Hounsfield units on CT were 0.41 before AX initiation, 0.71 at 3 months, and 0.94 at 6 months. No significant changes after AX intervention in hyaluronic acid, a marker of liver fibrosis; high-sensitivity C-reactive protein, a marker of inflammation; and MDA-modified LDL. |
| Ni Y. et al., 2015[108] | Randomized, single-blind, placebo-controlled, prospective study | 12 NASH patients | 12 mg/day | 24 weeks | Improved steatosis (p < 0.05), marginally improved lobular inflammation (p = 0.15) and NAFLD activity score (p = 0.08) |
| Choi H.D. et al., 2011 [40] | Randomized, double-blind, placebo-controlled, prospective study | 27 overweight subjects (BMI >25.0 kg/m2) | 0, 20 mg/day | 12 weeks | Decreased LDL chol and ApoB. (See Table 1. For other outcomes.) |
| Yoshida H. et al., 2010 [161] | Randomized, ouble-blind, placebo-controlled, prospective study | 61 non-obese subjects with fasting serum triglyceride of 120–200 mg/dL and without diabetes and hypertension | 0, 6, 12, 18 mg/day | 12 weeks | Multiple comparison: triglycerides were significantly decreased by 12 and 18 mg/day and HDL-cholesterol was significantly increased by 6 and 12 mg. Serum adiponectin was increased by AX (12 and 18 mg/day), and changes in adiponectin were positively correlated with changes in HDL-chol. |
| Satoh A. et al., 2009 [213] | Open-label, prospective study | 20 subjects at risk for developing metabolic syndrome (from 127 healthy subjects) | 4, (8, 20) mg/day | 4 weeks. | When subjects who met the diagnostic criteria for metabolic syndrome in Japan (SBP ≥ 130 mmHg, DBP ≥ 85 mmHg, TG ≥ 150 mg/dL, FG ≥ 100 mg/dL) at the start of the study were selected from 4 mg group, significant decreased in SBP(p < 0.01). On the other hand, there was no significant decrease in DBP. Reduced TG after treatment (218 mg/dL) than the baseline value (292 mg/dL), marginally reduced fasting glucose after the intervention (p < 0.1). |
| Uchiyama A. et al., 2008 [162] | Open-label, prospective study | 17 subjects at risk for developing metabolic syndrome | 8 mg twice day | 3 months | Significant decreases plasma HbAlc (p = 0.0433) and TNF-α levels (p = 0.0022) and increase adiponectin concentration (p = 0.0053). N.S: body weight, BMI and waist circumference. |
| Fukamauchi M. et al., 2007 [214] | Randomized, double-blind, placebo-controlled, prospective study | 32 healthy subjects | 0, 6 mg/day | 6 weeks | Synergistic effects of AX intake (12 mg/day, 6 weeks) and aerobic exercise (walking) were studied. AX contributed to reduction of body fat and suppressed the increase in blood lactate level after exercise. |
| Kim Y.K. et al., 2004 [50] | Open-label, prospective study | 15 healthy postmenopausal female subjects | 0, 2, 8 mg/day | 8 weeks | Increase HDL-chol levels in 2 mg and 8 mg group increased significantly after 8 weeks from 50.6 ± 5.8 to 60.4 ± 7.1 mg/dL, 44.4 ± 10.7 to 49.4 ± 2.7 mg/dL respectively (p < 0.05). In the 2 mg group, triglyceride decreased significantly from 171.6 ± 67.4 mg/dL to 145.8 ± 5.1 mg/dL (p < 0.05). (See Table 1. For other outcomes.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 2022, 14, 107. https://doi.org/10.3390/nu14010107
Nishida Y, Nawaz A, Hecht K, Tobe K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients. 2022; 14(1):107. https://doi.org/10.3390/nu14010107
Chicago/Turabian StyleNishida, Yasuhiro, Allah Nawaz, Karen Hecht, and Kazuyuki Tobe. 2022. "Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants" Nutrients 14, no. 1: 107. https://doi.org/10.3390/nu14010107
APA StyleNishida, Y., Nawaz, A., Hecht, K., & Tobe, K. (2022). Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients, 14(1), 107. https://doi.org/10.3390/nu14010107