Nutritional Therapies and Their Influence on the Intestinal Microbiome in Pediatric Inflammatory Bowel Disease
Abstract
:1. Introduction
Basic Concepts of Nutrition in Intestinal Inflammation
2. Diets with Proof of Clinical Efficacy for IBD
2.1. Exclusive Enteral Nutrition (EEN)
2.1.1. Mechanisms of Action of EEN
2.1.2. Clinical Efficacy of EEN
| Type of Diet | Study Design | Main Outcomes | Limitations OF Studies | Limitations of Dietary Therapy |
|---|---|---|---|---|
| EEN | Multiple study designs, including two systematic reviews [52,64]. | 80–85% remission rate Equivalent to CS for clinical remission Small studies show EEN is superior to CS for mucosal healing [52,65]. | Adult studies have shown decreased efficacy in ITT analyses due to high patient dropout rates | Very restrictive Difficult to maintain long term due to issues of tolerability Poor palatability |
| PEN [66,67,68] | Multiple study designs, induction of remission One systematic review, maintenance of remission | No evidence of efficacy for induction of remission The systematic review demonstrates efficacy for maintenance of remission | No consistent definition of PEN (percent caloric intake) Variable methodologies used across studies | Can be restrictive Difficult to maintain long term |
| CDED [48,69,70] | RCT: EEN versus CDED Two prospective, open-label studies | Week Six: EEN and CDED are both effective at achieving clinical remission Week 12: significantly higher CS-free remission in CDED group (76%) Earlier studies: 70–75% remission rate in children at week six | The primary outcome of RCT was tolerability Small numbers, nonrandomized, inconsistent protocol (not all participants took PEN) in prospective, open-label studies | Most effective for mild-moderate luminal CD The induction phase of treatment is relatively restrictive |
| SCD [68] | Exploratory multi-omic pilot study: SCD versus mSCD versus whole food | All patients showed clinical improvement and FC improvement SCD showed the greatest clinical improvement mSCD showed the greatest FC change Cannot conclude significance with sample size | Small sample size: 18 patients recruited; 10 patients completed the study Baseline mild disease (normal CRP, normal/mild increase ESR) Significant side effects among recipients of WF diet | Very restrictive May be difficult to adhere to without support for meal preparation |
| CD-TREAT [46] | RCT: EEN versus CD-TREAT in healthy adults Five children received CD-TREAT | 80% (four out of five) clinical improvement 60% (three out of five) clinical remission, improved FC | Very small sample size | Easier diet to follow Limited data |
| MD [47] | RCT: MD versus SCD | No significant difference in clinical symptoms, FC values between MD and SCD | The sample included patients with primarily mild disease Not all patients had elevated FC at baseline Lack of control arm | None Easiest diet to follow Miscellaneous health benefits may occur for conditions other than IBD |
| LFD [71,72] | Two RCTs | 52% decrease in symptoms 34% decrease in FC No effect on CRP | Small sample size Patient-reported outcomes (subjectivity, potential placebo effect) Patients primarily in remission (or with mild disease) | Can be restrictive |
2.1.3. Effects of EEN on the Microbiome
2.1.4. Summary
2.2. Partial Enteral Nutrition (PEN)
2.2.1. Mechanisms of Action of PEN
2.2.2. Clinical Efficacy of PEN
2.2.3. Summary
2.3. Crohn’s Disease Exclusion Diet (CDED)
2.3.1. Clinical Efficacy of CDED
2.3.2. Effects of CDED on the Intestinal Microbiome
2.3.3. Summary
3. Diets without Proven Clinical Efficacy for IBD
3.1. Specific Carbohydrate Diet
3.1.1. Clinical Efficacy of SCD
3.1.2. Effects of SCD on the Intestinal Microbiome
3.1.3. Summary
3.2. Crohn’s Disease Treatment with Eating Diet (CD-TREAT)
Summary
3.3. Mediterranean Diet (MD)
3.3.1. Clinical Efficacy and Effects on the Microbiome
3.3.2. Summary
3.4. Low FODMAP Diet (LFD)
3.4.1. Clinical Efficacy of LFD
3.4.2. Effects of LFD on the Intestinal Microbiome
3.4.3. Summary
3.5. Miscellaneous Diets
4. Further Considerations for Dietary Therapy
5. Conclusions
Funding
Conflicts of Interest
References
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; Chachu, K.; Day, L.; Lebwohl, B.; Muniraj, T.; et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P.; Cohen, B.L.; Chachu, K.; Day, L.; et al. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veltkamp, C.; Tonkonogy, S.L.; De Jong, Y.P.; Albright, C.; Grenther, W.B.; Balish, E.; Terhorst, C.; Sartor, R.B. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tgε26 mice. Gastroenterology 2001, 120, 900–913. [Google Scholar] [CrossRef]
- Ohkusa, T.; Okayasu, I.; Ogihara, T.; Morita, K.; Ogawa, M.; Sato, N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut 2003, 52, 79–83. [Google Scholar] [CrossRef]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2015, 65, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Janowitz, H.D.; Croen, E.C.; Sachar, D.B. The role of the fecal stream in Crohn’s disease: An historical and analytic review. Inflamm. Bowel Dis. 2007, 4, 29–39. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Peeters, M.; Hiele, M.; Vantrappen, G.; Pennincx, F.; Aerts, R.; Kerremans, R.; Goboes, K. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 1991, 338, 771–774. [Google Scholar] [CrossRef]
- Caputi, V.; Popov, J.; Giron, M.C.; O’Mahony, S. Gut Microbiota as a Mediator of Host Neuro-Immune Interactions: Implications in Neuroinflammatory Disorders. Mod. Trends Psychiatry 2021, 32, 40–57. [Google Scholar]
- Carloni, S.; Bertocchi, A.; Mancinelli, S.; Bellini, M.; Erreni, M.; Borreca, A.; Braga, D.; Giugliano, S.; Mozzarelli, A.M.; Manganaro, D.; et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439–448. [Google Scholar] [CrossRef]
- Narula, N.; Kassam, Z.; Yuan, Y.; Colombel, J.F.; Ponsioen, C.; Reinisch, W.; Moayyedi, P. Systematic Review and Meta-analysis: Fecal Microbiota Transplantation for Treatment of Active Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1702–1709. [Google Scholar] [CrossRef]
- Pai, N.; Popov, J.; Hill, L.; Hartung, E.; Grzywacz, K.; Moayyedi, P.; Surette, M.; Lee, C.; Godin, D.; Szamosi, J.; et al. Results of the First Pilot Randomized Controlled Trial of Fecal Microbiota Transplant in Pediatric Ulcerative Colitis: Lessons, Limitations, and Future Prospects. Gastroenterology 2021, 161, 388–393.e3. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nat. Cell Biol. 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, 6275. [Google Scholar] [CrossRef] [Green Version]
- Gehrig, J.L.; Venkatesh, S.; Chang, H.-W.; Hibberd, M.C.; Kung, V.L.; Cheng, J.; Chen, R.Y.; Subramanian, S.; Cowardin, C.A.; Meier, M.; et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 2019, 365, eaau4732. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.Y.; Mostafa, I.; Hibberd, M.C.; Das, S.; Mahfuz, M.; Naila, N.N.; Islam, M.M.; Huq, S.; Alam, M.A.; Zaman, M.U.; et al. A Microbiota-Directed Food Intervention for Undernourished Children. N. Engl. J. Med. 2021, 384, 1517–1528. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.-H.; Lochhead, P.; Khalili, H.; Song, M.; Tabung, F.K.; Burke, K.E.; Richter, J.M.; Giovannucci, E.L.; Chan, A.T.; Ananthakrishnan, A.N. Dietary Inflammatory Potential and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2020, 159, 873–883.e1. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Sacks, F.M.; Campos, H. Dietary Therapy in Hypertension. N. Engl. J. Med. 2010, 362, 2102–2112. [Google Scholar] [CrossRef] [Green Version]
- McNaughton, S.A.; Mishra, G.D.; Brunner, E.J. Dietary Patterns, Insulin Resistance, and Incidence of Type 2 Diabetes in the Whitehall II Study. Diabetes Care 2008, 31, 1343–1348. [Google Scholar] [CrossRef] [Green Version]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Peters, V.; Spooren, C.E.G.M.; Pierik, M.J.; Weersma, R.K.; van Dullemen, H.M.; Festen, E.A.M.; Visschedijk, M.C.; Masclee, A.A.M.; Hendrix, E.M.B.; Almeida, R.J.; et al. Dietary Intake Pattern is Associated with Occurrence of Flares in IBD Patients. J. Crohn’s Colitis 2021, 15, 1305–1315. [Google Scholar] [CrossRef]
- Kinsey, L.; Burden, S. A survey of people with inflammatory bowel disease to investigate their views of food and nutritional issues. Eur. J. Clin. Nutr. 2016, 70, 852–854. [Google Scholar] [CrossRef]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [Green Version]
- Daïen, C.I.; Pinget, G.V.; Tan, J.K.; Macia, L. Detrimental Impact of Microbiota-Accessible Carbohydrate-Deprived Diet on Gut and Immune Homeostasis: An Overview. Front. Immunol. 2017, 8, 548. [Google Scholar] [CrossRef] [Green Version]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal Protein Intake and Risk of Inflammatory Bowel Disease: The E3N Prospective Study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.L.; Keita, Å.V.; Duncan, S.H.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn’s disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BioRender. 2021. Available online: https://biorender.com (accessed on 1 November 2021).
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis 2021, 15, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Horwat, P.; Kopeć, S.; Garczyk, A.; Kaliciak, I.; Staręga, Z.; Drogowski, K.; Mardas, M.; Stelmach-Mardas, M. Influence of Enteral Nutrition on Gut Microbiota Composition in Patients with Crohn’s Disease: A Systematic Review. Nutrients 2020, 12, 2551. [Google Scholar] [CrossRef]
- Ashton, J.; Gavin, J.; Beattie, R.M. Exclusive enteral nutrition in Crohn’s disease: Evidence and practicalities. Clin. Nutr. 2018, 38, 80–89. [Google Scholar] [CrossRef]
- Lawley, M.; Wu, J.W.; Navas-López, V.M.; Huynh, H.Q.; Carroll, M.W.; Chen, M.; Medvedev, P.; Day, A.S.; Hussey, S.; Sigall-Boneh, R.; et al. Global Variation in Use of Enteral Nutrition for Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, e22–e29. [Google Scholar] [CrossRef]
- Rubio, A.; Pigneur, B.; Garnier-Lengliné, H.; Talbotec, C.; Schmitz, J.; Canioni, D.; Goulet, O.; Ruemmele, F.M. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment. Pharmacol. Ther. 2011, 33, 1332–1339. [Google Scholar] [CrossRef]
- Swaminath, A.; Feathers, A.; Ananthakrishnan, A.; Falzon, L.; Ferry, S.L. Systematic review with meta-analysis: Enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 46, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Chen, K.-C.; Chen, J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: A meta-analysis. World J. Pediatr. 2019, 15, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Huang, Y.; Shi, P.; Wang, Y.; Zhang, Y.; Xue, A.; Tang, Z.; Hu, W.; Sun, H.; Zhang, P.; et al. Effect of Exclusive Enteral Nutrition on the Disease Process, Nutrition Status, and Gastrointestinal Microbiota for Chinese Children with Crohn’s Disease. J. Parenter. Enter. Nutr. 2021, 45, 826–838. [Google Scholar] [CrossRef]
- Ramaswamy, P.K. Exclusive enteral nutrition with oral polymeric diet helps in inducing clinical and biochemical remission in adults with active Crohn’s Disease. J. Parenter. Enter. Nutr. 2021. [Google Scholar] [CrossRef]
- Mitrev, N.; Huang, H.; Hannah, B.; Kariyawasam, V.C. Review of exclusive enteral therapy in adult Crohn’s disease. BMJ Open Gastroenterol. 2021, 8, e000745. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Ben-Moshe, T.; Abitbol, G.; Ledder, O.; Peleg, S.; Millman, P.; Shaoul, R.; Kori, M.; Assa, A.; Cohen, S.; et al. The Effect of Nutritional Therapy on Bone Mineral Density and Bone Metabolism in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 877–882. [Google Scholar] [CrossRef]
- Day, A.; Wood, J.; Melton, S.; Bryant, R.V. Exclusive enteral nutrition: An optimal care pathway for use in adult patients with active Crohn’s disease. JGH Open 2020, 4, 260–266. [Google Scholar] [CrossRef]
- Boneh, R.S.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients with Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 752–759. [Google Scholar] [CrossRef]
- Panaccione, R.; Steinhart, A.H.; Bressler, B.; Khanna, R.; Marshall, J.K.; Targownik, L.; Afif, W.; Bitton, A.; Borgaonkar, M.; Chauhan, U.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Luminal Crohn’s Disease. J. Can. Assoc. Gastroenterol. 2019, 2, e1–e34. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Kunisaki, R.; Nakamura, S.; Tsujikawa, T.; Hirai, F.; Nakase, H.; Watanabe, K.; Yokoyama, K.; Nagahori, M.; Kanai, T.; et al. Effect of elemental diet combined with infliximab dose escalation in patients with Crohn’s disease with loss of response to infliximab: CERISIER trial. Intest. Res. 2018, 16, 494–498. [Google Scholar] [CrossRef]
- Buchanan, E.; Gaunt, W.W.; Cardigan, T.; Garrick, V.; McGrogan, P.; Russell, R.K. The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment. Pharmacol. Ther. 2009, 30, 501–507. [Google Scholar] [CrossRef]
- Sahu, P.; Kedia, S.; Vuyyuru, S.K.; Bajaj, A.; Markandey, M.; Singh, N.; Singh, M.; Kante, B.; Kumar, P.; Ranjan, M.; et al. Randomised clinical trial: Exclusive enteral nutrition versus standard of care for acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 2021, 53, 568–576. [Google Scholar] [CrossRef]
- Wong, K.; Isaac, D.M.; Wine, E. Growth Delay in Inflammatory Bowel Diseases: Significance, Causes, and Management. Dig. Dis. Sci. 2021, 66, 954–964. [Google Scholar] [CrossRef]
- Mehta, P.; Pan, Z.; Furuta, G.T.; Kim, D.Y.; De Zoeten, E.F. Parent Perspectives on Exclusive Enteral Nutrition for the Treatment of Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 744–748. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E. Effects of enteral nutrition on Crohn’s Disease: Clues to the impact of diet on disease pathogenesis. Inflamm. Bowel Dis. 2013, 19, 1322–1329. [Google Scholar] [CrossRef]
- Budd, G.R.; Aitchison, A.; Day, A.S.; Keenan, J.I. The effect of polymeric formula on enterocyte differentiation. Innate Immun. 2017, 23, 240–248. [Google Scholar] [CrossRef]
- Johnson, T.; Macdonald, S.; Hill, S.M.; Thomas, A.; Murphy, M.S. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: A randomised controlled trial. Gut 2006, 55, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease with an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults with Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Levine, A.; Lomer, M.; Wierdsma, N.; Allan, P.; Fiorino, G.; Gatti, S.; Jonkers, D.; Kierkus, J.; Katsanos, K.H.; et al. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1407–1419. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 15, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.R.; Gomez-Nguyen, A.; LaSalla, A.; Buttó, L.; Kulpins, D.; Warner, A.; Di Martino, L.; Ponzani, G.; Osme, A.; Rodriguez-Palacios, A.; et al. Replacing Animal Protein with Soy-Pea Protein in an “American Diet” Controls Murine Crohn Disease-Like Ileitis Regardless of Firmicutes: Bacteroidetes Ratio. J. Nutr. 2021, 151, 579–590. [Google Scholar]
- Logan, M.; Gkikas, K.; Svolos, V.; Nichols, B.; Milling, S.; Gaya, D.R.; Seenan, J.P.; Macdonald, J.; Hansen, R.; Ijaz, U.Z.; et al. Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn’s disease-new insights into dietary disease triggers. Aliment. Pharmacol. Ther. 2020, 51, 935–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2021, 8, 924. [Google Scholar] [CrossRef]
- Gatti, S.; Galeazzi, T.; Franceschini, E.; Annibali, R.; Albano, V.; Verma, A.K.; De Angelis, M.; Lionetti, M.E.; Catassi, C. Effects of the Exclusive Enteral Nutrition on the Microbiota Profile of Patients with Crohn’s Disease: A Systematic Review. Nutrients 2017, 9, 832. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2015, 18, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in Presumptively Protective Gut Bacterial Species and Metabolites Are Paradoxically Associated with Disease Improvement in Pediatric Crohn’s Disease During Enteral Nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Day, A.S.; Leach, S.T.; Lemberg, D.; Nielsen, S.; Mitchell, H.M. Effect of Exclusive Enteral Nutrition on the Microbiota of Children with Newly Diagnosed Crohn’s Disease. Clin. Transl. Gastroenterol. 2015, 6, e71. [Google Scholar] [CrossRef]
- Quince, C.; Ijaz, U.Z.; Loman, N.; Eren, A.M.; Saulnier, D.; Russell, J.; Haig, S.; Calus, S.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children with Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1729. [Google Scholar] [CrossRef] [Green Version]
- Leach, S.T.; Mitchell, H.M.; Eng, W.R.; Zhang, L.; Day, A.S. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 28, 724–733. [Google Scholar] [CrossRef]
- Andoh, A.; Inoue, R.; Kawada, Y.; Morishima, S.; Inatomi, O.; Ohno, M.; Bamba, S.; Nishida, A.; Kawahara, M.; Naito, Y. Elemental diet induces alterations of the gut microbial community in mice. J. Clin. Biochem. Nutr. 2019, 65, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Courtney, J.; Adams, M. Infectious Threats in “Healthy” Donors: Results of a Donor Screening Program for a Next-Generation FMT. 2016. Available online: https://www.rebiotix.com/infectious-threats-in-healthy-donors-results-of-a-donor-screening-program-for-a-next-generation-fmt (accessed on 26 October 2021).
- Hart, L.; Farbod, Y.; Szamosi, J.C.; Yamamoto, M.; Britz-McKibbin, P.; Halgren, C.; Zachos, M.; Pai, N. Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 1691. [Google Scholar] [CrossRef]
- Diederen, K.; Li, J.V.; Donachie, G.E.; De Meij, T.G.; De Waart, D.R.; Hakvoort, T.B.M.; Kindermann, A.; Wagner, J.; Auyeung, V.; Velde, A.A.T.; et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Feng, R.; Li, T.; Xu, S.; Hao, X.; Qiu, Y.; Chen, M. Systematic review with meta-analysis of partial enteral nutrition for the maintenance of remission in Crohn’s disease. Nutr. Res. 2020, 81, 7–18. [Google Scholar] [CrossRef]
- Urlep, D.; Benedik, E.; Brecelj, J.; Orel, R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Nucl. Med. Mol. Imaging 2019, 179, 431–438. [Google Scholar] [CrossRef]
- Lee, D.; Baldassano, R.N.; Otley, A.R.; Albenberg, L.; Griffiths, A.M.; Compher, C.; Chen, E.Z.; Li, H.; Gilroy, E.; Nessel, L.; et al. Comparative effectiveness of nutritional and biological therapy in North American children with active Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 1786–1793. [Google Scholar] [CrossRef]
- Duncan, H.; Buchanan, E.; Cardigan, T.; Garrick, V.; Curtis, L.; McGrogan, P.; Barclay, A.; Russell, R.K. A retrospective study showing maintenance treatment options for paediatric CD in the first year following diagnosis after induction of remission with EEN: Supplemental enteral nutrition is better than nothing! BMC Gastroenterol. 2014, 14, 50. [Google Scholar] [CrossRef] [Green Version]
- Konno, M.; Takahashi, M.; Toita, N.; Fujiwara, S.-I.; Nojima, M. Long-term therapeutic effectiveness of maintenance enteral nutrition for Crohn’s disease. Pediatr. Int. 2015, 57, 276–280. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Kim, S.Y.; Koh, H. Effect of short-term partial enteral nutrition on the treatment of younger patients with severe Crohn’s disease. Gut Liver 2015, 9, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Schulman, J.M.; Pritzker, L.; Shaoul, R. Maintenance of Remission with Partial Enteral Nutrition Therapy in Pediatric Crohn’s Disease: A Retrospective Study. Can. J. Gastroenterol. Hepatol. 2017, 2017, 5873158. [Google Scholar] [CrossRef] [PubMed]
- Wilschanski, M.; Sherman, P.; Pencharz, P.; Davis, L.; Corey, M.; Griffiths, A. Supplementary enteral nutrition maintains remission in paediatric Crohn’s disease. Gut 1996, 38, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, Y.; Cho, J.M.; Oh, S.H.; Kim, K.M. Therapeutic Efficacy of Oral Enteral Nutrition in Pediatric Crohn’s Disease: A Single Center Non-Comparative Retrospective Study. Yonsei Med. J. 2016, 57, 1185–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavin, J.J.; Ashton, J.J.; Heather, N.; Marino, L.V.; Beattie, R.M. Nutritional support in paediatric Crohn’s disease: Outcome at 12 months. Acta Paediatr. 2017, 107, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Brückner, A.; Werkstetter, K.J.; Frivolt, K.; Shokry, E.; Ahmed, M.; Metwaly, A.; Marques, J.G.; Uhl, O.; Krohn, K.; Hajji, M.; et al. Partial enteral nutrition has no benefit on bone health but improves growth in paediatric patients with quiescent or mild Crohn’s disease. Clin. Nutr. 2020, 39, 3786–3796. [Google Scholar] [CrossRef]
- Pigneur Arnaud, B.; Martinez-Vinson, C.; Bourmaud, A.; Swellen, G.; Duclaux-Loras, R.; Hugot, J.P.; Roman, C.; Dumant, C.; Spyckerelle, C.; Guinard Samuel, V.; et al. OP15 Cyclic exclusive enteral nutrition to maintain longterm drug-free remission in Paediatric Crohn’s Disease: The CD HOPE study of the GETAID pédiatrique. J. Crohn’s Colitis 2021, 15, S015. [Google Scholar] [CrossRef]
- Nahidi, L.; Corley, S.M.; Wilkins, M.R.; Wei, J.; Alhagamhmad, M.; Day, A.S.; Lemberg, D.A.; Leach, S.T. The major pathway by which polymeric formula reduces inflammation in intestinal epithelial cells: A microarray-based analysis. Genes Nutr. 2015, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Marques, J.G.; Shokry, E.; Frivolt, K.; Werkstetter, K.J.; Brückner, A.; Schwerd, T.; Koletzko, S.; Koletzko, B. Metabolomic Signatures in Pediatric Crohn’s Disease Patients with Mild or Quiescent Disease Treated with Partial Enteral Nutrition: A Feasibility Study. SLAS Technol. 2020, 26, 165–177. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef]
- Boneh, R.S.; Shabat, C.S.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary Therapy with the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohn’s Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Niseteo, T.; Sila, S.; Trivić, I.; Mišak, Z.; Kolaček, S.; Hojsak, I. Modified Crohn’s disease exclusion diet is equally effective as exclusive enteral nutrition: Real-world data. Nutr. Clin. Pract. 2021. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild to moderate Crohn’s disease: The CDED-AD prospective pilot trial. Lancet Gastroenterol. Hepatol. 2021, 7, 49–59. [Google Scholar] [CrossRef]
- Verburgt, C.M.; Dunn, K.A.; Ghiboub, M. Unpublished data. In Proceedings of the United European Gastroenterology Week, Virtual, 2–6 October 2021. [Google Scholar]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Suskind, D.L.; Lee, D.; Kim, Y.-M.; Wahbeh, G.; Singh, N.; Braly, K.; Nuding, M.; Nicora, C.D.; Purvine, S.O.; Lipton, M.S.; et al. The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn’s Disease: A Randomized Diet Controlled Trial. Nutrients 2020, 12, 3749. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B.; Forbes, B.A. Increased Proportions of Bifidobacterium and the Lactobacillus Group and Loss of Butyrate-Producing Bacteria in Inflammatory Bowel Disease. J. Clin. Microbiol. 2013, 52, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased e coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Walters, S.S.; Quiros, A.; Rolston, M.; Grishina, I.; Li, J.; Fenton, A.; DeSantis, T.Z.; Thai, A.; Andersen, G.L.; Papathakis, P.; et al. Analysis of Gut Microbiome and Diet Modification in Patients with Crohn’s Disease. SOJ Microbiol. Infect. Dis. 2014, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Britto, S.; Kellermayer, R. Carbohydrate Monotony as Protection and Treatment for Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 942–948. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, E.D.; Zheng, H.; Joglekar, P.; Higginbottom, S.K.; Firbank, S.J.; Bolam, D.N.; Sonnenburg, J.L. Specificity of Polysaccharide Use in Intestinal Bacteroides Species Determines Diet-Induced Microbiota Alterations. Cell 2010, 141, 1241–1252. [Google Scholar] [CrossRef] [Green Version]
- Papada, E.; Amerikanou, C.; Forbes, A.; Kaliora, A.C. Adherence to Mediterranean diet in Crohn’s disease. Eur. J. Nutr. 2019, 59, 1115–1121. [Google Scholar] [CrossRef]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Hanauer, S.B. Which Diet for Crohn’s Disease? Food for Thought on the Specific Carbohydrate Diet, Mediterranean Diet, and Beyond. Gastroenterology 2021, 161, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2020, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Nimptsch, K.; Wu, K.; Chan, A.T. High School Diet and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 2311–2319. [Google Scholar]
- Strisciuglio, C.; Cenni, S.; Serra, M.R.; Dolce, P.; Martinelli, M.; Staiano, A.; Miele, E. Effectiveness of Mediterranean Diet’s Adherence in children with Inflammatory Bowel Diseases. Nutrients 2020, 12, 3206. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Wilson, A.; Teft, W.A.; Morse, B.L.; Choi, Y.-H.; Woolsey, S.; DeGorter, M.K.; Hegele, R.A.; Tirona, R.G.; Kim, R.B. Trimethylamine-N-oxide: A Novel Biomarker for the Identification of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 3620–3630. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Xiao, Y.; Tian, L.; Cui, B.; Ji, G.; Liu, Y.-Y.; Zhang, F. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease. Appl. Microbiol. Biotechnol. 2018, 103, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; Macdonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [Green Version]
- Bodini, G.; Zanella, C.; Crespi, M.; Pumo, S.L.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A Diet Low in Red and Processed Meat Does Not Reduce Rate of Crohn’s Disease Flares. Gastroenterology 2019, 157, 128–136.e5. [Google Scholar] [CrossRef] [Green Version]
- Strisciuglio, C.; Giannetti, E.; Martinelli, M.; Sciorio, E.; Staiano, A.; Miele, E. Does cow’s milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis? Acta Paediatr. Int. J. Paediatr. 2013, 102, e273–e278. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.; Truelove, S.C. A Controlled Therapeutic Trial of Various Diets in Ulcerative Colitis. BMJ 1965, 2, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Sarbagili-Shabat, C.; Albenberg, L.; Van Limbergen, J.; Pressman, N.; Otley, A.; Yaakov, M.; Wine, E.; Weiner, D.; Levine, A. A Novel UC Exclusion Diet and Antibiotics for Treatment of Mild to Moderate Pediatric Ulcerative Colitis: A Prospective Open-Label Pilot Study. Nutrients 2021, 13, 3736. [Google Scholar] [CrossRef]
- Turrini, A. Food data quality in nutritional surveys: Which issues are to be tackled? J. Food Compos. Anal. 2000, 13, 597–609. [Google Scholar] [CrossRef]
- Verburgt, C.M.; Ghiboub, M.; Benninga, M.A.; De Jonge, W.J.; Van Limbergen, J.E. Nutritional Therapy Strategies in Pediatric Crohn’s Disease. Nutrients 2021, 13, 212. [Google Scholar] [CrossRef]
- Yu, T.; Yu, Q.; Chen, X.; Zhou, L.; Wang, Y.; Yu, C. Exclusive enteral nutrition protects against inflammatory bowel disease by inhibiting NF-κB activation through regulation of the p38/MSK1 pathway. Int. J. Mol. Med. 2018, 42, 1305–1316. [Google Scholar] [CrossRef]
- Zachos, M.; Tondeur, M.; Griffiths, A.M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2007, 1, CD000542. [Google Scholar] [CrossRef]
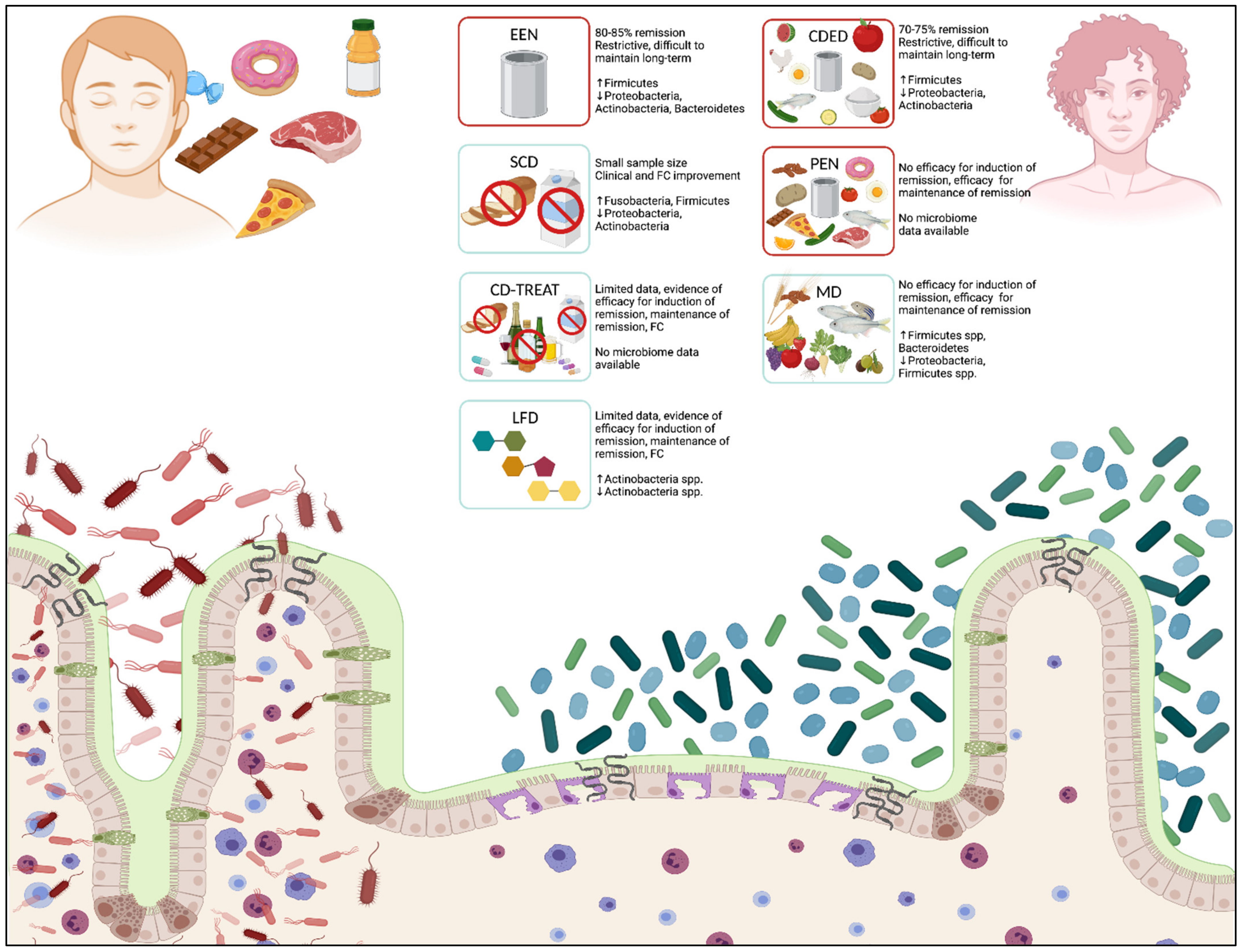
| Type of Diet | Taxa Increased | Taxa Decreased | Clinical Efficacy |
|---|---|---|---|
| EEN [52,65,77,79,81,82] | Firmicutes (p): Clostridiales (c), Erysipelotrichaceae (c), Veillonellaceae (f) | Proteobacteria (p) Actinobacteria (p): Bifidobacterium (g), Ruminococcus (g), Faecalibacterium (g), Faecalibacterium prausnitzii (s) Bacteroidetes (p): Bacteroides (g), Prevotella (g) | 80–85% remission rate Equivalent to CS for clinical remission Small studies show EEN superior to CS for mucosal healing |
| PEN | No data available | No data available | No evidence of efficacy for induction of remission The systematic review demonstrates efficacy for maintenance of remission |
| CDED [69,70] | Firmicutes (p): Clostridiales (c), Roseburia (g), Oscillibacter (g), Anaerotruncus (g), Ruminococcus (g) | Actinobacteria (p) Proteobacteria (p): Gammaproteobacteria (c) | 70–75% remission rate in children at week six 76% remission rate in children at week 12 |
| SCD [68] | Fusobacteria (p): Fusobacterium ulcerans (s) Firmicutes (p): Clostridiales (c), Eubacterium (g), Blautia (s), Lachnospiraceae (f), Roseburia (g), Anaerobutyricum (g), Faecalibacterium (g) | Proteobacteria (p): Escherichia coli (s) Actinobacteria (p): Faecalibacterium prausnitzii (s) | All patients showed clinical improvement and FC improvement SCD showed the greatest clinical improvement mSCD showed the greatest FC change Cannot conclude significance with sample size |
| CD-TREAT | No data available | No data available | 80% (four out of five) clinical improvement 60% (three out of five) clinical remission, improved FC |
| MD [83] | Firmicutes (p) Bacteroidetes (p) | Proteobacteria (p) Firmicutes (p): Bacillaceae (f) | No significant difference in clinical symptoms, FC values between MD and SCD |
| LFD [71,72] | Actinobacteria (p): Bifidobacterium dentium (s) | Actinobacteria (p): Faecalibacterium prausnitzii (s), Bifidobacterium longum (s), Bifidobacterium adolescentis (s) | 52% decrease in symptoms 34% decrease in FC |
| Name | Intervention | Country |
|---|---|---|
| The Intensive Post Exclusive Enteral Nutrition Study | CD-Treat | UK |
| Diet for Induction and Maintenance of Remission and Rebiosis in Crohn’s Disease | EEN, mEEN, PEN, CDED | Canada, Ireland, Israel, Spain, Netherlands |
| “Tasty & Healthy” Dietary Approach for Crohn’s Disease | Whole food diet | Israel |
| The Challenge Study: A Dietary Personalization Protocol for Patients with Crohn’s Disease and Deep Remission | CDED + milk fat and gluten | Israel |
| Exclusive Enteral Nutrition versus Infliximab in Chinese CD Patients | EEN | China |
| Biologics and Partial Enteral Nutrition Study | PEN | UK, Scotland |
| Adherence to Exclusive Enteral Nutrition in Patients with Crohn’s Disease | EEN | China |
| Based on the Special Disease Management of Crohn’s Disease Diet Studies | CD-C food | China |
| Diet in Paediatric Crohn’s Disease Treated with Biologics | CDED | Argentina |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, L.; Verburgt, C.M.; Wine, E.; Zachos, M.; Poppen, A.; Chavannes, M.; Van Limbergen, J.; Pai, N. Nutritional Therapies and Their Influence on the Intestinal Microbiome in Pediatric Inflammatory Bowel Disease. Nutrients 2022, 14, 4. https://doi.org/10.3390/nu14010004
Hart L, Verburgt CM, Wine E, Zachos M, Poppen A, Chavannes M, Van Limbergen J, Pai N. Nutritional Therapies and Their Influence on the Intestinal Microbiome in Pediatric Inflammatory Bowel Disease. Nutrients. 2022; 14(1):4. https://doi.org/10.3390/nu14010004
Chicago/Turabian StyleHart, Lara, Charlotte M. Verburgt, Eytan Wine, Mary Zachos, Alisha Poppen, Mallory Chavannes, Johan Van Limbergen, and Nikhil Pai. 2022. "Nutritional Therapies and Their Influence on the Intestinal Microbiome in Pediatric Inflammatory Bowel Disease" Nutrients 14, no. 1: 4. https://doi.org/10.3390/nu14010004






