Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Outcomes
2.5. Quality Assessment
2.6. Data Synthesis and Analysis
3. Results
3.1. Search Results
3.2. Study and Patient Characteristics
3.3. Meta-Analysis of the Effect of Magnesium on Glucose and Insulin-Sensitivity Parameters
3.3.1. People with Diabetes
3.3.2. People at High Risk of Diabetes
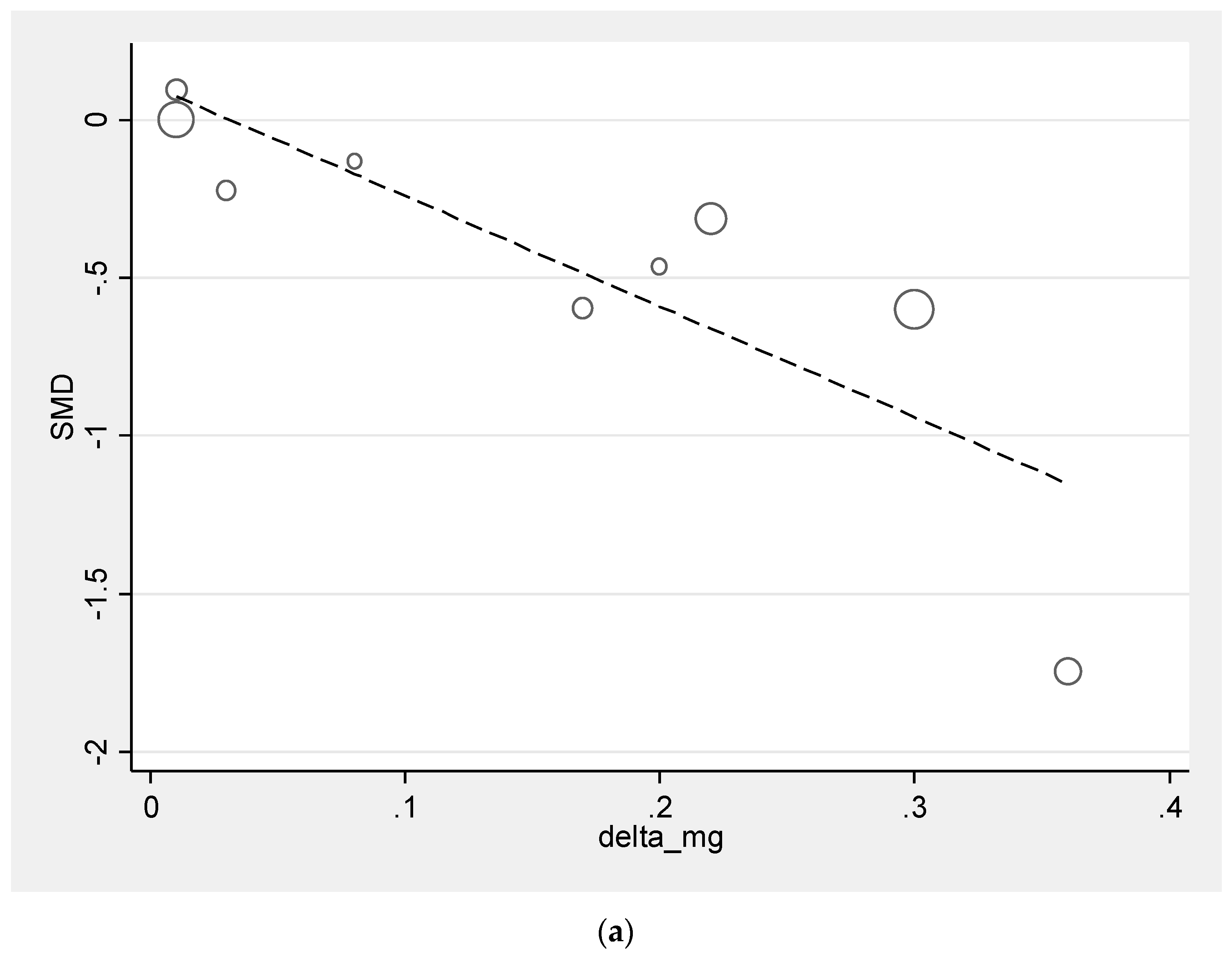
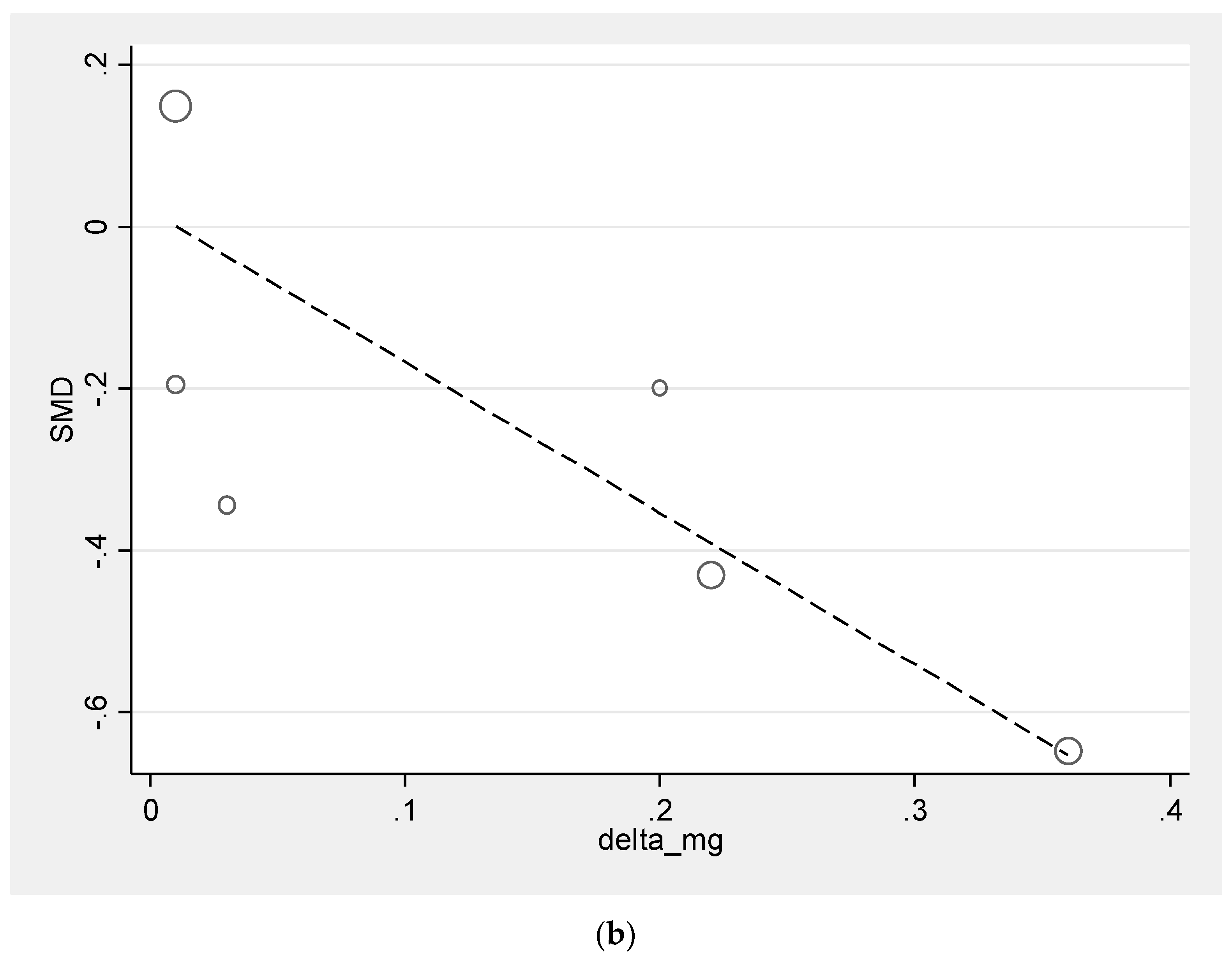
3.4. Meta-Regression Analysis
3.5. Compliance and Adverse Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Informed Consent Statement
Conflicts of Interest
References
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2019, 59, 263–272. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium Metabolism and its Disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Molnar, B.; Huhn, E.; Bodis, L. Magnesium substitution in pregnancy. A prospective, randomized double-blind study. Geburtshilfe und Frauenheilkunde 1988, 48, 595–600. [Google Scholar] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in Infectious Diseases in Older People. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Watutantrige-Fernando, S.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.-Y.; Xun, P.; He, K.; Qin, L.-Q. Magnesium intake and risk of type 2 diabetes: Meta-analysis of prospective cohort studies. Diabetes Care 2011, 34, 2116–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Song, Y.; He, K.; Levitan, E.; Manson, J.E.; Liu, S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet. Med. 2006, 23, 1050–1056. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Association, A.D. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadad, A.R. The merits of measuring the quality of clinical trials: Is it becoming a Byzantine discussion? Transpl. Int. 2009, 22, 1028. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Talari, H.R.; Zakizade, M.; Soleimani, A.; Bahmani, F.; Ghaderi, A.; Mirhosseini, N.; Eslahi, M.; Babadi, M.; Mansournia, M.A.; Asemi, Z. Effects of magnesium supplementation on carotid intima–media thickness and metabolic profiles in diabetic haemodialysis patients: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2019, 121, 809–817. [Google Scholar] [CrossRef]
- Asemi, Z.; Karamali, M.; Jamilian, M.; Foroozanfard, F.; Bahmani, F.; Heidarzadeh, Z.; Benisi-Kohansal, S.; Surkan, P.J.; Esmaillzadeh, A. Retracted: Magnesium supplementation affects metabolic status and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2015, 102, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamilian, M.; Samimi, M.; Faraneh, A.E.; Aghadavod, E.; Shahrzad, H.D.; Chamani, M.; Mafi, A.; Asemi, Z. Magnesium supplementation affects gene expression related to insulin and lipid in patients with gestational diabetes. Magnes. Res. 2017, 30, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Plat, J.; Bakker, S.J.L.; Mensink, R.P. Effects of long-term magnesium supplementation on endothelial function and cardiometabolic risk markers: A randomized controlled trial in overweight/obese adults. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Solini, A.; Seghieri, M.; Giannini, L.; Biancalana, E.; Parolini, F.; Rossi, C.; Dardano, A.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. The Effects of Dapagliflozin on Systemic and Renal Vascular Function Display an Epigenetic Signature. J. Clin. Endocrinol. Metab. 2019, 104, 4253–4263. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Azadbakht, L.; Khalili, N.; Mortazavi, M.; Esmaillzadeh, A. Oral Magnesium Supplementation Improved Lipid Profile but Increased Insulin Resistance in Patients with Diabetic Nephropathy: A Double-Blind Randomized Controlled Clinical Trial. Biol. Trace Element Res. 2019, 193, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.R.; D’El-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J. Hypertens. 2017, 35, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Park, H.; Son, S.; Lee, C.W.; Kim, I.; Kim, H. Effects of oral magnesium supplementation on insulin sensitivity and blood pressure in normo-magnesemic nondiabetic overweight Korean adults. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 781–788. [Google Scholar] [CrossRef]
- Chacko, S.A.; Sul, J.; Song, Y.; Li, X.; Leblanc, J.; You, Y.; Butch, A.; Liu, S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: A randomized, double-blind, controlled, crossover trial in overweight individuals. Am. J. Clin. Nutr. 2010, 93, 463–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.D.L.L.D.S.E.; Cruz, T.; Rodrigues, L.E.; Ladeia, A.M.; Bomfim, O.; Olivieri, L.; Melo, J.; Correia, R.; Porto, M.; Cedro, A. Magnesium Replacement Does Not Improve Insulin Resistance in Patients With Metabolic Syndrome: A 12-Week Randomized Double-Blind Study. J. Clin. Med. Res. 2014, 6, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Navarrete-Cortes, A.; Ble-Castillo, J.L.; Guerrero-Romero, F.; Cordova-Uscanga, R.; Rojop, I.E.J.; Aguilar-Mariscal, H.; Tovilla-Zárate, C.A.; Lopez-Guevara, M.D.R. No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes. Res. 2014, 27, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. Oral Magnesium Supplementation Decreases C-reactive Protein Levels in Subjects with Prediabetes and Hypomagnesemia: A Clinical Randomized Double-blind Placebo-controlled Trial. Arch. Med. Res. 2014, 45, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.; Hernández-Ronquillo, G.; Rodriguez-Morán, M. Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: A double-blind placebo-controlled randomized trial. Diabetes Metab. 2015, 41, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.D.L.; Cruz, T.; Pousada, J.C.; Rodrigues, L.E.; Barbosa, K.; Canguçu, V. The Effect of Magnesium Supplementation in Increasing Doses on the Control of Type 2 Diabetes. Diabetes Care 1998, 21, 682–686. [Google Scholar] [CrossRef] [Green Version]
- De Valk, H.; Verkaaik, R.; Van Rijn, H.; Geerdink, R.; Struyvenberg, A. Oral magnesium supplementation in insulin-requiring Type 2 diabetic patients. Diabet. Med. 1998, 15, 503–507. [Google Scholar] [CrossRef]
- Paolisso, G.; Scheen, A.; Cozzolino, D.; Di Maro, G.; Varricchio, M.; D’Onofrio, F.; Lefèbvre, P.J. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J. Clin. Endocrinol. Metab. 1994, 78, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Guerrero-Romero, F. Oral Magnesium Supplementation Improves Insulin Sensitivity and Metabolic Control in Type 2 Diabetic Subjects: A randomized double-blind controlled trial. Diabetes Care 2003, 26, 1147–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solati, M.; Ouspid, E.; Hosseini, S.; Soltani, N.; Keshavarz, M.; Dehghani, M. Oral magnesium supplementation in type II diabetic patients. Med. J. Islam. Repub. Iran 2014, 28, 67. [Google Scholar] [PubMed]
- Eibl, N.L.; Nowak, H.R.; Schnack, C.J.; Hopmeier, P.G.; Schernthaner, G.; Kopp, H.-P. Hypomagnesemia in Type II Diabetes: Effect of a 3-Month Replacement Therapy. Diabetes Care 1995, 18, 188–192. [Google Scholar] [CrossRef]
- Corica, F.; Allegra, A.; Di Benedetto, A.; Giacobbe, M.S.; Romano, G.; Cucinotta, D.; Buemi, M.; Ceruso, D. Effects of oral magnesium supplementation on plasma lipid concentrations in patients with non-insulin-dependent diabetes mellitus. Magnes. Res. 1994, 7, 43–47. [Google Scholar]
- Gullestad, L.; Jacobsen, T.; Dolva, L.O. Effect of Magnesium Treatment on Glycemic Control and Metabolic Parameters in NIDDM Patients. Diabetes Care 1994, 17, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Hägg, E.; Carlberg, B.C.; Hillörn, V.S.; Villumsen, J. Magnesium therapy in type 1 diabetes. A double blind study concerning the effects on kidney function and serum lipid levels. Magnes. Res. 1999, 12, 123–130. [Google Scholar] [PubMed]
- Purvis, J.R.; Cummings, D.M.; Landsman, P.; Carroll, R.; Barakat, H.; Bray, J.; Whitley, C.; Horner, R.D. Effect of Oral Magnesium Supplementation on Selected Cardiovascular Risk Factors in Non--Insulin-Dependent Diabetics. Arch. Fam. Med. 1994, 3, 503. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.; Krüger, K.; Völker, K.; Golf, S.; Wadepuhl, M.; Kraus, A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects–a double-blind, placebo-controlled, randomized trial. Diabetes Obes. Metab. 2011, 13, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Toprak, O.; Kurt, H.; Sarı, Y.; Şarkış, C.; Us, H.; Kırık, A. Magnesium Replacement Improves the Metabolic Profile in Obese and Pre-Diabetic Patients with Mild-to-Moderate Chronic Kidney Disease: A 3-Month, Randomised, Double-Blind, Placebo-Controlled Study. Obes. Surg. 2017, 42, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Karandish, M.; Jafarabadi, M.A.; Heidari, L.; Nikbakht, R.; Rezaei, H.B.; Mousavi, R. Metabolic and hormonal effects of melatonin and/or magnesium supplementation in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Nutr. Metab. 2021, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Farsinejad-Marj, M.; Azadbakht, L.; Mardanian, F.; Saneei, P.; Esmaillzadeh, A. Clinical and Metabolic Responses to Magnesium Supplementation in Women with Polycystic Ovary Syndrome. Biol. Trace Element Res. 2020, 196, 349–358. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Günther, T. The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes. Res. 2010, 23, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Doi, Y.; Ninomiya, T.; Mukai, N.; Hirakawa, Y.; Hata, J.; Ozawa, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Magnesium intake decreases Type 2 diabetes risk through the improvement of insulin resistance and inflammation: The Hisayama Study. Diabet. Med. 2013, 30, 1487–1494. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; dos Santos, L.R.; Melo, S.R.D.S.; Santos, R.D.O.; de Oliveira, A.R.S.; Cruz, K.J.C.; Marreiro, D.D.N. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Element Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Rayssiguier, Y.; Mazur, A. Magnesium and inflammation: Lessons from animal models. Clin. Calcium 2005, 15, 245–248. [Google Scholar] [PubMed]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Hamilton, K.P.; Zelig, R.; Parker, A.R.; Haggag, A. Insulin Resistance and Serum Magnesium Concentrations among Women with Polycystic Ovary Syndrome. Curr. Dev. Nutr. 2019, 3, nzz108. [Google Scholar] [CrossRef] [PubMed]
- Andretta, A.; Schieferdecker, M.E.M.; Petterle, R.R.; Paiva, E.D.S.; Boguszewski, C. Relations between serum magnesium and calcium levels and body composition and metabolic parameters in women with fibromyalgia. Adv. Rheumatol. 2020, 60, 18. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qian, Z.-Y.; Zhou, P.-H.; Zhou, X.-L.; Zhang, D.-L.; He, N.; Zhang, J.; Liu, Y.-H.; Gu, Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari, M.; Mozaffari, H.; Jafari, A.; Ghanbari, M.; Mofrad, M.D. The effects of magnesium supplementation on obesity measures in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 2921–2937. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Johnson, L.K. Magnesium (Mg) supplementation improves magnesium status and decreases elevated C-reactive protein in adults older than 51 years with poor quality sleep. FASEB J. 2010, 24, 325.8. [Google Scholar] [CrossRef]

| Diabetes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | Number of Studies | Number of Participants | Meta-Analysis | Heterogeneity (I2) | Publication Bias | |||||
| Mg | Placebo | SMD | 95% CI | p-Value | Egger’s Bias and p-Value | Trim and Fill (95%CI) | Classic Fail Safe N | |||
| FPG | 11 | 325 | 331 | −0.426 | −0.782; −0.07 | 0.02 | 79.0% | −5.84; p = 0.02 | Unchanged | 65 |
| HbA1c | 10 | 301 | 307 | −0.134 | −0.409; 0.141 | 0.34 | 63.7% | 5.02; p = 0.06 | −0.25 (−0.52; 0.03) [2 L] | 6 |
| Insulin | 4 | 153 | 149 | 0.596 | −0.576; 1.767 | 0.32 | 96.0% | −0.16; p = 0.99 | Unchanged | 4 |
| HOMA-IR | 4 | 153 | 149 | −0.169 | −0.656; 0.319 | 0.50 | 76.9% | −3.98; p = 0.63 | Unchanged | 4 |
| High risk of diabetes | ||||||||||
| FPG | 12 | 482 | 485 | −0.344 | −0.655; −0.03 | <0.0001 | 81.2% | 1.18; p = 0.63 | −0.565 (−0.860; −0.271) [4 L] | 71 |
| 2hOGTT | 3 | 105 | 105 | −0.35 | −0.62; −0.07 | 0.01 | 0% | 1.38; p = 0.15 | −0.41 (−0.64 to −0.18) [2 L] | 2 |
| HbA1c | 2 | 70 | 74 | −0.275 | −1.032; 0.481 | 0.48 | 69.3% | Only two studies | ||
| Insulin | 9 | 296 | 296 | −0.059 | −0.234; 0.116 | 0.51 | 11.0% | −2.17; p = 0.09 | Unchanged | 0 |
| HOMA-IR | 9 | 340 | 344 | −0.234 | −0.443; −0.025 | 0.028 | 43.2% | −0.57; p = 0.77 | Unchanged | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronese, N.; Dominguez, L.J.; Pizzol, D.; Demurtas, J.; Smith, L.; Barbagallo, M. Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2021, 13, 4074. https://doi.org/10.3390/nu13114074
Veronese N, Dominguez LJ, Pizzol D, Demurtas J, Smith L, Barbagallo M. Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients. 2021; 13(11):4074. https://doi.org/10.3390/nu13114074
Chicago/Turabian StyleVeronese, Nicola, Ligia J. Dominguez, Damiano Pizzol, Jacopo Demurtas, Lee Smith, and Mario Barbagallo. 2021. "Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials" Nutrients 13, no. 11: 4074. https://doi.org/10.3390/nu13114074






