Nutrition as Prevention Factor of Gestational Diabetes Mellitus: A Narrative Review
Abstract
:1. Introduction
2. Risk Factors for GDM
3. Materials and Methods
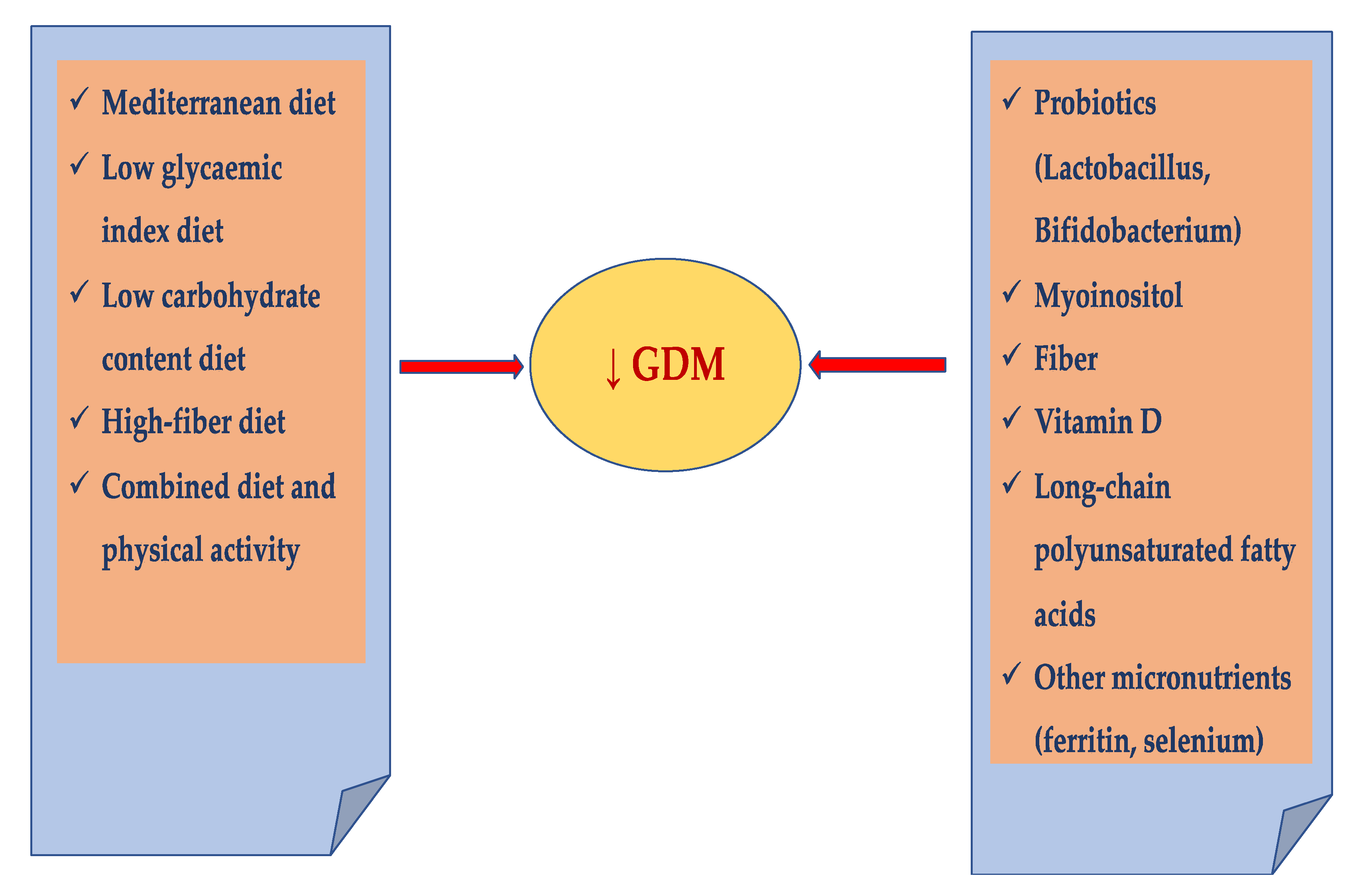
4. Prevention of GDM
4.1. Individualized Diet
Mediterranean Diet
4.2. Physical Activity
4.3. Combined Diet and Physical Activity
4.4. Probiotics
4.5. Myoinositol
4.6. Fiber
4.7. Vitamin D
4.8. Long-Chain Polyunsaturated Fatty Acids
4.9. Other Micronutrients
5. Conclusions and Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association Gestational Diabetes Mellitus. Diabetes Care 2003, 26, S103–S105. [CrossRef] [Green Version]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Agarwal, M.M.; Boulvain, M.; Coetzee, E.; Colagiuri, S.; Falavigna, M.; Hod, M.; Meltzer, S.; Metzger, B.; Omori, Y.; Rasa, I.; et al. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- Kanguru, L.; Bezawada, N.; Hussein, J.; Bell, J. The burden of diabetes mellitus during pregnancy in low- and middle-income countries: A systematic review. Glob. Health Action 2014, 7, 23987. [Google Scholar] [CrossRef] [Green Version]
- Gilmartin, A.B.; Ural, S.H.; Repke, J.T. Gestational diabetes mellitus. Rev. Obstet. Gynecol. 2008, 1, 129–134. [Google Scholar]
- Poomalar, G.K. Changing trends in management of gestational diabetes mellitus. World J. Diabetes 2015, 6, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Petry, C.J. Gestational diabetes: Risk factors and recent advances in its genetics and treatment. Br. J. Nutr. 2010, 104, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [Green Version]
- Blüher, M. Clinical Relevance of Adipokines. Diabetes Metab. J. 2012, 36, 317–327. [Google Scholar] [CrossRef] [Green Version]
- El Hajj, N.; Schneider, E.; Lehnen, H.; Haaf, T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 2014, 148, R111–R120. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D.; Ma, R.C.; Matzen, P.; Biesma, R.G. Early life opportunities for prevention of diabetes in low and middle income countries. BMC Public Health 2012, 12, 1025. [Google Scholar] [CrossRef] [Green Version]
- Graves, E.; Hill, D.J.; Evers, S.; Van Aarsen, K.; Yama, B.; Yuan, S.; Campbell, M.K. The Impact of Abnormal Glucose Tolerance and Obesity on Fetal Growth. J. Diabetes Res. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, S.; Dunger, D.B.; Norris, S. Gestational Diabetes Mellitus in Africa: A Systematic Review. PLoS ONE 2014, 9, e97871. [Google Scholar] [CrossRef] [PubMed]
- Kanthimathi, S.; Chidambaram, M.; Liju, S.; Bhavadharini, B.; Bodhini, D.; Prakash, V.G.; Amutha, A.; Bhavatharini, A.; Anjana, R.M.; Mohan, V.; et al. Identification of Genetic Variants of Gestational Diabetes in South Indians. Diabetes Technol. Ther. 2015, 17, 462–467. [Google Scholar] [CrossRef]
- Schwartz, N.; Nachum, Z.; Green, M.S. The prevalence of gestational diabetes mellitus recurrence—Effect of ethnicity and parity: A metaanalysis. Am. J. Obstet. Gynecol. 2015, 213, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Morisset, A.-S.; Veillette, J.; Weisnagel, S.J.; Tchernof, A.; Robitaille, J.; St-Yves, A. Prevention of gestational diabetes mellitus: A review of studies on weight management. Diabetes/Metab. Res. Rev. 2010, 26, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Elsea, S.; Romero, R.; Chaiworapongsa, T.; Francis, G.L. Prolactin Receptor Gene Polymorphisms Are Associated with Gestational Diabetes. Genet. Test. Mol. Biomark. 2013, 17, 567–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Cheong, H.S.; Park, B.-L.; Baik, S.H.; Park, S.; Lee, S.W.; Kim, M.-H.; Chung, J.H.; Choi, J.S.; Kim, M.-Y.; et al. Melatonin receptor 1 B polymorphisms associated with the risk of gestational diabetes mellitus. BMC Med. Genet. 2011, 12, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Badri, M.R.; Zantout, M.S.; Azar, S.T. The role of adipokines in gestational diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2015, 6, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mierzyński, R.; Poniedziałek-Czajkowska, E.; Dłuski, D.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Majsterek, M.; Leszczyńska-Gorzelak, B. Nesfatin-1 and Vaspin as Potential Novel Biomarkers for the Prediction and Early Diagnosis of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 159. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, M.; Sheikhan, F.; Arabipoor, A.; Hosseini, R.; Nourbakhsh, F.; Zolfaghari, Z. Gestational diabetes mellitus risk factors in women with polycystic ovary syndrome (PCOS). Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gracia, T.; Duran, A.; Fuentes, M.; Rubio, M.A.; Runkle, I.; Carrera, E.F.; Torrejón, M.J.; Bordiú, E.; del Valle, L.; de la Torre, N.G.; et al. Lifestyle patterns in early pregnancy linked to gestational diabetes mellitus diagnoses when using IADPSG criteria. The St Carlos gestational study. Clin. Nutr. 2016, 35, 699–705. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Silva-Zolezzi, I.; Samuel, T.M.; Spieldenner, J. Maternal nutrition: Opportunities in the prevention of gestational diabetes. Nutr. Rev. 2017, 75, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Donazar-Ezcurra, M.; Burgo, C.L.-D.; Bes-Rastrollo, M. Primary prevention of gestational diabetes mellitus through nutritional factors: A systematic review. BMC Pregnancy Childbirth 2017, 17, 30. [Google Scholar] [CrossRef] [Green Version]
- Glueck, C.; Wang, P.; Kobayashi, S.; Phillips, H.; Sieve-Smith, L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil. Steril. 2002, 77, 520–525. [Google Scholar] [CrossRef]
- Syngelaki, A.; Nicolaides, K.H.; Balani, J.; Hyer, S.; Akolekar, R.; Kotecha, R.; Pastides, A.; Shehata, H. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N. Engl. J. Med. 2016, 374, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Dornhorst, A.; Frost, G. The principles of dietary management of gestational diabetes: Reflection on current evidence. J. Hum. Nutr. Diet. 2002, 15, 145–156. [Google Scholar] [CrossRef]
- Bowers, K.; Tobias, D.K.; Yeung, E.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am. J. Clin. Nutr. 2012, 95, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hu, F.B.; Yeung, E.; Willett, W.; Zhang, C. Prospective Study of Pre-Gravid Sugar-Sweetened Beverage Consumption and the Risk of Gestational Diabetes Mellitus. Diabetes Care 2009, 32, 2236–2241. [Google Scholar] [CrossRef] [Green Version]
- Hamer, M.; Chida, Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: Systematic review and meta-analysis. J. Hypertens. 2007, 25, 2361–2369. [Google Scholar] [CrossRef]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.B.; Zhang, C. Prepregnancy Dietary Protein Intake, Major Dietary Protein Sources, and the Risk of Gestational Diabetes Mellitus. Diabetes Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Middleton, P.; Crowther, C.A. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2012, 7, CD009021. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, S.B.; Rönö, K.; Klemetti, M.; Roine, R.P.; Lindström, J.; Erkkola, M.; Kaaja, R.J.; Pöyhönen-Alho, M.; Tiitinen, A.; Huvinen, H.; et al. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL). Diabetes Care 2015, 39, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Markovic, T.P.; Muirhead, R.; Overs, S.; Ross, G.P.; Louie, J.C.Y.; Kizirian, N.; Denyer, G.; Petocz, P.; Hyett, J.; Brand-Miller, J.C. Randomized Controlled Trial Investigating the Effects of a Low–Glycemic Index Diet on Pregnancy Outcomes in Women at High Risk of Gestational Diabetes Mellitus: The GI Baby 3 Study. Diabetes Care 2015, 39, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, F.; Dante, G.; Petrella, E.; Neri, I. Dietary Interventions, Lifestyle Changes, and Dietary Supplements in Preventing Gestational Diabetes Mellitus. Obstet. Gynecol. Surv. 2014, 69, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Committee on Practice Bulletins—Obstetrics ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [CrossRef] [PubMed]
- Fraser, R.B.; Ford, F.A.; Lawrence, G.F. Insulin sensitivity in third trimester pregnancy. A randomized study of dietary effects. BJOG: Int. J. Obstet. Gynaecol. 1988, 95, 223–229. [Google Scholar] [CrossRef]
- Griffith, R.J.; Alsweiler, J.; Moore, A.E.; Brown, S.; Middleton, P.; Shepherd, E.; Crowther, C.A. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 2020, CD012394. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, L.A. Unresolved controversies in gestational diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L.; Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines (Eds.) Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press (US): Washington, DC, USA, 2009; PMID: 20669500. [Google Scholar]
- apur, K.; Kapur, A.; Hod, M. Nutrition Management of Gestational Diabetes Mellitus. Ann. Nutr. Metab. 2021, 76, 1–13. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; De La Torre, N.G.; Calle-Pascual, A.L.; Durán, A.; Jiménez, I.; Rubio, M.; Herraíz, M.; Izquierdo, N.; Pérez, N.; Garcia, A.S.; et al. Detection, treatment and prevention programs for gestational diabetes mellitus: The St Carlos experience. Endocrinol. Diabetes Nutr. 2019, 67, 342–350. [Google Scholar] [CrossRef]
- Olmedo-Requena, R.; Gómez-Fernández, J.; Amezcua-Prieto, C.; Mozas-Moreno, J.; Khan, K.S.; Jiménez-Moleón, J.J. Pre-Pregnancy Adherence to the Mediterranean Diet and Gestational Diabetes Mellitus: A Case-Control Study. Nutrients 2019, 11, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melero, V.; De La Torre, N.G.; Assaf-Balut, C.; Jimenez, I.; Del Valle, L.; Durán, A.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; Izquierdo, N.; et al. Effect of a Mediterranean Diet-Based Nutritional Intervention on the Risk Incidence of Developing Gestational Diabetes Mellitus and other Maternal-Fetal Adverse Events in Hispanic Women Residents in Spain. Nutrients 2020, 12, 3505. [Google Scholar] [CrossRef]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of Dietary Proteins and Amino Acids in the Pathogenesis of Insulin Resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Assaf-Balut, C.; De La Torre, N.G.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, N.G.; Assaf-Balut, C.; Varas, I.J.; Del Valle, L.; Durán, A.; Fuentes, M.; Del Prado, N.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; et al. Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos Study. Nutrients 2019, 11, 1210. [Google Scholar] [CrossRef] [Green Version]
- Rogozińska, E.; Chamillard, M.; Hitman, G.A.; Khan, K.S.; Thangaratinam, S. Nutritional Manipulation for the Primary Prevention of Gestational Diabetes Mellitus: A Meta-Analysis of Randomised Studies. PLoS ONE 2015, 10, e0115526. [Google Scholar] [CrossRef] [Green Version]
- Dodd, J.M.; Turnbull, D.; McPhee, A.J.; Deussen, A.R.; Grivell, R.; Yelland, L.N.; Crowther, C.A.; Wittert, G.; Owens, J.A.; Robinson, J.S.; et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014, 348, g1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffries, K.; Shub, A.; Walker, S.P.; Hiscock, R.; Permezel, M. Reducing excessive weight gain in pregnancy: A randomised controlled trial. Med J. Aust. 2009, 191, 429–433. [Google Scholar] [CrossRef]
- Tieu, J.; Shepherd, E.; Middleton, P.; Crowther, C.A. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2017, 2017, CD006674. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic Association; Nutrition, A.S.O.; Siega-Riz, A.M.; King, J.C. Position of the American Dietetic Association and American Society for Nutrition: Obesity, Reproduction, and Pregnancy Outcomes. J. Am. Diet. Assoc. 2009, 109, 918–927. [Google Scholar] [CrossRef]
- Russo, L.; Nobles, C.; Ertel, K.A.; Chasan-Taber, L.; Whitcomb, B.W. Physical Activity Interventions in Pregnancy and Risk of Gestational Diabetes Mellitus. Obstet. Gynecol. 2015, 125, 576–582. [Google Scholar] [CrossRef]
- Sanabriamartinez, G.; Garcia-Hermoso, A.; Poyatosleon, R.; Alvarez-Bueno, C.; López, M.S.; Vizcaino, V.M. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Crowther, C.A.; Middleton, P.; Heatley, E. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst. Rev. 2013, 3, CD009275. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Duda, W.; Borowiack, E.; Roseboom, T.; Tomlinson, J.; Walczak, J.; Kunz, R.; et al. Interventions to reduce or prevent obesity in pregnant women: A systematic review. Health Technol. Assess. 2012, 16, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, E.; Crane, M.; Tieu, J.; Han, S.; Crowther, C.A.; Middleton, P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2015, 4, CD010443. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homayouni, A.; Bagheri, N.; Mohammad-Alizadeh-Charandabi, S.; Kashani, N.; Mobaraki-Asl, N.; Mirghafurvand, M.; Asgharian, H.; Ansari, F.; Pourjafar, H. Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr. Diabetes Rev. 2020, 16, 538–545. [Google Scholar] [CrossRef]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-blind Randomized Controlled Trial. Diabetes Care 2019, 42, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.-R.; Wu, T.-W.; Chao, Y.-C. Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials. Medicina 2018, 54, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 496.e1–496.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, Y.; Li, Z.; Sun, L.; Guo, W. The efficacy of myo-inositol supplementation to prevent gestational diabetes onset: A meta-analysis of randomized controlled trials. J. Matern. Neonatal Med. 2018, 32, 2249–2255. [Google Scholar] [CrossRef]
- Matarrelli, B.; Vitacolonna, E.; D’Angelo, M.; Pavone, G.; Mattei, P.A.; Liberati, M.; Celentano, C. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: A randomized controlled trial. J. Matern. Neonatal Med. 2013, 26, 967–972. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women. Obstet. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Corrado, F.; D’Anna, R.; Di Vieste, G.; Giordano, D.; Pintaudi, B.; Santamaria, A.; Di Benedetto, A. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet. Med. 2011, 28, 972–975. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, R.; Scilipoti, A.; Giordano, D.; Caruso, C.; Cannata, M.L.; Interdonato, M.L.; Corrado, F.; Di Benedetto, A. myo-Inositol Supplementation and Onset of Gestational Diabetes Mellitus in Pregnant Women with a Family History of Type 2 Diabetes: A prospective, randomized, placebo-controlled study. Diabetes Care 2013, 36, 854–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agha-Jaffar, R.; Oliver, N.; Johnston, R.A.-J.N.O.D.; Robinson, S. Gestational diabetes mellitus: Does an effective prevention strategy exist? Nat. Rev. Endocrinol. 2016, 12, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J.-P.; Iuorno, M.J.; Apridonidze, T.; Nestler, J.E. Uncoupling Between Insulin and Release of ad-Chiro-Inositol–Containing Inositolphosphoglycan Mediator of Insulin Action in Obese Women With Polycystic Ovary Syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary Fiber Intake, Dietary Glycemic Load, and the Risk for Gestational Diabetes Mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gong, Y.; Della Corte, K.; Yu, D.; Xue, H.; Shan, S.; Tian, G.; Liang, Y.; Zhang, J.; He, F.; et al. Relevance of dietary glycemic index, glycemic load and fiber intake before and during pregnancy for the risk of gestational diabetes mellitus and maternal glucose homeostasis. Clin. Nutr. 2021, 40, 2791–2799. [Google Scholar] [CrossRef]
- McIntosh, M.; Miller, C. A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr. Rev. 2001, 59, 52–55. [Google Scholar] [CrossRef]
- Lu, M.; Xu, Y.; Lv, L.; Zhang, M. Association between vitamin D status and the risk of gestational diabetes mellitus: A meta-analysis. Arch. Gynecol. Obstet. 2016, 293, 959–966. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Pan, G.-T.; Guo, J.-F.; Li, B.-Y.; Qin, L.-Q.; Zhang, Z.-L. Vitamin D Deficiency Increases the Risk of Gestational Diabetes Mellitus: A Meta-Analysis of Observational Studies. Nutrients 2015, 7, 8366–8375. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Song, Y.; Bertrand, K.A.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.E.; Mills, J.L.; Hu, F.B.; Zhang, C. Prepregnancy habitual intake of vitamin D from diet and supplements in relation to risk of gestational diabetes mellitus: A prospective cohort study. J. Diabetes 2017, 10, 373–379. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2016, CD008873. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, F.R.; Pasupuleti, V.; Mezones-Holguin, E.; Zapata, V.A.B.; Thota, P.; Deshpande, A.; Hernandez, A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2015, 103, 1278–1288.e4. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Ashraf, A. Role of Vitamin D in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int. J. Endocrinol. 2009, 2010, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Radesky, J.S.; Oken, E.; Rifas-Shiman, S.L.; Kleinman, K.P.; Rich-Edwards, J.W.; Gillman, M.W. Diet during early pregnancy and development of gestational diabetes. Paediatr. Périnat. Epidemiol. 2007, 22, 47–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szajewska, H.; Horvath, A.; Koletzko, B. Effect of n−3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006, 83, 1337–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, S.; Menato, G.; Villois, P.; Gambino, R.; Cassader, M.; Cotrino, I.; Cavallo-Perin, P. Iron supplementation and gestational diabetes in midpregnancy. Am. J. Obstet. Gynecol. 2009, 201, 158.e1–158.e6. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, F.; Zhou, J.; Liu, Z. The Relationship Between Body Iron Status, Iron Intake and Gestational Diabetes. Medicine 2016, 95, e2383. [Google Scholar] [CrossRef]
- Tan, M.; Sheng, L.; Qian, Y.; Ge, Y.; Wang, Y.; Zhang, H.; Jiang, M.; Zhang, G. Changes of Serum Selenium in Pregnant Women with Gestational Diabetes Mellitus. Biol. Trace Element Res. 2001, 83, 231–237. [Google Scholar] [CrossRef]
- Askari, G.; Iraj, B.; Salehi-Abargouei, A.; Fallah, A.A.; Jafari, T. The association between serum selenium and gestational diabetes mellitus: A systematic review and meta-analysis. J. Trace Elements Med. Biol. 2015, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierzyński, R.; Poniedziałek-Czajkowska, E.; Sotowski, M.; Szydełko-Gorzkowicz, M. Nutrition as Prevention Factor of Gestational Diabetes Mellitus: A Narrative Review. Nutrients 2021, 13, 3787. https://doi.org/10.3390/nu13113787
Mierzyński R, Poniedziałek-Czajkowska E, Sotowski M, Szydełko-Gorzkowicz M. Nutrition as Prevention Factor of Gestational Diabetes Mellitus: A Narrative Review. Nutrients. 2021; 13(11):3787. https://doi.org/10.3390/nu13113787
Chicago/Turabian StyleMierzyński, Radzisław, Elżbieta Poniedziałek-Czajkowska, Maciej Sotowski, and Magdalena Szydełko-Gorzkowicz. 2021. "Nutrition as Prevention Factor of Gestational Diabetes Mellitus: A Narrative Review" Nutrients 13, no. 11: 3787. https://doi.org/10.3390/nu13113787





