Abstract
Quantitative assessments of the health risk of the constituents of alcoholic beverages including ethanol are reported in the literature, generally with hepatotoxic effects considered as the endpoint. Risk assessment studies on minor compounds such as mycotoxins, metals, and other contaminants are also available on carcinogenicity as the endpoint. This review seeks to highlight population cancer risks due to alcohol consumption using the margin of exposure methodology. The individual and cumulative health risk contribution of each component in alcoholic beverages is highlighted. Overall, the results obtained consistently show that the ethanol contributes the bulk of harmful effects of alcoholic beverages, while all other compounds only contribute in a minor fashion (less than 1% compared to ethanol). Our data provide compelling evidence that policy should be focused on reducing total alcohol intake (recorded and unrecorded), while measures on other compounds should be only secondary to this goal.
1. Introduction
The epidemiological association of alcoholic beverages with cancer remains a topic that has continued to attract global attention for over a century with the first documented cases, cancer of the esophagus, being reported in 1910 [1,2]. Later in 1988, the World Health Organization (WHO)/International Agency for Research on Cancer (IARC) classified “alcohol drinking” as carcinogenic to humans (group 1) after establishing a causal link between alcohol use and malignancies of the oral pharynx, esophagus, and liver [1]. The promoters or causative factors in alcoholic beverages for developing carcinogenic lesions are a matter of continuing debate among scientists. However, alcohol being a multicomponent mixture, the potential contribution of each or all the compounds to carcinogenesis should not be overlooked. These substances occur as residues, contaminants, or even adulterants, in addition to being naturally occurring in either raw materials or fermentation by-products.
Ethanol, the principal component of alcoholic beverages, is classified as a human carcinogen (group 1) by IARC. Other than ethanol, other IARC-classified carcinogenic compounds such as acetaldehyde, formaldehyde, acrylamide, aflatoxins, ochratoxin A, arsenic, lead, cadmium, ethyl carbamate, furan, safrole, 4-methylimidazole, N-nitrosodimethylamine (NDMA), 3-Monochloropropane-1,2-diol (3-MCPD), and benzene have occurred in alcoholic beverages. The contribution of these compounds to cancer is either synergistic or independent of each other. Understanding the contribution of each component is important in disentangling the mechanisms of carcinogenicity due to alcohol and ultimately aids in alcohol control policies. Nevertheless, epidemiological research has reported that only ethanol achieves the requisite threshold to explain the carcinogenic risk of alcoholic beverages. This review seeks to highlight population cancer risks due to alcohol consumption using the margin of exposure (MOE) approach with emphasis on the cancer-risk contribution of individual components of alcoholic beverages. This review identifies ethanol as the main oncogenic component in alcoholic beverages and lays emphasis on the need for policy geared towards the reduction in drinking per se and not target on other minor carcinogens that may require strict implementation of industry best practices, i.e., as low as reasonably achievable (ALARA) guidelines and good manufacturing practices.
2. The Margin of Exposure Method and Its Application to Alcoholic Beverages
Despite there being other methods for evaluating the health risks associated with alcohol intake, the margin of exposure (MOE) method is recommended for comparing the risks of different alcoholic beverage components [1]. MOE compares exposure levels to a toxicological threshold. The toxicological thresholds are derived from the dose–response evaluations for both carcinogens and non-carcinogens.
The ratio between the benchmark dose’s lower one-sided confidence limit (BMDL) and predicted human consumption/exposure of the same substance is known as the margin of exposure. MOE is typically used to compare the health risks of various chemicals and, as a result, to prioritize risk management efforts. The lower the MOE, the greater the risk to people; typically, a value of less than 10,000 is used to indicate health risk.
The benchmark dose (BMD) is the dose of a chemical that, based on the dose–response modeling, causes a specified change in the response rate (benchmark response) of an undesirable impact compared to the background. The benchmark response is typically suggested to be set near the lower limit of what can be measured (e.g., for animal experiment in the 1–10% range). BMD–response modeling results can then be used with exposure data to create a MOE for quantitative risk assessment. No observed effect level (NOEL) or no observed adverse effect level (NOAEL) values can be used as surrogate thresholds where BMDL values are unavailable in the literature. Consequently, the MOEs can be determined by dividing the NO(A)EL by estimated human intake [1].
The human intakes for each beverage group (i.e., beer, wine, spirits, and unrecorded alcohol) for various drinking scenarios (e.g., low risk drinking and heavy drinking) can be based on drinking guidelines such as the Canadian ones, which consider 13.6 g of pure alcohol a standard drink [1]. MOEs for average and maximum contamination with the various substances can also be determined for both drinking scenarios to give a range for average and worst-case contamination scenarios [1].
The most recent detailed IARC reviews were suggested to be used to select compounds and their levels in alcoholic beverages. For the established and probable human carcinogens, toxicological endpoints and BMD are primarily based on literature data [1]. Suitable risk assessment studies, including endpoints and dose–response modeling results, were typically identified in monographs published by national and international risk assessment bodies such as the United States Environmental Protection Agency (US EPA), the World Health Organization International Programme on Chemical Safety (WHO-IPCS), the Joint FAO/WHO Expert Committee on Food Additives (JECFA), and the European Food Safety Authority (EFSA). Data from peer-reviewed scientific research can be used for compounds without accessible monographs or those with missing data on dose–response modeling findings [1].
3. Occurrence of Carcinogenic Compounds in Alcoholic Beverages
Ethanol and acetaldehyde (ethanal), both categorized by IARC as group 1 carcinogens, are the primary carcinogens occurring in alcoholic beverages accounting for approximately 5.5% of all cancer cases worldwide [3]. Although the inherent cancer risk of alcoholic beverages parallels consumption volumes, even light alcohol drinking has been associated with cancer with ethanol and acetaldehyde being central to the pathogenicity. At the molecular level, ethanol and acetaldehyde are postulated to cause cancer in similar mechanistic fashion, since acetaldehyde, a genotoxic compound, is a metabolite of ethanol resulting from the alcohol dehydrogenase or CYP 450 E1 pathways. Since ethanol and acetaldehyde have similar carcinogenesis mechanisms, the computation of cancer risk can be be undertaken cumulatively. Additionally, ethanol plays a promoting role in oncogenesis by solvating other carcinogens [1].
Besides metabolism, acetaldehyde occurs naturally, albeit in small amounts in alcoholic beverages with the highest contents reported to be in fortified wines (118 mg/L) and some spirit drinks (66 mg/L) [4]. Additionally, acetaldehyde occurs at high levels in certain unrecorded alcohols [5]. The average daily acetaldehyde exposure from alcoholic beverages has been calculated to be 0.112 mg/kg body weight, with a MOE of 498 [5].
IARC has classified formaldehyde (methanal), a naturally occurring substance found in various plants, mainly fruits and vegetables, and animal products such as meat, dairy products, and fish [1], as a group 1 carcinogen [6]. Formaldehyde is a carcinogen linked to the development of leukemia and naso-pharyngeal cancer in humans. Alcoholic beverages contain a substantial quantity of formaldehyde [7]. In a sampling of 500 beverages including wine, beer, spirits, and unrecorded alcohol, lower formaldehyde contamination (1.8 percent) was found, which was however more than the WHO IPCS permissible concentrations [7]. To surpass the daily US EPA reference dose (RfD) of 0.2 mg/kg bodyweight [8], a person weighing 60 kg would need to partake daily 800 mL of alcohol containing 14.37 mg/L formaldehyde. Even in the worst-case scenario, this level of exposure is exceedingly unlikely.
Acrylamide, considered by IARC as probably carcinogenic, may produce cancer through its metabolite, glycidamide, that forms DNA adducts [9]. Nevertheless, there are only a few reports on the occurrence of acrylamide in alcoholic beverages with one study reporting acrylamide levels of 22 µg/kg [10]. The group 2B carcinogen, 3-monochloropropane-1,2-diol, is a heat-induced contaminant resulting from the thermal processing of malt [11]. In experimental animals, 3-MCPD causes renal tubule adenocarcinomas. Although 3-MCPD is detected in some dark specialty malts used for beer production [11,12,13], it only occurs in low levels in most beers. It typically ranges from <10 μg/L to 14 µg/L [14,15].
IARC has classified the mycotoxins, aflatoxin B1 and ochratoxin A, found in some alcoholic beverages as carcinogenic to humans (group 1) and possibly carcinogenic to humans (group 2B), respectively [16]. Aflatoxin B1, as well as other aflatoxins (B2, G1, and G2), is a naturally occurring toxin in barley, corn, and sorghum malts that enters beer due to the use of contaminated cereals [17,18,19]. The occurrence of aflatoxins is climate-related with aflatoxins thriving in warm climates, especially in the tropics. Indeed, higher contamination of beer is reported to be in warm climatic countries such as South Africa, India, Mexico, and Kenya, among others [19,20]. Aflatoxin B1 has been found in the greatest concentrations (up to 6.8 µg/L) in artisanal beers from Kenya [20,21]. Similarly, ochratoxin A (OTA) occurs as a contaminant in grapes and in raw materials for beer, such as barley, malt, or cereal derivatives. Unlike aflatoxin B1, OTA is partially detoxified during fermentation [22], and its concentration remains unchanged in wine for one year [23].
Among heavy metals, arsenic, cadmium, and lead are possibly the ones of carcinogenic concern. The IARC classifies metalloid arsenic and inorganic arsenic compounds as group 1 carcinogens [24]. Lung, skin, liver, kidney, prostate, and urinary bladder malignancies have all been linked to inorganic arsenic compounds [24]. The reported levels of arsenic in beer are 0–102.4 µg/L [24], while those in spirits and wines are 0–27 and 0–14.6 µg/L, respectively [25]. The IARC designated cadmium as a group 1 carcinogenic agent because it causes cancers of the lungs, kidneys, and prostate [26]. According to an EFSA report [25], the amount of arsenic in various beverages varies. Fortified and liqueur wines had a Cd concentration of 0.5 µg/L, whereas liqueur had a level of 6.0 µg/L. The average concentration of Cd in wines and beers is 1.2 and 1.8 µg/L, respectively [25]. Organic lead compounds are “not classifiable as to their carcinogenicity to humans” (group 3) [27], whereas inorganic lead and lead compounds are “probably carcinogenic” (group 2A) [28]. The concentrations of lead vary across alcohol types. The average content of Pb in wines is 29 µg/kg with no significant differences in the amounts between the red and white varieties. Beer and beer-like beverages contain 12 µg/kg Pb on average [29].
Benzene, a heat-induced contaminant, is classified as a group I carcinogen, and it arises in alcoholic beverages. Benzene is a genotoxic compound that targets pluripotent hematopoietic stem cells leading to a raft of chromosomal aberrations [30]. The compound can occur in soft beverages that contain benzoic acid (a preservative) [31,32,33] or in beers manufactured with benzene-contaminated industrial carbon dioxide [34,35].
Furan, a group 2B carcinogen [36], is touted to intercalate with DNA via its cytochrome P-450-mediated metabolite, cis-2-butene-1,4-dial [37,38] leading to carcinogenesis. Furan has been found in beer samples at amounts as high as 28 µg/kg. Lower furan concentrations have been reported in wines and liqueurs, 6.5 and 28 µg/kg, respectively [39].
In 2015, IARC classified the controversial herbicide glyphosate as “probably carcinogenic to humans” based on some evidence in humans due to a correlation with non-Hodgkin lymphoma and significant evidence for glyphosate’s carcinogenicity in experimental animals [40]. In 2013, Nagatomi et al. observed that glyphosate content in 15 commercial canned beers from Japan was below the limit of quantitation (10 µg/L) [41]. From a risk assessment standpoint, these observed amounts are unlikely to cause harm.
Ethyl carbamate, a probable human carcinogen (group 2A) [42], has been found in small concentrations in wines and beers (in µg/L) [43] and in larger proportions in stone-fruit spirits (in mg/L) [43]. Another group 2A carcinogenic compound, N-nitrosodimethylamine (NDMA), is hepatotoxic [27]. Ethanol through its solvation effect or via alteration of cellular metabolism and suppression of DNA repair, enhances the carcinogenicity of NDMA [44]. NDMA in alcoholic beverages can arise from the manufacturing processes or from storage. During the production process, N-nitroso compounds can emerge by activities such as when malt is directly heated or when polluted water is used, or when foods and beverages are stored [45,46]. In a follow-up screening of German beers conducted between 1992 and 2006, NDMA was found in 29 malt samples (43%) and 81 beer samples (7%), with only 4% of the beer samples (n = 1242) having concentrations above the technical threshold value [47].
Pulegone, a component of essential oil-containing plants of the mint family, is found in mint-flavored alcoholic beverages [48]. Pulegone has been linked to liver and bladder cancer in animal models, prompting the IARC to classify it as probably carcinogenic to humans (group 2B) [48]. Despite being recognized as a potential carcinogen, occurrence data on pulegone are still scanty with only the National Toxicology Programme (NTP) reporting a mean value of 10.5 µg/L [49].
Safrole, a substituted benzodioxole, is a genotoxic agent that naturally occurs in several spices such as sweet basil, black pepper, cinnamon nutmeg, mace, cinnamon, and aniseed. Moreover, safrole occurs in food and beverages that are flavored with it. The IARC categorizes safrole as “possibly carcinogenic to humans” (group 2B) [27]. Since safrole occurs in cola drinks [50], it has the potential to occur in alcoholic beverages [51] especially admixtures of cola and alcohol. On average, humans consume 0.3 mg of safrole per day, with the 97.5th percentile consuming 0.5 mg. The presence of possibly carcinogenic compounds in alcoholic beverages is summarized in Table 1.

Table 1.
Summary of the occurrence of potentially carcinogenic compounds in alcoholic beverages (reprinted with modifications with permission from Springer Nature, Archives of Toxicology, Pflaum et al. [1], copyright 2016).
4. Comparative Risk Assessment of Compounds in Alcoholic Beverages
The presence of a carcinogenic compound in an alcoholic beverage does not directly impute an inherent risk of consumers of the drink. However, the quantitative risk assessment serves to ascribe harm due to a compound if it exceeds the toxicological threshold. The margin of exposure (MOE) methodology as described in the literature is applicable to conduct a comparative risk assessment for compounds in alcoholic beverages [1,5,60,61,62]. Where human data were unavailable, animal data were used instead for risk assessment. Moreover, non-cancer endpoints were chosen for substances such as Pb where there was no dose–response modeling data for cancer effects available. However, non-cancer endpoints may be more sensitive than cancer endpoints. Additionally, the most sensitive endpoint was chosen if dose–response data for several organ sites were available. Table 2 lists the toxicological endpoints and points of departure used in dose–response modeling and risk assessment.

Table 2.
Dose–response modeling for potential human carcinogens occurring in alcoholic beverages (reprinted with permission from Springer Nature, Archives of Toxicology, Pflaum et al. [1] copyright 2016).
For daily consumption of four standard alcoholic drinks, MOEs were calculated for the average and worst-case scenarios. For ethanol, the lowest MOE was achieved (0.8). Inorganic lead and arsenic showed MOEs ranging from 10 to 300, while acetaldehyde, cadmium, ethyl carbamate, and pulegone had MOEs ranging from 1000 to 10,000. Safrole, ochratoxin A, NDMA, 4-methylimidazole, 3-MCPD, glyphosate, furan, formaldehyde, and acrylamide had average MOEs exceeding 10,000 even in these extreme contexts such as binge drinking (Figure 1). However, the MOE for aflatoxin B1 from Kenyan artisanal beer that was significantly tainted ranged from 15 to 58 with a mean of 36. As a result, ethanol is the most significant carcinogen found in alcoholic beverages, with a clear dose–response relationship. Other contaminants (lead, arsenic, ethyl carbamate, acetaldehyde) may pose risks below those tolerated for food contaminants, but from a cost-effectiveness standpoint, the focus should be on reducing alcohol consumption in general rather than on mitigative actions for some contaminants that contribute only a small (if any) portion of the total health risk. This review again highlights the fact that ethanol remains the compound with the highest carcinogenic potential that is present in alcoholic beverages. This finding is consistent with other studies reported in the literature [1,21,60,62]. Aflatoxin B1 also emerged as a compound of interest in unrecorded artisanal beers that clearly requires attention in the warm climatic countries where the consumption of such beers is prevalent [20,21]. Figure 1 shows the comparative MOEs for carcinogens.
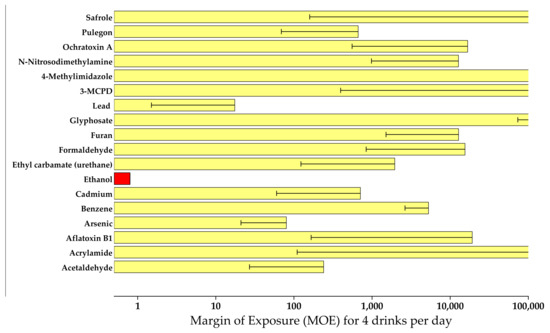
Figure 1.
Comparative MOEs for IARC-classified carcinogens in alcoholic beverages (reprinted with permission from Springer Nature, Archives of Toxicology, Pflaum et al. [1], copyright 2016).
5. Overall Toxic Effects of Alcoholic Beverages
According to studies, no amount of alcohol use promotes health [94]. Alcohol consumption significantly contributes to death, disability, and ill health worldwide [94,95,96]. Alcohol is the sixth most common cause of mortality and disability-adjusted life years (DALYs) in both men and women, accounting for 22% of female fatalities and 68% of male deaths [94]. There is a link between harmful alcohol consumption and various mental and behavioral illnesses, as well as other non-communicable diseases such as tuberculosis and HIV/AIDS and injuries. Injuries constitute the greatest negative consequence of alcohol consumption after cancer. Cardiovascular disease accounts for 15% of alcohol-attributable morbidity, while liver cirrhosis accounts for 13% of all alcohol-attributable deaths [97]. Besides the health risks, irresponsible alcohol use results in social and economic losses for consumers and the community as a whole [98,99].
6. Conclusions
Despite there being other methods for evaluating the health risks associated with alcohol intake, the margin of exposure method is recommended for comparing the risks of different alcoholic beverage components. From this review, ethanol remains the most prominent carcinogen in alcoholic beverages, according to quantitative comparative risk assessment. Therefore, the reduction in alcohol intake ought to be prioritized in combating harm due to alcoholic beverages [100]. Since the dose–response relationship holds for alcohol harm, reduction in alcoholic strength would be beneficial in minimizing the harmful effects of alcohol [101]. For illustration, drinking four bottles of 5.5 percent vol. ethanol beer generates a MOE of 0.5, whereas drinking the same volume of light beer (1.5 percent vol. ethanol) yields a substantially greater MOE of 1.9 [1]. Moreover, consumers may not be typically able to discriminate different alcohol strengths in beer and, thus, may not ingest more volumes to compensate for the lower alcoholic strength beer [102,103].
Other carcinogens besides ethanol require mitigative steps as well, which may require strict adoption of industry best practices such as keeping contaminants/components as low as can reasonably be achieved (ALARA). We urge the relevant regulatory authorities to implement the available mitigative measures to protect consumers from potentially carcinogenic substances.
Author Contributions
D.W.L. was in charge of conceptualization; D.W.L. and A.O.O. were in charge of methodology; A.O.O. was in charge of writing—original draft preparation; D.W.L. was in charge of writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Extracted data are presented in the main tables.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L. Etude de statistique clinique de 134 cas de cancer de l’ oesophage en du cardia. Arch. Mal. Appar. Dig. 1910, 4, 451–475. [Google Scholar]
- Praud, D.; Rota, M.; Rehm, J.; Shield, K.; Zatoński, W.; Hashibe, M.; La Vecchia, C.; Boffetta, P. Cancer incidence and mortality attributable to alcohol consumption. Int. J. Cancer 2016, 138, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Sohnius, E.-M. The role of acetaldehyde outside ethanol metabolism in the carcinogenicity of alcoholic beverages: Evidence from a large chemical survey. Food Chem. Toxicol. 2008, 46, 2903–2911. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Kanteres, F.; Rehm, J. Carcinogenicity of acetaldehyde in alcoholic beverages: Risk assessment outside ethanol metabolism. Addiction 2009, 104, 533–550. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Formaldehyde. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 88, 39–325. [Google Scholar]
- Monakhova, Y.B.; Jendral, J.A.; Lachenmeier, D.W. The margin of exposure to formaldehyde in alcoholic beverages. Arch. Ind. Hyg. Toxicol. 2012, 63, 227–237. [Google Scholar] [CrossRef]
- Jendral, J.A.; Monakhova, Y.B.; Lachenmeier, D.W. Formaldehyde in Alcoholic Beverages: Large Chemical Survey Using Purpald Screening Followed by Chromotropic Acid Spectrophotometry with Multivariate Curve Resolution. Int. J. Anal. Chem. 2011, 2011, 797604. [Google Scholar] [CrossRef]
- Boettcher, M.I.; Schettgen, T.; Kütting, B.; Pischetsrieder, M.; Angerer, J. Mercapturic acids of acrylamide and glycidamide as biomarkers of the internal exposure to acrylamide in the general population. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2005, 580, 167–176. [Google Scholar] [CrossRef]
- Mo, W.; He, H.; Xu, X.; Huang, B.; Ren, Y. Simultaneous determination of ethyl carbamate, chloropropanols and acrylamide in fermented products, flavoring and related foods by gas chromatography–triple quadrupole mass spectrometry. Food Control 2014, 43, 251–257. [Google Scholar] [CrossRef]
- Wenzl, T.; Lachenmeier, D.W.; Gökmen, V. Analysis of heat-induced contaminants (acrylamide, chloropropanols and furan) in carbohydrate-rich food. Anal. Bioanal. Chem. 2007, 389, 119–137. [Google Scholar] [CrossRef]
- Breitling-Utzmann, C.M.; Köbler, H.; Harbolzheimer, D.; Maier, A. 3-MCPD - Occurrence in bread crust and various food groups as well as formation in toast. Dtsch. Leb. 2003, 99, 280–285. [Google Scholar]
- Svejkovská, B.; Novotný, O.; Divinová, V.; Réblová, Z.; Doležal, M.; Velíšek, J. Esters of 3-chloropropane-1,2-diol in foodstuffs. Czech J. Food Sci. 2018, 22, 190–196. [Google Scholar] [CrossRef]
- Baxter, E.D.; Booer, C.D.; Muller, R.E.; O’Shaugnessy, C.; Slaiding, I.R. Minimizing acrylamide and 3-MCPD in crystal malts; effects on flavour. Proc. Congr. Eur. Brew Conv. 2005, 30, 163-1–163-6. [Google Scholar]
- Sadowska-Rociek, A.; Surma, M. A survey on thermal processing contaminants occurrence in dark craft beers. J. Food Compos. Anal. 2021, 99, 103888. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Aflatoxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100F, 225–248. [Google Scholar]
- Mably, M.; Mankotia, M.; Cavlovic, P.; Tam, J.; Wong, L.; Pantazopoulos, P.; Calway, P.; Scott, P.M. Survey of aflatoxins in beer sold in Canada. Food Addit. Contam. 2005, 22, 1252–1257. [Google Scholar] [CrossRef]
- Gilbert, J.; Michelangelo, P. Analytical methods for mycotoxins in the wheat chain. In Mycotoxin Reduction in Grain Chain; Leslie, J.F., Logrieco, A.F., Eds.; Wiley Blackwell: Oxford, UK, 2014; pp. 169–188. [Google Scholar]
- Odhav, B.; Naicker, V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002, 19, 55–61. [Google Scholar] [CrossRef]
- Okaru, A.O.; Abuga, K.O.; Kibwage, I.O.; Hausler, T.; Luy, B.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Aflatoxin contamination in unrecorded beers from Kenya – A health risk beyond ethanol. Food Control 2017, 79, 344–348. [Google Scholar] [CrossRef]
- Okaru, A.O.; Rehm, J.; Sommerfeld, K.; Kuballa, T.; Walch, S.G.; Lachenmeier, D.W. The Threat to Quality of Alcoholic Beverages by Unrecorded Consumption. In Alcoholic Beverages; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–34. [Google Scholar]
- Esti, M.; Benucci, I.; Liburdi, K.; Acciaro, G. Monitoring of ochratoxin A fate during alcoholic fermentation of wine-must. Food Control 2012, 27, 53–56. [Google Scholar] [CrossRef]
- Lopez de Cerain, A.; González-Peñas, E.; Jiménez, A.M.; Bello, J. Contribution to the study of ochratoxin A in Spanish wines. Food Addit. Contam. 2002, 19, 1058–1064. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic and arsenic compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100C, 41–93. [Google Scholar]
- Barbaste, M.; Medina, B.; Perez-Trujillo, J.P. Analysis of arsenic, lead and cadmium in wines from the Canary Islands, Spain, by ICP/MS. Food Addit. Contam. 2021, 4, 141–148. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Cadmium and cadmium compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100C, 121–145. [Google Scholar]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Suppl. S7; International Agency for Research on Cancer: Lyon, France, 1987.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Inorganic and organic lead compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 87, 39–468. [Google Scholar]
- EFSA. Scientifc opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Benzene. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100F, 249–294. [Google Scholar]
- Lachenmeier, D.W.; Kuballa, T.; Reusch, H.; Sproll, C.; Kersting, M.; Alexy, U. Benzene in infant carrot juice: Further insight into formation mechanism and risk assessment including consumption data from the DONALD study. Food Chem. Toxicol. 2010, 48, 291–297. [Google Scholar] [CrossRef]
- Loch, C.; Reusch, H.; Ruge, I.; Godelmann, R.; Pflaum, T.; Kuballa, T.; Schumacher, S.; Lachenmeier, D.W. Benzaldehyde in cherry flavour as a precursor of benzene formation in beverages. Food Chem. 2016, 206, 74–77. [Google Scholar] [CrossRef]
- Steinbrenner, N.; Löbell-Behrends, S.; Reusch, H.; Kuballa, T.; Lachenmeier, D.W. Benzol in Lebensmitteln – ein Überblick. J. Verbrauchersch. Lebensm. 2010, 5, 443–452. [Google Scholar] [CrossRef]
- Long, D.G. From cobalt to chloropropanol: De tribulationibus aptis cerevisiis imbibendis. J. Inst. Brew. 1999, 105, 79–84. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Lin, H.; Fan, W.; Dong, J.-J.; Chen, H.-L. Investigation into Benzene, Trihalomethanes and Formaldehyde in Chinese Lager Beers. J. Inst. Brew. 2006, 112, 291–294. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Furan. IARC Monogr. Eval. Carcinog. Risks Hum. 1995, 63, 393–407. [Google Scholar]
- Chen, L.J.; Hecht, S.S.; Peterson, L.A. Identification of cis-2-butene-1,4-dial as a microsomal metabolite of furan. Chem. Res. Toxicol. 1995, 8, 903–906. [Google Scholar] [CrossRef]
- Peterson, L.A.; Cummings, M.E.; Vu, C.C.; Matter, B.A. Glutathione trapping to measure microsomal oxidation of furan toto cis-2-butene-1,4-dial. Drug Metab. Dispos. 2005, 33, 1453–1458. [Google Scholar] [CrossRef]
- EFSA. Update on furan levels in food from monitoring years 2004–2010 and exposure assessment. EFSA J. 2011, 9, 2347. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Glyphosate. IARC Monogr. Eval. Carcinog. Risks Hum. 2015, 112, 321–399. [Google Scholar]
- Nagatomi, Y.; Yoshioka, T.; Yanagisawa, M.; Uyama, A.; Mochizuki, N. Simultaneous LC-MS/MS Analysis of Glyphosate, Glufosinate, and Their Metabolic Products in Beer, Barley Tea, and Their Ingredients. Biosci. Biotechnol. Biochem. 2013, 77, 2218–2221. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 96, 1–1428. [Google Scholar]
- Uthurry, C.A.; Varela, F.; Colomo, B.; Suárez Lepe, J.A.; Lombardero, J.; García del Hierro, J.R. Ethyl carbamate concentrations of typical Spanish red wines. Food Chem. 2004, 88, 329–336. [Google Scholar] [CrossRef]
- Anderson, L.M.; Souliotis, V.L.; Chhabra, S.K.; Moskal, T.J.; Harbaugh, S.D.; Kyrtopoulos, S.A. N-nitrosodimethylamine-derived O(6)-methylguanine in DNA of monkey gastrointestinal and urogenital organs and enhancement by ethanol. Int. J. Cancer 1996, 66, 130–134. [Google Scholar] [CrossRef]
- Lijinsky, W. N-Nitroso compounds in the diet. Mutat. Res. Toxicol. Environ. Mutagen. 1999, 443, 129–138. [Google Scholar] [CrossRef]
- Tricker, A.R.; Kubacki, S.J. Review of the occurrence and formation of non-volatile N -nitroso compounds in foods. Food Addit. Contam. 1992, 9, 39–69. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Fügel, D. Reduction of nitrosamines in beer—Review of a success story. Brew Sci. 2007, 60, 84–89. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Pulegone. IARC Monogr. Eval. Carcinog. Risks Hum. 2015, 108, 141–154. [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of pulegone (CAS No. 89-82-7) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2011, 563, 1–201. [Google Scholar]
- SCF. Opinion of the Scientifc Committee on Food on the Safety of the Presence of Safrole (1-allyl-3,4-methylene Dioxy Benzene) in Flavouring and Other Food Ingredients with Flavouring Properties; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Curro, P.; Micali, G.; Lanuzza, F. Determination of beta-asarone, safrole, isosafrole and anethole in alcoholic drinks by high-performance liquid chromatography. J Chromatogr. 1987, 404, 273–278. [Google Scholar] [CrossRef]
- Gutsche, B.; Weißhaar, R.; Buhlert, J. Acrylamide in food - Screening results from food control in Baden-Württemberg. Deut. Lebensm. Rundsch. 2002, 98, 437–443. [Google Scholar]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the European Commission on Ethyl Carbamate and Hydrocyanic Acid in Food and Beverages (Question No EFSA-Q-2006-076). EFSA J. 2007, 1–44. [Google Scholar]
- Donhauser, S.; Wagner, D.J.F. Critical trace-elements in brewing technology. 2. Occurrence of arsenic, lead, cadmium, chromium, mercury and selenium in beer. Monatsschr. Brauwiss. 1987, 40, 328–333. [Google Scholar]
- Andrey, D. A simple gas chromatography method for the determination of ethylcarbamate in spirits. Z. Lebensm. Unters. Forsch. 1987, 185, 21–23. [Google Scholar] [CrossRef]
- Klejdus, B.; Moravcová, J.; Lojková, L.; Vacek, J.; Kubán, V. Solid-phase extraction of 4(5)-methylimidazole (4MeI) and 2-acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl)-imidazole (THI) from foods and beverages with subsequent liquid chromatographic-electrospray mass spectrometric quantification. J. Sep. Sci. 2006, 29, 378–384. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Fujiwara, M. Determination of 4 (5)-Methylimidazole in Food by Thin Layer Chromatography. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1981, 22, 189–196. [Google Scholar] [CrossRef][Green Version]
- European Commission. European Commission Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC). Off. J. Eur. Union 2008, L 354/34, 34–50. [Google Scholar]
- BfR Provisional Assessment of Glyphosate in Beer; BfR Communication No. 005/2016; Bundesinstitut für Risikobewertung (BfR): Berlin, Germany, 25 February 2016.
- Lachenmeier, D.W.; Przybylski, M.C.; Rehm, J. Comparative risk assessment of carcinogens in alcoholic beverages using the margin of exposure approach. Int. J. Cancer 2012, 131, 995–1003. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Gill, J.S.; Chick, J.; Rehm, J. The total margin of exposure of ethanol and acetaldehyde for heavy drinkers consuming cider or vodka. Food Chem. Toxicol. 2015, 83, 210–214. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Rehm, J. Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Sci. Rep. 2015, 5, 8126. [Google Scholar] [CrossRef]
- Mueller, U.; Agudo, A.; Carrington, C.; Doerge, D.; Hellenäs, K.E.; Leb-lanc, J.C.; Rao, M.; Renwick, A.; Slob, W.; Wu, Y. Acrylamide (Addendum). In Safety Evaluation of Certain Contaminants in Food. Prepared by the Seventysecond Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series 63; WHO and FAO: Geneva, Switzerland, 2011; pp. 1–51. [Google Scholar]
- National Toxology Program NTP. Technical Report on the Toxicology and Carcinogenesis Studies of Acrylamide in F344/N Rats and B6C3F1 Mice (Feed and Drinking Water Studies). Natl. Toxicol. Progr. Tech. Rep. Ser. 2012, 575, 1–236. [Google Scholar]
- EFSA. Opinion of the scientific panel on contaminants in the food chain [CONTAM] related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived prod. EFSA J. 2007, 446, 1–127. [Google Scholar]
- Yeh, F.S.; Yu, M.C.; Mo, C.C.; Luo, S.; Tong, M.J.; Henderson, B.E. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989, 49, 2506–2509. [Google Scholar] [CrossRef]
- Benford, D.J.; Alexander, J.; Baines, J.; Bellinger, D.C.; Carrington, C.; Peréz, V.A.; Uxbury, J.; Fawell, J.; Hailemariam, K.; Montoro, R.; et al. Arsenic (Addendum). In Safety Evaluation of Certain Contaminants in Food. Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series 63; WHO and FAO: Geneva, Switzerland, 2011; pp. 153–316. [Google Scholar]
- Chen, C.-L.; Chiou, H.-Y.; Hsu, L.-I.; Hsueh, Y.-M.; Wu, M.-M.; Chen, C.-J. Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan. Environ. Res. 2010, 110, 455–462. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Benzene (CASRN 71-43-2). Integrated Risk Information System. Document 0276; US Environmental Protection Agency: Washington, DC, USA, 2003.
- Rothman, N.; Li, G.L.; Dosemeci, M.; Bechtold, W.E.; Marti, G.E.; Wang, Y.Z.; Linet, M.; Xi, L.Q.; Lu, W.; Smith, M.T.; et al. Hematotoxicity among Chinese workers heavily exposed to benzene. Am. J. Ind. Med. 1996, 29, 236–246. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Kanteres, F.; Rehm, J. Epidemiology-based risk assessment using the benchmark dose/margin of exposure approach: The example of ethanol and liver cirrhosis. Int. J. Epidemiol. 2011, 40, 210–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beland, F.A.; Benson, R.W.; Mellick, P.W.; Kovatch, R.M.; Roberts, D.W.; Fang, J.-L.; Doerge, D.R. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. 2005, 43, 1–19. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP technical report on the toxicology and carcino- gensis. Studies of urethane, ethanol, and urethane/ethanol (urethane, CAS No. 51-79-6; ethanol, CAS No. 64-17-5) in B6C3F1 mice (drinking water studies). Natl. Toxicol. Progr. Tech. Rep. Ser. 2004, 510, 1–346. [Google Scholar]
- Vavasour, E.; Renwick, A.G.; Engeli, B.; Barlow, S.; Castle, L.; DiNovi, M.; Slob, W.; Schlatter, J.; Bolger, M. Ethyl carbamate. In Safety Evaluation of Certain Contaminants in Food. Prepared by the Sixty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series 55; WHO and FAO: Geneva, Switzerland, 2006. [Google Scholar]
- IPCS. Environmental Health Criteria 239: Principles for Modelling Dose-Response for the Risk Assessment of Chemicals; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Til, H.P.; Woutersen, R.A.; Feron, V.J.; Hollanders, V.H.; Falke, H.E.; Clary, J.J. Two-year drinking-water study of formaldehyde in rats. Food Chem. Toxicol. 1989, 27, 77–87. [Google Scholar] [CrossRef]
- Williams, G.M.; Arisseto, A.P.; Baines, J.; DiNovi, M.; Feeley, M.; Schlatter, J.; Slob, W.; Toledo, M.C.F.; Vavasour, E. Furan. In Safety Evaluation of Certain Contaminants in food. Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series 63; WHO and FAO: Geneva, Switzerland, 2011. [Google Scholar]
- Moser, G.J.; Foley, J.; Burnett, M.; Goldsworthy, T.L.; Maronpot, R. Furan-induced dose–response relationships for liver cytotoxicity, cell proliferation, and tumorigenicity (furan-induced liver tumorigenicity). Exp. Toxicol. Pathol. 2009, 61, 101–111. [Google Scholar] [CrossRef]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 4302. [Google Scholar]
- Navas-Acien, A.; Tellez-Plaza, M.; Guallar, E.; Muntner, P.; Silbergeld, E.; Jaar, B.; Weaver, V. Blood Cadmium and Lead and Chronic Kidney Disease in US Adults: A Joint Analysis. Am. J. Epidemiol. 2009, 170, 1156–1164. [Google Scholar] [CrossRef]
- Abraham, K.; Mielke, H.; Lampen, A. Hazard characterization of 3-MCPD using benchmark dose modeling: Factors influencing the outcome. Eur. J. Lipid Sci. Technol. 2012, 114, 1225–1226. [Google Scholar] [CrossRef]
- Cho, W.S.; Han, B.S.; Nam, K.T.; Park, K.; Choi, M.; Kim, S.H.; Jeong, J.; Jang, D.D. Carcinogenicity study of 3-monochloropropane-1,2-diol in Sprague-Dawley rats. Food Chem. Toxicol. 2008, 46, 3172–3177. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the re-evaluation of caramel colours (E 150 a,b,c,d) as food additives. EFSA J. 2011, 9, 2004. [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of 4-methylimidazole (Cas No. 822-36-6) in F344/N rats and B6C3F1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser. 2007, 535, 1–274. [Google Scholar]
- Zeilmaker, M.J.; Bakker, M.I.; Schothorst, R.; Slob, W. Risk Assessment of N-nitrosodimethylamine Formed Endogenously after Fish-with-Vegetable Meals. Toxicol. Sci. 2010, 116, 323–335. [Google Scholar] [CrossRef]
- Peto, R.; Gray, R.; Brantom, P.; Grasso, P. Dose and time relationships for tumor induction in the liver and esophagus of 4080 inbred rats by chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine. Cancer Res. 1991, 51, 6452–6469. [Google Scholar]
- Peto, R.; Gray, R.; Brantom, P.; Grasso, P. Effects on 4080 rats of chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine: A detailed dose-response study. Cancer Res. 1991, 51, 6415–6451. [Google Scholar]
- Barlow, S.; Bolger, M.; Pitt, J.I.; Verger, P. Ochratoxin A (addendum). In Safety Evaluation of Certain Contaminants in Food. Prepared by the Sixty-Eighth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series 59; WHO and FAO: Geneva, Switzerland, 1989. [Google Scholar]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Ochratoxin A (CAS No. 303-47-9) in F344/N Rats (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1989, 358, 1–142. [Google Scholar]
- E.M.A. Public Statement on the Use of Herbal Medicinal Products Containing Pulegone and Menthofuran. EMEA/HMPC/138386/2005; European Medicines Agency: London, UK, 2016.
- Martati, E.; Boersma, M.G.; Spenkelink, A.; Khadka, D.B.; Punt, A.; Vervoort, J.; Bladeren, P.J.; Rietjens, I.M. Physiologically based biokinetic (PBBK) model for safrole bioactivation and detoxification in rats. Chem. Res. Toxicol. 2011, 24, 818–834. [Google Scholar] [CrossRef]
- Boberg, E.W.; Miller, E.C.; Miller, J.A.; Poland, A.; Liem, A. Strong evidence from studies with brachymorphic mice and pentachlorophenol that 1’-sulfoöxysafrole is the major ultimate electrophilic and carcinogenic metabolite of 1’-hydroxysafrole in mouse liver. Cancer Res. 1983, 43, 5163–5173. [Google Scholar]
- Miller, E.C.; Swanson, A.B.; Phillips, D.H.; Fletcher, T.L.; Liem, A.; Miller, J.A. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983, 43, 1124–1134. [Google Scholar]
- Burton, R.; Sheron, N. No level of alcohol consumption improves health. Lancet 2018, 392, 987–988. [Google Scholar] [CrossRef]
- Astrup, A.; Estruch, R. Alcohol and the global burden of disease. Lancet 2019, 393, 2390. [Google Scholar] [CrossRef]
- Room, R.; Babor, T.; Rehm, J. Alcohol and public health. Lancet 2005, 365, 519–530. [Google Scholar] [CrossRef]
- Rehm, J.; Room, R.; Monteiro, M.; Gmel, G.; Graham, K.; Rehn, N.; Sempos, C.T.; Jernigan, D. Alcohol as a risk factor for global burden of disease. Eur. Addict. Res. 2003, 9, 157–164. [Google Scholar] [CrossRef]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Bardach, A.E.; Alcaraz, A.O.; Ciapponi, A.; Garay, O.U.; Riviere, A.P.; Palacios, A.; Cremonte, M.; Augustovski, F. Alcohol consumption’s attributable disease burden and cost-effectiveness of targeted public health interventions: A systematic review of mathematical models. BMC Public Health 2019, 19, 1378. [Google Scholar] [CrossRef]
- Babor, T.; Holder, H.; Caetano, R.; Homel, R.; Casswell, S.; Livingston, M.; Edwards, G.; Österberg, E.; Giesbrecht, N.; Rehm, J.; et al. Strategies and interventions to reduce alcohol-related harm. In Alcohol: No Ordinary Commodity; Oxford University Press Inc.: New York, NY, USA, 2010; Volume 58, pp. 103–108. [Google Scholar]
- Rehm, J.; Lachenmeier, D.W.; Llopis, E.J.; Imtiaz, S.; Anderson, P. Evidence of reducing ethanol content in beverages to reduce harmful use of alcohol. Lancet Gastroenterol. Hepatol. 2016, 1, 78–83. [Google Scholar] [CrossRef]
- Segal, D.S.; Stockwell, T. Low alcohol alternatives: A promising strategy for reducing alcohol related harm. Int. J. Drug Policy 2009, 20, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Geller, E.S.; Kalsher, M.J.; Clarke, S.W. Beer versus mixed-drink consumption at fraternity parties: A time and place for low-alcohol alternatives. J. Stud. Alcohol 1991, 52, 197–204. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
