Elective Surgery in Adult Patients with Excess Weight: Can Preoperative Dietary Interventions Improve Surgical Outcomes? A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Analysis
3. Results
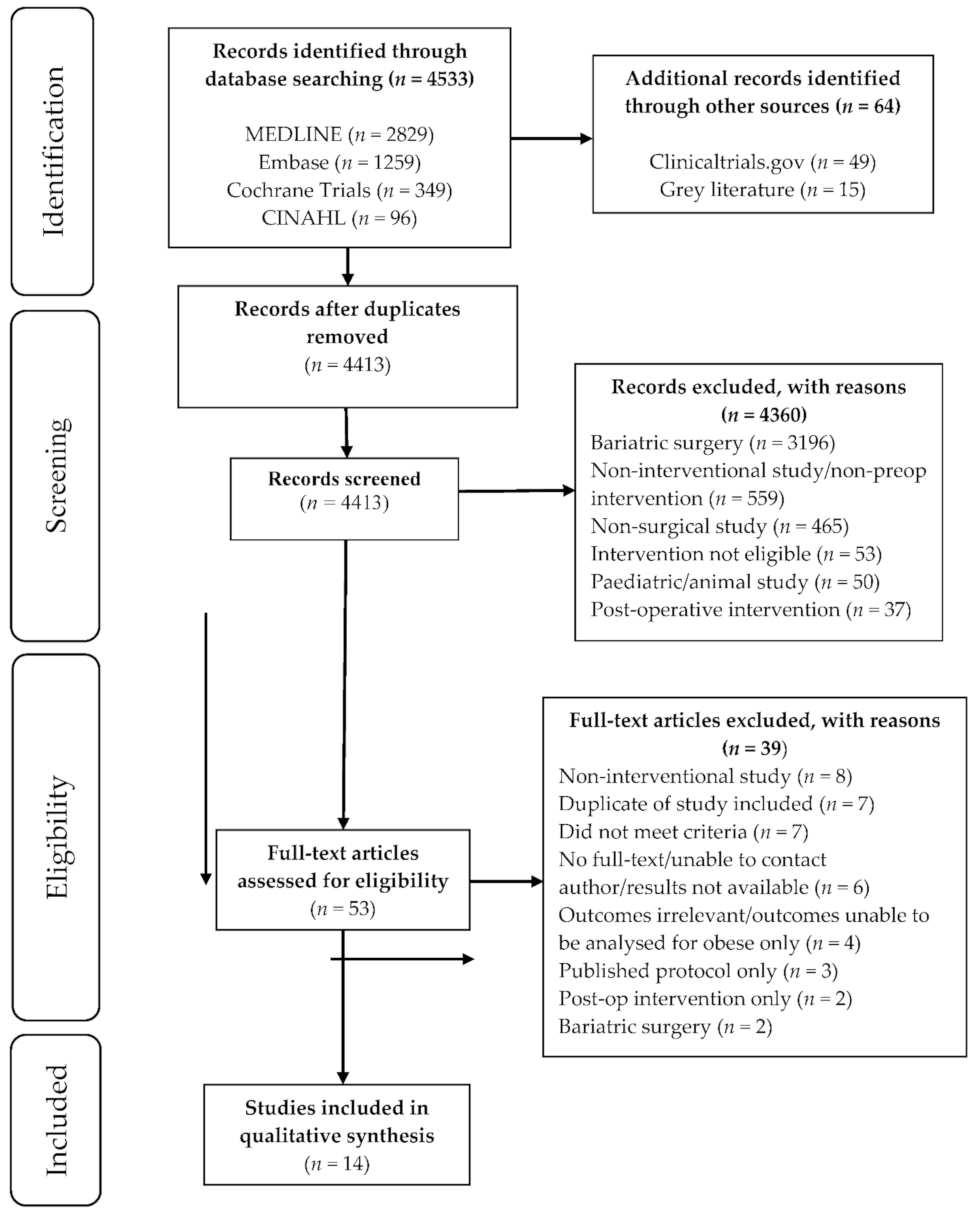
3.1. Literature Search
3.2. Study Characteristics
3.3. Intervention and Comparator Characteristics
3.4. Weight Change and Body Composition
3.5. Impact on Surgical Outcomes
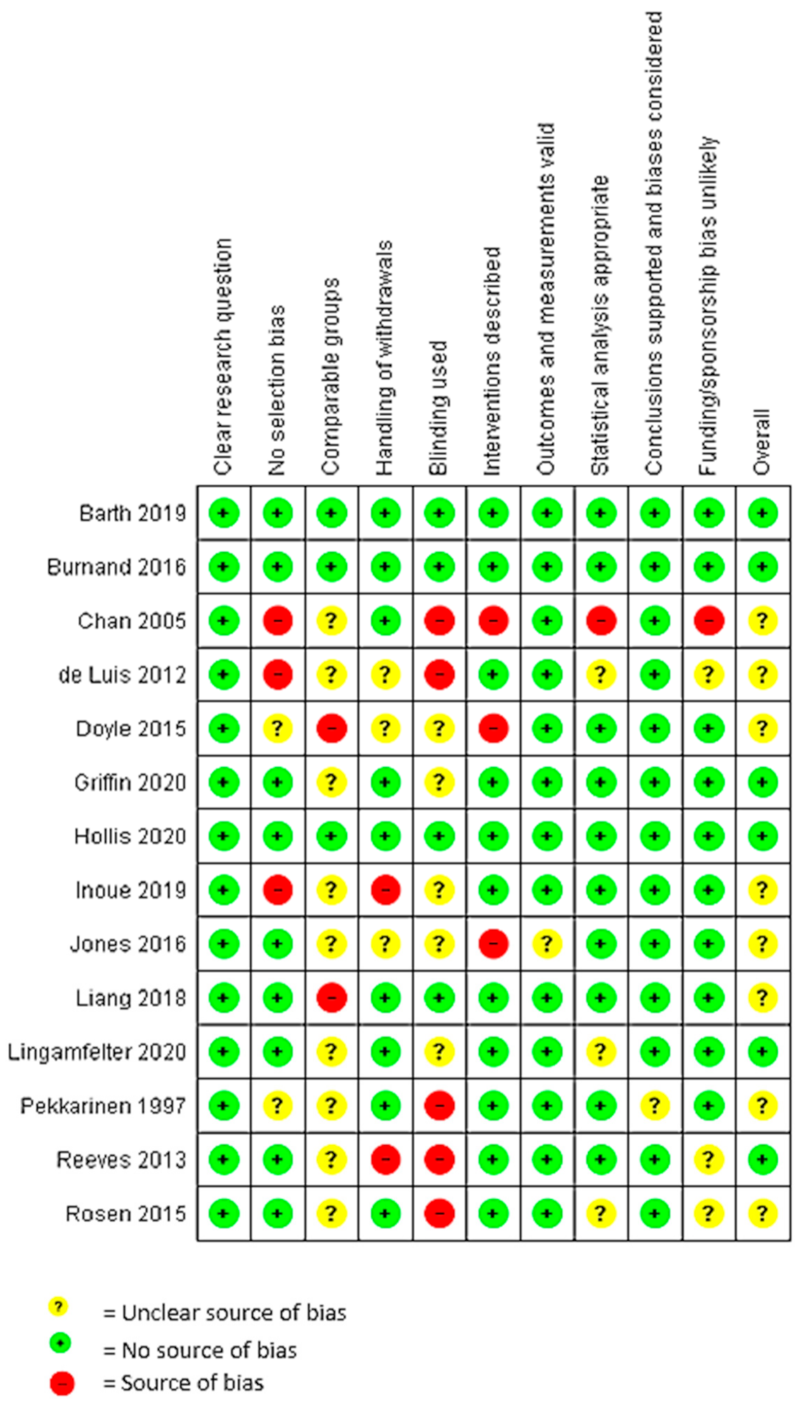
3.6. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Australian Insitute for Health and Welfare. Australia’s Health. 2020. Available online: https://www.aihw.gov.au/getmedia/2aa9f51b-dbd6-4d56-8dd4-06a10ba7cae8/aihw-aus-232.pdf.aspx?inline=true (accessed on 22 April 2021).
- Erlinger, S. Gallstones in obesity and weight loss. Eur. J. Gastroen. Hepat. 2000, 12, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Polednak, A.P. Trends in incidence rates for obesity-associated cancers in the US. Cancer Detect. Prev. 2003, 27, 415–421. [Google Scholar] [CrossRef]
- Doyle, S.L.; Lysaght, J.; Reynolds, J.V. Obesity and post-operative complications in patients undergoing non-bariatric surgery. Obes. Rev. 2010, 11, 875–886. [Google Scholar] [CrossRef]
- Alizadeh, R.F.; Moghadamyeghaneh, Z.; Whealon, M.D.; Hanna, M.H.; Mills, S.D.; Pigazzi, A.; Stamos, M.J.; Carmichael, J.C. BMI significantly impacts outcomes of colorectal surgery. Am. Surgeon. 2016, 82, 930–935. [Google Scholar] [CrossRef]
- Tsukada, K.; Miyazaki, T.; Kato, H.; Masuda, N.; Fukuchi, M.; Fukai, Y.; Nakajima, M.; Ishizaki, M.; Motegi, M.; Mogi, A.; et al. Body fat accumulation and postoperative complications after abdominal surgery. Am. Surgeon. 2004, 70, 347–351. [Google Scholar] [PubMed]
- Kama, N.A.; Kologlu, M.; Doganay, M.; Reis, E.; Atli, M.; Dolapci, M. A risk score for conversion from laparoscopic to open cholecystectomy. Am. J. Surg. 2001, 181, 520–525. [Google Scholar] [CrossRef]
- Wang, J.L.; Gadinsky, N.E.; Yeager, A.M.; Lyman, S.L.; Westrich, G.H. The Increased Utilization of Operating Room Time in Patients with Increased BMI during Primary Total Hip Arthroplasty. J. Arthroplast. 2013, 28, 680–683. [Google Scholar] [CrossRef]
- He, A.-Q.; Li, C.-Q.; Zhang, Q.; Liu, T.; Liu, J.; Liu, G. Visceral-to-Subcutaneous Fat Ratio Is a Potential Predictor of Postoperative Complications in Colorectal Cancer. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2021, 27, e930329. [Google Scholar]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, T.W.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update. Obesity 2013, 21, S1–S27. [Google Scholar] [CrossRef]
- Ibrahim, S.; Chen, C.L.; Lin, C.C.; Yang, C.H.; Wang, C.C.; Wang, S.H.; Liu, Y.W.; Yong, C.C.; Concejero, A.; Jawan, B.; et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transpl. 2006, 12, 950–957. [Google Scholar] [CrossRef]
- Clausen, E.S.; Frankel, C.; Palmer, S.M.; Snyder, L.D.; Smith, P.J. Pre-transplant weight loss and clinical outcomes after lung transplantation. J. Heart Lung Transplant. 2018, 37, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Geiker, N.R.W.; Horn, J.; Astrup, A. Preoperative weight loss program targeting women with overweight and hypertrophy of the breast—A pilot study. Clin. Obes. 2017, 7, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ochner, C.N.; Dambkowski, C.L.; Yeomans, B.L.; Teixeira, J.; Xavier-PiSunyer, F. Pre-bariatric surgery weight loss requirements and the effect of preoperative weight loss on postoperative outcome. Int. J. Obes. 2012, 36, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Ross, L.; Wallin, S.; Osland, E.; Memon, M.A. Commercial Very Low Energy Meal Replacements for Preoperative Weight Loss in Obese Patients: A Systematic Review. Obes. Surg. 2016, 26, 1343–1351. [Google Scholar] [CrossRef]
- Roman, M.; Monaghan, A.; Serraino, G.F.; Miller, D.; Pathak, S.; Lai, F.; Zaccardi, F.; Ganchi, A.; Khunti, K.; Davies, M.J.; et al. Meta-analysis of the influence of lifestyle changes for preoperative weight loss on surgical outcomes. Br. J. Surg. 2019, 106, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.H.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. J. Br. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Mullen, J.L.; Gertner, M.H.; Buzby, G.P.; Goodhart, G.L.; Rosato, E.F. Implications of malnutrition in the surgical patient. Arch. Surg. 1979, 114, 121–125. [Google Scholar] [CrossRef]
- Moctezuma-Velázquez, P.; Vergara-Fernández, O.; Salgado-Nesme, N.; Anguilar-Frasco, J.L.; Sainz-Hernandez, J.C.; Moctezuma-Velazquez, C. Influence of Muscle Mass Area and Visceral Obesity on 30-day Mortality After Colorectal Surgery with Primary Anastomosis. Rev. Investig. Clin. 2021. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Academy of Nutrition and Dietetics. Quality Criteria Checklist: Primary Research: American Dietetic Association’s Evidence Analysis Library; Academy of Nutrition and Dietetics: Chigaco, IL, USA, 2016. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, J.; Higgins, J.; Altman, D. Chapter 10: Analysing data and undetaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (Updated February 2021); Cochrane: London, UK, 2021. [Google Scholar]
- Barth, R.J., Jr.; Mills, J.B.; Suriawinata, A.A.; Putra, J.; Tosteson, T.D.; Axelrod, D.; Freeman, R.; Whalen, G.F.; LaFemina, J.; Tarczewski, S.M.; et al. Short-term Preoperative Diet Decreases Bleeding After Partial Hepatectomy: Results from a Multi-institutional Randomized Controlled Trial. Ann. Surg. 2019, 269, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.K.; Bernardi, K.; Holihan, J.L.; Cherla, D.V.; Escamilla, R.; Lew, D.F.; Berger, D.H.; Ko, T.C.; Kao, L.S. Modifying Risks in Ventral Hernia Patients with Prehabilitation: A Randomized Controlled Trial. Ann. Surg. 2018, 268, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Burnand, K.M.; Lahiri, R.P.; Burr, N.; Jansen van Rensberg, L.; Lewis, M.P. A randomised, single blinded trial, assessing the effect of a two week preoperative very low calorie diet on laparoscopic cholecystectomy in obese patients. HPB 2016, 18, 456–461. [Google Scholar] [CrossRef] [Green Version]
- de Luis, D.A.; Izaola, O.; García Alonso, M.; Aller, R.; Cabezas, G.; de la Fuente, B. Effect of a commercial hypocaloric diet in weight loss and post surgical morbidities in obese patients with chronic arthropathy, a randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1814–1820. [Google Scholar] [PubMed]
- Hollis, G.; Franz, R.; Bauer, J.; Bell, J. Implementation of a very low calorie diet program into the pre-operative model of care for obese general elective surgery patients: Outcomes of a feasibility randomised control trial. Nutr. Diet. 2020, 77, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, S.B.; Ross, L.J.; Burstow, M.J.; Desbrow, B.; Palmer, M.A. Efficacy of a dietitian-led very low calorie diet (VLCD) based model of care to facilitate weight loss for obese patients prior to elective, non-bariatric surgery. J. Hum. Nutr. Diet. 2021, 34, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.D.; Waterland, P.W.; Powell-Brett, S.; Super, P.; Richardson, M.; Bowley, D. Preoperative Very Low-Calorie Diet Reduces Technical Difficulty during Laparoscopic Cholecystectomy in Obese Patients. Surg. Laparosc. Endosc. Percutan. Technques 2016, 26, 226–229. [Google Scholar] [CrossRef]
- Rosen, M.J.; Aydogdu, K.; Grafmiller, K.; Petro, C.C.; Faiman, G.H.; Prabhu, A. A Multidisciplinary Approach to Medical Weight Loss Prior to Complex Abdominal Wall Reconstruction: Is it Feasible? J. Gastrointest. Surg. 2015, 19, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Chan, C.K. A review of incisional hernia repairs: Preoperative weight loss and selective use of the mesh repair. Hernia 2005, 9, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, T.; Mustajoki, P. Use of very low-calorie diet in preoperative weight loss: Efficacy and safety. Obes. Res. 1997, 5, 595–602. [Google Scholar] [CrossRef]
- Reeves, J.G.; Suriawinata, A.A.; Ng, D.P.; Holubar, S.D.; Mills, J.B.; Barth, R.J., Jr. Short-term preoperative diet modification reduces steatosis and blood loss in patients undergoing liver resection. Surgery 2013, 154, 1031–1037. [Google Scholar] [CrossRef]
- Doyle, A.; Dillon, J.; Adeyi, O.; Fischer, S.E.; MacArthur, M.; Marquez, M.; Smith, R.; Grant, D.; Cattral, M.S.; McGilvray, I.; et al. Treatment with optifast reduces hepatic steatosis and safely increases candidacy rates for live donor liver transplantation. Hepatology 2015, 62, 213A. [Google Scholar]
- Inoue, K.; Yoshiuchi, S.; Yoshida, M.; Nakamura, N.; Nakajima, S.; Kitamura, A.; Mouri, K.; Michiura, T.; Mukaide, H.; Osaki, T.; et al. Preoperative weight loss program involving a 20-day very low-calorie diet for obesity before laparoscopic gastrectomy for gastric cancer. Asin. J. Endosc. Surg. 2019, 12, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Lingamfelter, M.; Orozco, F.R.; Beck, C.N.; Harrer, M.F.; Post, Z.D.; Ong, A.C.; Ponzio, D.Y. Nutritional Counseling Program for Morbidly Obese Patients Enables Weight Optimization for Safe Total Joint Arthroplasty. Orthopedics 2020, 43, e316–e322. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health and Care Excellence (NICE). Obesity: Identification, Assessment and Management Clinical Guideline [CG189]. 2014. Available online: https://www.nice.org.uk/guidance/cg189 (accessed on 30 July 2020).
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Simopoulos, C.; Polychronidis, A.; Botaitis, S.; Perente, S.; Pitakoudis, M. Laparoscopic Cholecystectomy in Obese Patients. Obes. Surg. 2005, 15, 243–246. [Google Scholar] [CrossRef]
- Van Nieuwenhove, Y.; Dambrauskas, Z.; Campillo-Soto, A.; van Dielen, F.; Wiezer, R.; Janssen, I.; Kramer, M.; Thorell, A. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch. Surg. 2011, 146, 1300–1305. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-J.; Li, H.-R.; Zhang, W.-H.; Lui, K.; Zhang, D.; Sun, L.; Chen, X.; Zhao, L.; Chen, X.-Z.; Yang, K.; et al. Visceral Fat Area (VFA) Superior to BMI for Predicting Postoperative Complications After Radical Gastrectomy: A Prospective Cohort Study. J. Gastrointest. Surg. 2020, 24, 1298–1306. [Google Scholar] [CrossRef]
- Holderbaum, M.; Casagrande, D.S.; Sussenbach, S.; Buss, C. Effects of very low calorie diets on liver size and weight loss in the preoperative period of bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2018, 14, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, I.N.; Graves, S.E.; Wicks, I.P.; Bennell, K.L.; Osborne, R.H. Severely compromised quality of life in women and those of lower socioeconomic status waiting for joint replacement surgery. Arthritis Care Res. 2005, 53, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Oudhoff, J.P.; Timmermans, D.R.M.; Knol, D.L.; Binjen, A.B.; van der Wal, G. Waiting for elective general surgery: Impact on health related quality of life and psychosocial consequences. BMC Public Health 2007, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Primatesta, P.; Goldacre, M.J. Inguinal Hernia Repair: Incidence of Elective and Emergency Surgery, Readmission and Mortality. Int. J. Epidemiol. 1996, 25, 835–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, B.; Mercer, D.; Brown, P.; FitzGerald, M.; Jalink, D.; Shaw, R. Risk of emergency admission while awaiting elective cholecystectomy. Can. Med. Assoc. J. 2003, 169, 662. [Google Scholar]
- Mullen, M.G.; Michaels, A.D.; Mehaffey, J.H.; Guidry, C.A.; Turrentine, F.E.; Hedrick, T.L.; Friel, C.M. Risk Associated with Complications and Mortality After Urgent Surgery vs Elective and Emergency Surgery: Implications for Defining “Quality” and Reporting Outcomes for Urgent Surgery. JAMA Surg. 2017, 152, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Hirose, K.; Shore, A.D.; Clark, J.M.; Gearhart, S.L.; Efron, J.; Makary, M.A. Surgical site infections and cost in obese patients undergoing colorectal surgery. Arch. Surg. 2011, 146, 1068–1072. [Google Scholar] [CrossRef] [Green Version]
- Meller, M.M.; Toossi, N.; Gonzalez, M.H.; Son, M.; Lau, E.C.; Johanson, N. Surgical Risks and Costs of Care are Greater in Patients Who Are Super Obese and Undergoing THA. Clin. Orthop. Relat. Res. 2016, 474, 2472–2481. [Google Scholar] [CrossRef] [Green Version]
- New South Wales Health. Operating Theatre Efficiency. 2021. Available online: https://aci.health.nsw.gov.au/resources/surgical-services/efficiency/theatre-efficiency#program-content (accessed on 17 June 2021).
- Australian Commission on Safety and Quality in Health Care. Laparoscopic Cholecystectomy Hospitalisations. 2015. Available online: https://www.safetyandquality.gov.au/sites/default/files/migrated/4.4-Laparoscopic-cholecystectomy-1.pdf (accessed on 17 June 2021).
- Australian Commission on Safety and Quality in Health Care. Hospital-Acquired Complications (HACs). 2021. Available online: https://www.safetyandquality.gov.au/our-work/indicators/hospital-acquired-complications#hospital-acquired-complications-resources (accessed on 18 June 2021).
- Schiesser, M.; Kirchhoff, P.; Müller, M.K.; Schafer, M.; Clavien, P.A. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery 2009, 145, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, M.A.; Terzioğlu, H.; Genç, V.; Erkek, A.B.; Ozban, M.; Sonyurek, P.; Elhan, A.H.; Torun, N. Preoperative nutritional risk assessment in predicting postoperative outcome in patients undergoing major surgery. World J. Surg. 2006, 30, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.T.; Mullen, J.L.; Buzby, G.P. The link between nutritional status and clinical outcome: Can nutritional intervention modify it? Am. J. Clin. Nutr. 1988, 47 (Suppl. S2), 352–356. [Google Scholar] [CrossRef] [PubMed]
- Pichard, C.; Kyle, U.G.; Morabia, A.; Perrier, A.; Vermeulen, B.; Unger, P. Nutritional assessment: Lean body mass depletion at hospital admission is associated with an increased length of stay. Am. J. Clin. 2004, 79, 613–618. [Google Scholar] [CrossRef] [Green Version]
- American College of Surgeons. ACS National Surgical Quality Improvement Program. 2021. Available online: https://www.facs.org/quality-programs/acs-nsqip (accessed on 18 June 2021).
| Parameter | Criteria | Search Terms (Example Taken from MEDLINE Search) |
|---|---|---|
| Participants | Adults with obesity or excess weight/fat who are undergoing elective, non-bariatric surgery | obes * or overweight or “body size” or “visceral fat” or “central obesity” or “high body mass index” or adipos * or fat |
| Interventions | Dietary intervention(s) with/without exercise that were aimed at weight/fat loss prior to surgery | diet or “weight reduc *” or “weight loss” or “preoperative care” or “diet therapy” or “healthy lifestyle” |
| Comparisons | - | - |
| Outcomes | Surgical outcome(s) of interest | “postoperative complication *” or “surgery outcome *” or “intraoperative complication *” or “length of operation” or "length of surgery" or “length of stay” or “operat * time” or “intraoperative time” or “surgical procedures, operative” or ischemia or anesthesia or necrosis |
| Study design | All study types considered | - |
| Author (Year) | Country | Study Design and Quality | Outpatient Setting | Surgery Type and Procedure | Inclusion Criteria (Aged ≥ 18 Years unless Stated) | Exclusion Criteria | Baseline Demographics, Mean Unless Stated (Range) | Sample Size for Intervention (I) and Comparator (C) Groups, Dropouts |
|---|---|---|---|---|---|---|---|---|
| Griffin et al., 2021 [29] | Australia | Single cohort + | Large public hospital | Various-including general, orthopaedic, gynaecological | BMI ≥ 30, referred by surgeon to specialist dietitian clinic | Contraindications to VLCD/medically unsafe/unsuitable for VLCD | 90% female Age: 45 ± 13.1 years BMI 44.3 ± 6.2 (33.4–60.4) | I: n = 78 No comparator Dropout n = 1 |
| Hollis et al., 2020 [28] | Australia | Randomised controlled trial (pilot study) + | Tertiary public hospital | General Surgery-hernia repair or laparoscopic cholecystectomy | BMI ≥ 30 awaiting surgery | Contraindications to VLCD | 63% female Age: 51.6 ± 13.1 years BMI 40.5 ± 5.9 (range NR) | I: n = 25 C: n = 25 n = 4 dropouts (n = 23 completed in each group) |
| Lingamfelter et al., 2020 [37] | United States of America | Retrospective case series + | Tertiary specialist institution | Orthopaedic-hip or knee replacement | BMI ≥ 40 requiring surgery | NR | 60% female Age: 62.6 ± 8.5 years BMI 44.6 ± 3.9 (40–61.9) | I: n = 111 No comparator Dropouts NR |
| Barth et al., 2019 [24] | United States of America | Randomised controlled trial + | Two large medical centres | Hepatobiliary-liver resection | BMI ≥ 25 requiring surgery | NR | 45% female Age: 56 ± 12 years BMI 32.5 ± 6.4 (range NR) | I: n = 31 C: n = 32 (n = 60 analysed, n = 3 did not receive surgery) |
| Inoue et al., 2019 [36] | Japan | Retrospective cohort Ø | Hospital | Upper gastrointestinal-laparoscopic gastrectomy for gastric cancer | Age ≥ 20, BMI ≥ 25 OR waist circumference ≥ 85 cm (men), ≥ 90 cm (women), planned for surgery for stage 1 or 2 cancer | ASA β score ≥ III, inadequate organ function, hx upper abdominal open surgery, “uncontrolled” diabetes, active infectious disease, on steroids, allergy to shake ingredient(s) | 7% female Age: 71 (median) years BMI 26 (median) (23.5–31) | I: n = 33 (n = 27 had surgical outcomes analysed) C: n = 23 Dropouts NR |
| Liang et al., 2018 [25] | United States of America | Randomised controlled trial Ø | Safety-net academic institution | General surgery-ventral hernia repair | BMI 30–40, hernia 3–20 cm, willing to undergo preoperative optimisation | Severe co-morbidities limiting survival, requiring emergency surgery, pregnant / intending to become pregnant | 83% female Age: 49.5 ± 10.1 years BMI 36.8 ± 2.6 (range NR) | I: n = 59 (n = 3 dropouts, n = 12 no surgery), final n = 44 C: n = 59 (n = 1 dropout, n = 24 no surgery), final n = 34 |
| Burnand et al., 2016 [26] | United Kingdom | Randomised controlled trial + | Hospital | General-laparoscopic cholecystectomy | BMI ≥ 30, symptomatic gallstones | Pre-existing liver disease, diabetes, bile duct stones, previous abdominal surgery | 91% female Age: 46 (median) years BMI 33.8 ± 3.4 (range NR) | I: n = 21 C: n = 25 n = 0 dropouts |
| Jones et al., 2016 [30] | United Kingdom | Single cohort Ø | Hospital | General-laparoscopic cholecystectomy | Any BMI, requiring surgery for biliary colic | Previous cholecystitis or treatment for gallstones, previous upper midline abdominal surgery | 87% female Age:45 (median) years BMI 30.3 (median) (22.5–44.1) | I: n = 38 (n = 19 BMI ≥ 30) No comparator Dropouts NR |
| Doyle et al., 2015 [35] | Canada | Retrospective cohort Ø | Hospital | Organ transplant-liver | Any BMI, liver donors with >10% liver steatosis (put into intervention group), with <10% steatosis (put into control group) | Suspected non-alcoholic steatohepatitis | 58% female Age: 39(mean) years BMI 29.6 (25.4–34.9) | I: n = 16 (n = 14 had surgery) C: n = 53 |
| Rosen et al., 2015 [31] | United States of America | Single cohort Ø | Comprehensive specialty hernia centre | General surgery-complex incisional hernia repair | BMI > 35 who presented to author, evaluated by medical weight-loss specialist | NR | 80% female Age: 54 ± 9 years BMI 49 ± 10 (36–85) | I: n = 25 No comparator Dropouts NR |
| Reeves et al., 2013 [34] | United States of America | Retrospective cohort + | Hospital | Hepatobiliary-liver resection | Any BMI, patients who underwent major hepatic resection | NR | 42% female Age: 60 (mean) years BMI 27.2 (18.5–47.6) | I: n = 51 C: n = 60 Dropouts NR |
| de Luis et al., 2012 [27] | Spain | Randomised controlled trial Ø | Clinical nutrition unit within hospital | Orthopaedic-hip/knee replacement | BMI > 30, indication for surgery for chronic osteoarthritis | Heart disease or stroke in last 3 months, elevated blood lipids or blood pressure > 140/90 mmHg, medications for diabetes, blood pressure, taking steroids | 83% female Age: 65 ± 8.5 years BMI 38.6 ± 4.7 (range NR) | I: n = 20 C: n = 20 (n = 42 recruited, n = 40 completed, dropout group NR) |
| Chan and Chan, 2005 [32] | Canada | Single cohort Ø | Hospital specialising in abdominal hernias | General surgery-ventral/incisional hernia repair | Any BMI but BMI > 30 given intervention, undergoing ventral/incisional hernia repair | NR | 51% female Age: 56 (mean) years BMI 28.9 ± 13.1 (range NR) | I: n = 188 No comparator Dropouts NR |
| Pekkarinen and Mustajoki, 1997 [33] | Finland | Single cohort Ø | Six metropolitan surgical/ gynaecological hospitals | Mixed, including gynaecological, general, orthopaedic | BMI ≥ 35 | Contraindications to VLCD | 60% female Age: 50 ± 10.5 years BMI 44 (median) (35–58) | I: n = 30 No comparator Dropouts NR |
| Author (Year) | Diet Type, Product, Funding | Dietary Profile | Dietary Prescription | Duration | Service Provided | Frequency of Contact and Attendance | ADHERENCE TO DIET | Tolerance/Acceptance | Weight Change for I (Intervention) and C (Comparator) Group (Loss Unless Stated) |
|---|---|---|---|---|---|---|---|---|---|
| Griffin, et al., 2021 [29] | VLCD-based-Optifast®/Optislim® shakes, bars, soups, or desserts. Participants paid for own products. | 800 kcal–1200 kcal, 0.8–1 g/kg adjusted body weight protein, fat NR. | 1–4 meal replacements, >2 cups non-starch vegetables, 2 L energy-free fluids, protein-rich foods to meet requirements. | Median 10 weeks to reach target weight. Duration based on amount of weight loss required or time-frame until surgery. | Diet prescription by dietitian individualised to aid adherence. Evidence-based care provided: managing symptoms, education on progression to healthy eating post-surgery. | Fortnightly dietitian appointments, 93% of appointments attended. | Adherence not formally measured but informed the nutrition care plan. | n = 1 could not tolerate products, n = 2 preferred food, 56% reported ≥1 mild side effect, 90% resolved by end of treatment. | I: 9 ± 6.4kg 7.4 ± 5.3% BW, BMI = 3.3 kg/m2. No comparator. |
| Hollis et al., 2020 [28] | VLCD using Optifast® shakes. Products free of charge. | 700–800 kcal with 0.75 g/kg adjusted body weight protein, fat NR. | VLCD shakes (number dependent on meeting protein requirements) >2 cups non-starch vegetables, 2 L energy-free fluids, 1 tsp oil. | 8 weeks. | Dietitian-led. Individualised program with advice on managing symptoms. | Fortnightly dietitian appointments, 93% attendance in intervention group vs. 39% in controls (p < 0.001). | Urinary ketones found in 56% of intervention participants. | n = 1 drop-out due to “gastrointestinal upset”. | I: 6.5 kg ± 3.8, 5.5%BW. C: gain 0.1 kg ± 2.6. |
| Lingamfelter et al., 2020 [37] | Healthy eating advice. | NR | NR | Mean duration 22 weeks, based on goals BMI < 40. | Individualized meal plans and recommendations for diet changes led by dietitian. | Monthly dietitian appointments; 83% of participants attended >1 appointment. | NR | NR | I: 5.8 ± 5.3 kg %BW = NR BMI = 2.1 kg/m2. No comparator. |
| Barth et al., 2019 [24] | VLCD using Optifast 800® shakes, liquid-only diet. Products free of charge. | 800 kcal, 70 g protein, 100 g CHO, 20 g fat. | Five shakes, unlimited energy-free fluids. | 1 week. | Dietitian-led. Provided with food-based equivalent VLCD if products not tolerated. | Initial appointment plus two phone calls during the week to ensure adherence and accurate food recording. | Food diaries analysed by dietitian: 28/30 (94%) adherence (consumed solely Optifast®, n = 1 had diet-adherent food), mean intake 805 kcal and 70 g protein. | n = 1/31 pts never started diet (reason NR). | NR |
| Inoue et al., 2019 [36] | VLCD using Obecure® shake (one per day). Product free of charge. | NR—only product profile reported (178 kcal, 22 g protein, 15 g CHO, 2 g fat per product). | One meal replacement to replace “main meal”, no restriction on other two meals. Low-calorie vegetables with the replaced meal were allowed. | 3 weeks (20 days). | Dietitian-led. Dietitian provided “nutritional counselling” for 7 days, then VLCD for 20 days with information on a “nutritionally-balanced” diet, low-calorie foods, and appropriate mealtimes. | Unclear—at least twice during the one month preoperative period. | Adherence reported to dietitian and surgeon = 96.9%. | n = 1 stopped VLCD due to taste “intolerable”, no adverse events associated with VLCD. | I: 3.2 kg 4.2% BW BMI = 1.2 kg/m2. C: NR. |
| Liang et al., 2018 [25] | Healthy eating and exercise-“prehabilitation”. | NR | Dietary modifications, daily goals checklist (servings of fruit and vegetables). | Up to 26 weeks, based on body-weight-loss goal of 7%. | Multidisciplinary team: surgical specialists, medical weight-loss experts, dietitians, physical therapists, health educators, nurse practitioners, and study coordinators. | Weekly group meetings plus monthly assessments. | NR | NR | I: 2.72 ± 5.3 kg C: 2 ± 3.8 kg (Non-significant between groups, p = 0.308). |
| Burnand et al., 2016 [26] | VLCD using SlimFast® shakes. Funding source NR. | 800 kcal. Protein, CHO, and fat NR. | Two shakes, one ready-made meal of <3% fat (participants’ choice-not described). | 2 weeks. | Diet sheet given with diet instructions. Dietitian available to participants via phone. | NR | Via detailed dietary survey, 2-week duration-mean intake 947 kcal/day. | VLCD “well tolerated”. Limited detail. | I: 3.48 ± 1.98 kg BMI = 1.29 ± 0.74 kg/m2. 3.8%BW C: 0.98 ± 1.67 kg BMI= 0.36 ± 0.61 kg/m2 1.1%BW (kg and BMI difference between groups p < 0.0001). |
| Jones et al., 2016 [30] | VLCD, product not named. Funding source NR. | 800 kcal, protein/ CHO/fat NR. | NR | 2 weeks. | Verbal advice to adhere to VLCD (unclear by whom), diet sheets with dietary suggestions provided. | Once at beginning. | NR | NR | I: Median −1.4 kg (range gain 3 kg to 4.2 kg loss). No comparator. |
| Doyle et al., 2015 [35] | VLCD using Optifast 900®. Funding source NR. | 900 kcal, 90 g protein, 67 g CHO, fat NR. | Four shakes, up to 2 L water plus coffee/tea without milk or sugar. No other foods/ drinks allowed. | 4 to 15 weeks, median 7.3 weeks, guided by BMI reduction target of 10%. | Dietitian saw participants prior to starting diet. No other details given. | Unclear, seen by dietitian at least once at beginning. | NR | Constipation n = 2, no adverse effects, no diet dropouts. | I: BMI = 4.4 kg/m2 C: NR. |
| Rosen et al., 2015 [31] | “Protein sparing modified fast” (food-based VLCD). | 800 kcal, 1.2–1.4 g protein/kg ideal body weight, 40 mg (authors question accuracy) CHO, fat NR, vitamin and mineral supplementation. | Food-based using “high biological value” protein. | Mean 68 weeks (range 26–144), guided by patient-directed weight-loss goal aiming BMI < 40. | Care led by medical weight-loss specialist (unclear if nutritionally trained). “Nutritional team” administered the diet. | Goals and progress discussed at 3-monthly appointments with surgeon. | NR | NR | I: 24 ± 21 kg (range 2–80 kg) BMI = 9 ± 8 kg/m2 (range 0.6–33 kg/m2) 18 ± 12%BW. No comparator. |
| Reeves et al., 2013 [34] | Low-calorie diet using SlimFast® shake. Funding source NR. | 900 kcal, 33 g protein, fat 20–40%, and carbohydrate 30–50% of total daily calories. | Breakfast: 1 cup oatmeal/ SlimFast; Lunch: ¾ cup cottage cheese/SlimFast; Dinner: 85 g lean meat/fish/ SlimFast; Allowed ½ cup fruit per meal. | 1 week. | Diet information provided by surgeon. | Seen once by surgeon at beginning. | Adherence measured by surgeon asking patient on day of surgery- ”Nearly all” participants indicated they were completely adherent. | NR | NR |
| De Luis et al., 2012 [27] | Low-calorie diet using Optisource®, formula unclear-“envelope”. Funding source NR. | 1190 kcal, 63 g protein, 166 g CHO, 21 g fat. | Lunch and dinner meals replaced with Optisource, no limitations on other meals. | 12 weeks. | NR | NR | Dietary intake measured via 3-day food records: mean intake 1248 kcal/day (reduction of 543 kcal from baseline), 70 g protein, 163 g CHO, 35 g fat. | NR | I: 7.6 kg BMI = 3.1 kg/m2 C: 4.3 kg BMI= 2.1 kg/m2. |
| Chan and Chan et al., 2005 [32] | “Weight loss program”-low-calorie diet (food-based). | 1500 kcal, “low carbohydrate”, protein and fat NR. | Diet “limits carbohydrate” and encourages fruit and vegetables. | Median 17 weeks (range 1–530 weeks), guided by weight-loss goals of BMI < 28. | Weight-loss target and diet were explained at initial office visit, unclear by whom. | Once at beginning. | NR | NR | I: 11.5 ± 3.5 kg. 63% of obese reduced their BMI to <30 kg/m2. No comparator. |
| Pekkarinen and Mustajoki et al., 1997 [33] | VLCD using Modifast Sandoz® shake. Funding source NR. | 458 kcal, 52 g protein, 45 g CHO, 7 g fat. | 3 shakes, small amount of low-CHO vegetables, 2 L water/low-energy fluids. “Re-feeding” period commenced 1 month prior to surgery—reduced to 1 shake per day for 1 week, then to normal food. | Mean 14 weeks (range 7–24), guided by initial weight, planned operation, and weight-loss progress. | “Therapist” provided care, tailored appointments, including “nutritional education and behaviour therapy to create new eating habits and to prevent weight gain”. | Fortnightly. | Listed for those who experienced adverse outcomes/problems with adherence. n = 1 depression, n = 3 excess alcohol intake. n = 6 “suspected” intake of normal food. | n = 2 ceased VLCD within “first weeks”, reason NR. | I: 19.6 kg (range 4.3–44.7 kg) BMI = 7 kg/m2 15%BW. No comparator. |
| Author (Year), Surgery Performed | Intervention (I) and Comparator (C) Group Diets | Body Measurement/Composition Changes | Mortality | Wound/Other Complications | Subjective/Qualitative Outcomes |
|---|---|---|---|---|---|
| Griffin et al., 2021 [29] Various procedures including gynaecological, general, and orthopaedic | I: VLCD-based (800–1200 kcal). No comparator. | NR | NR | NR | Surgeon survey: changes from VLCD (n = 12): Facilitated access to organ(s): 75% agreement Shortened operating time: 75% agreement Reduced blood loss: 58% agreement Easier laparoscopic access: 58% agreement |
| Hollis et al., 2020 [28] Hernia repair/laparoscopic cholecystectomy | I: VLCD (800–900 kcal). C: Generic healthy eating information sheet. | I: WC = 6.1 ± 4.8 cm loss MM = 1.53 kg loss FM: 4.48 kg loss C: WC = 1.3 cm gain MM = 0.1 kg loss FM = 0.16 kg loss Both MM and FM: sig. difference between groups (p < 0.05) | (Deaths at 30 days) Both groups: 0% | NR | Health-related quality of life: I: +18 from baseline (p < 0.05) (improved) C: −1.8 from baseline (NS) |
| Lingamfelter et al., 2020 [37] Total hip/knee arthroplasty | I: Healthy eating. No comparator. | NR | NR | Delayed wound healing n = 2 (2.7%) Superficial infection: n = 1 (1.4%) Haematoma n = 1 (1.4%) | NR |
| Barth et al., 2019 [24] Partial hepatectomy | I: VLCD using Optifast 800® (800 kcal). C: Usual intake. | Hepatocyte glycogen score β = 1.6 in diet group vs. 2.5 in comparators (p < 0.0001). No significant difference in steatosis/steatohepatitis between groups. | (Deaths at 30 days) Both groups: 0% | Abscess: 3% of entire cohort Wound dehiscence: 6% of entire cohort | Surgeon blinded to groups: Judged the liver to be easier to mobilise and manipulate in the diet group: 1.86 versus 2.90 (p < 0.004) |
| Inoue et al., 2019 [36] Gastric resection for gastric cancer | I: VLCD using Obecure® (one/day). C: Usual intake (historical controls). | I: WC = −2.74 cm Waist:hip ratio reduced 0.01 cm (both reduced from baseline (p < 0.05) C: NR | I: “Hospital mortality” 0% C: NR but reported as NS between groups | Complications only reported for intervention: Pancreatic fistula (12.1%) Anastomotic leak/haemorrhage n = 2 (3%) Gastroparesis/Hepatobiliary disorder n = 2 (3%) | NR |
| Liang et al., 2018 [25] Ventral hernia repair | I: Healthy eating and exercise. C: “Standard counselling” without specific dietary change. | I: WC 4.6 ± 16.7 cm loss Hip circumference: 2.1 ± 6.5 cm loss C: WC 1.6± 8.9 cm Hip circumference: 2.3 ±8.4 cm loss Between groups: Waist: NS, p = 0.188 Hip: NS, p = 0.239 | NR | Haematoma I: n = 1/44 (2%) C: n = 0. Wound dehiscence I: n = 1 (2%) C: n = 0. Seroma I: n = 1 (2%) C: n = 0 | NR |
| Burnand et al., 2016 [26] Laparoscopic cholecystectomy | I: VLCD using SlimFast® (800 kcal). C: Usual intake. | NR | NR | Haematoma n = 1 in intervention group Not analysed for significance | Surgeon blinded to groups judged the difficulty of procedure-scale 1 (very difficult) to 4 (easy): Dissection of Calot’s triangle easier in intervention group (p < 0.02) No difference for other measures: ease of gallbladder retraction, ease of liver displacement, and liver friability |
| Jones et al., 2016 [30] Laparoscopic cholecystectomy | I: VLCD (800 kcal). No comparator. | NR | NR | NR | Surgeon (blinded to dietary compliance-“compliant” = loss of >2 kg): Compliant participants had better (easier) scores for all areas of difficulty (p = 0.018) Difficulty of visualization of Calot’s triangle and gallbladder bed dissection was higher in non-compliant participants but was NS (p = 0.07) All cases where the surgeon was unable to retract the liver > 90° (15%, n = 6/38) were non-compliant No difficulties were reported in retracting the liver or visualising Calot’s triangle in compliant participants vs. difficulties reported for 42% of non-compliant participants. (p < 0.05) |
| Doyle et al., 2015 [35] Liver donation (recipients) | I: VLCD using Optifast 900® (900 kcal). C: Usual intake. | NR | (Deaths at 90 days) I: n = 1 (8%) C: n = 1 (2%) (NS difference) | Wound complications: I: n = 1(8%) C: n = 1 (2%) Abdominal bleed/infection/ bile leak: I: n = 6 (46%) C: n = 13 (37%) Not analysed for significance separately. Aggregate of all complications = p = 0.11 (NS) | NR |
| Rosen et al., 2015 [31] Complex incisional hernia repair | I: Food-based VLCD (<800 kcal). No comparator. | NR | NR | Seroma n = 1 (4%) Surgical site infection n = 1 (4%) Deep infection n = 1 (4%) | NR |
| Reeves et al., 2013 [34] Liver resection for liver cancer | I: LCD using SlimFast® and/or food (900 kcal). C: Usual intake. | NR | (Deaths at 30 days) n = 0 both groups | Complications per Clavien£ scale I: n = 12 (25%) C: n = 15 (25%) Infectious complications I: n = 9 (18%) C: n = 6 (10%) NS between groups | NR |
| de Luis et al., 2012 [27] Hip/knee arthroplasty | I: LCD using Optisource® (1190 kcal). C: Reduction of usual intake by 500 kcal (mean 1290 kcal). | I: WC = −5.5 cm Fat Free Mass = −700 g (NS) FM = −5.6 kg C: WC = −4.2 cm Fat Free Mass = −1.2 kg (NS) FM = −4 kg Waist: hip ratio change: both groups NS | (Time-frame NR) n = 0 both groups | DVT: n = 1 each group (NS) | NR |
| Chan and Chan et al., 2005 [32] Ventral incisional hernia repair | I: Food-based LCD (1500 kcal). No control group (but non-obese not given diet). | NR | Perioperative deaths n = 0 Postoperative deaths NR | Postoperative hernia recurrence: Obese (intervention group): n = 5/72 (7%) Non-obese: n = 8/116 (6.9%) | NR |
| Pekkarinen and Mustajoki et al., 1997 [33] Various procedures including orthopaedic, general, and cardiac | I: VLCD using Modifast Sandoz® (459 kcal). No comparator. | NR | NR | Haematoma n = 2 (13%) Surgical site infection n = 3 (20%) | NR |
| Operating Time (mins) | Blood Loss (mL) | Infection Rate (%) | Length of Stay (Days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Diet Used, Surgery Type | I n | C n | I | C | Difference for I Group € | I | C | Difference for I Group € | I | C | Difference for I Group € | I | C | Difference for I Group € |
| Hollis et al., 2020 [28], VLCD, Mixed General | 20 | 14 | 89.9 ± 29.3 | 107.5 ± 41.4 | −17.6 min NS, p = 0.16 a | NR | 0 | 14.2 | −14.2% NS, p = 0.16 b | 1.2 ± 0.5 | 1.4 ± 0.7 | −0.2 days NS, p = 0.47 a | ||
| BarthBarth et al., 2019 [24], VLCD, Liver resection | 30 | 30 | 246 ± 72 | 258 ± 90 | −12 min NS, p = 0.42 c | 452 | 863 | −411 mL (p < 0.05) c | NR | 5 (median) | 4 (median) | +1 day NS c | ||
| Inoue et al., 2019 [36] VLCD, Gastrectomy | 27 | 23 | 355 (196–567) (median, range) | 374 (258–482) (median, range) | −19 min NS, p = 0.35 d | 49 (1–282) (median, range) | 76 (34–914) (median, range) | −27 mL (p < 0.05) d | 6.1 | NR | NR | NR | ||
| Liang et al., 2018 [25], Healthy Eating, Ventral hernia repair | 44 | 34 | 102.3 ± 55.8 | 91.0 ± 49.2 | +11.3 min NS, p = 0.414 c | NR | 0 | 0 | nil | 0 (median) (0.2 IQR) | 0 (median) (0.1 IQR) | No difference NS, p = 0.687 e | ||
| Burnand et al., 2016 [26] VLCD Laparoscopic cholecystectomy | 21 | 25 | 25 (18–41) (median, range) | 31 (20–170) (median, range) | −6 min (p < 0.01) f | NR | NR | 0.4 (0.2–6.1) (median, range) | 0.3 (0.2–1.2) (median, range) | −0.1 day NS, p = 0.36 f | ||||
| Doyle et al., 2015 [35], VLCD Liver resection for donation | 14 | 53 | NR | 417 (range 255–579) | 358 (range 286–429) | +59 mL NS g | NR | 16 (mean) (7.8–24.7 95% CI) | 18 (mean) (11.4–23.9 95% CI) | −2 days NS, p > 0.99 g | ||||
| Reeves et al., 2013 [34], LCD, liver resection | 51 | 60 | NR | 600 (51), mean, SEM | 906 (76), mean, SEM | −306 mL (p < 0.05) h | 18% | 10% | −8% NS h | 6.3 (1.1), mean, SEM | 6.3(1.2), mean, SEM | No difference NS h | ||
| De Luis et al., 2012 [27], LCD, Hip/knee replacement | 20 | 20 | 86.5 ± 21.8 | 91.8 ± 41.7 | −5.3 min NS i | NR | 0 | 0 | nil | 8.1 ± 2.7 | 8.9 ± 6.7 | −0.8 days NS i | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffin, S.B.; Palmer, M.A.; Strodl, E.; Lai, R.; Burstow, M.J.; Ross, L.J. Elective Surgery in Adult Patients with Excess Weight: Can Preoperative Dietary Interventions Improve Surgical Outcomes? A Systematic Review. Nutrients 2021, 13, 3775. https://doi.org/10.3390/nu13113775
Griffin SB, Palmer MA, Strodl E, Lai R, Burstow MJ, Ross LJ. Elective Surgery in Adult Patients with Excess Weight: Can Preoperative Dietary Interventions Improve Surgical Outcomes? A Systematic Review. Nutrients. 2021; 13(11):3775. https://doi.org/10.3390/nu13113775
Chicago/Turabian StyleGriffin, Sally B., Michelle A. Palmer, Esben Strodl, Rainbow Lai, Matthew J. Burstow, and Lynda J. Ross. 2021. "Elective Surgery in Adult Patients with Excess Weight: Can Preoperative Dietary Interventions Improve Surgical Outcomes? A Systematic Review" Nutrients 13, no. 11: 3775. https://doi.org/10.3390/nu13113775






