The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Analysis of Thyroid-Axis Related Enzyme Activities
2.3. TE Analysis in Serum, Tissue, and Diet
2.4. Selenop Analysis in Serum
2.5. Determination of Serum Parameters by ELISA or Multiplex Assay
2.6. Analysis of Enzyme Activities
2.7. PCR Analysis of the Liver, Kidney, and Pituitary Gland
2.8. Statistical Analysis
3. Results
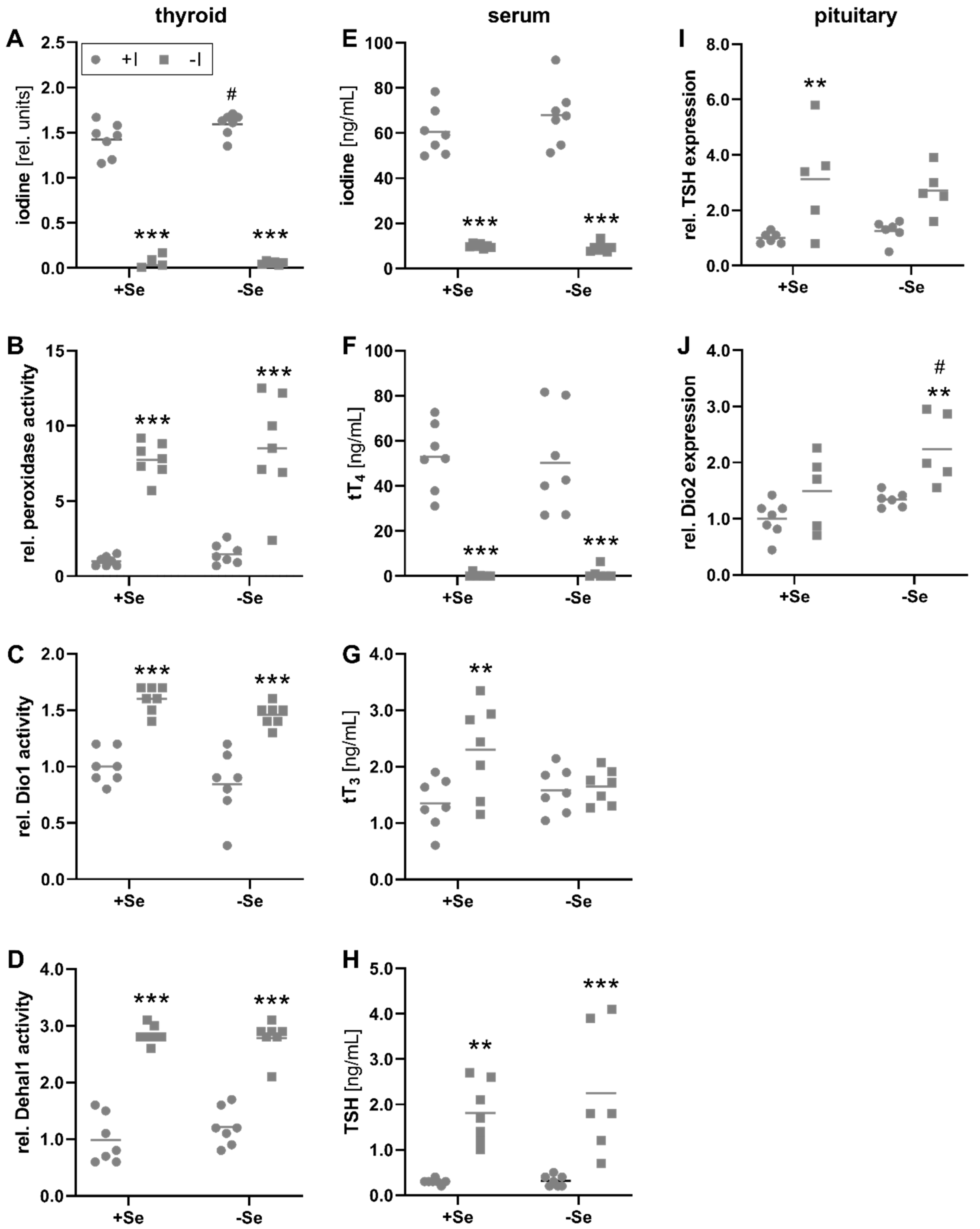
3.1. Biomarkers of Iodine Status in Relation to Iodine and Selenium Supply
3.2. The Systemic Selenium Status Is Unaffected by Iodine Deficiency
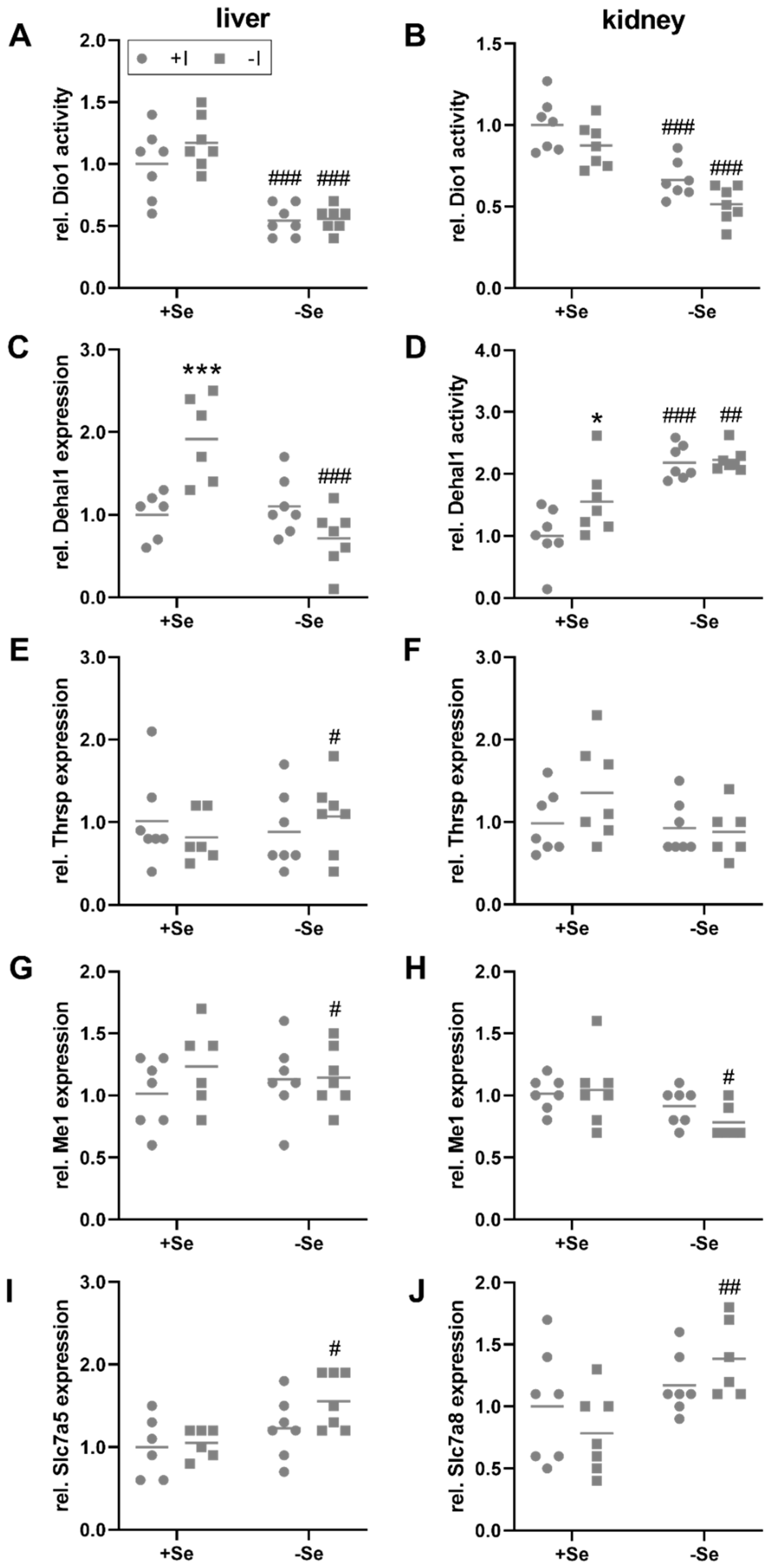
3.3. Peripheral Effects of Thyroid Hormone in Response to a Low Iodine and Selenium Supply
3.4. Selenium and Iodine Deficiency Have Almost No Effect on the Status of Other Trace Elements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigo, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.R. Iodine and thyroid function. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Kohrle, J. Thyroid hormone deiodinases—A selenoenzyme family acting as gate keepers to thyroid hormone action. Acta Med. Austriaca 1996, 23, 17–30. [Google Scholar]
- Zimmermann, M.B.; Andersson, M. Global Endocrinology: Global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 2021, 185, R13–R21. [Google Scholar] [CrossRef]
- Biban, B.G.; Lichiardopol, C. Iodine Deficiency, Still a Global Problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar] [CrossRef]
- Aburto, N.; Abudou, M.; Candeias, V.; Wu, T. Effect and Safety of Salt Iodization to Prevent Iodine Deficiency Disorders: A Systematic Review with Meta-Analyses; WHO eLibrary of Evidence for Nutrition Actions (eLENA); World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [Green Version]
- Hoeflich, J.; Hollenbach, B.; Behrends, T.; Hoeg, A.; Stosnach, H.; Schomburg, L. The choice of biomarkers determines the selenium status in young German vegans and vegetarians. Br. J. Nutr. 2010, 104, 1601–1604. [Google Scholar] [CrossRef]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimaki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [Green Version]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef]
- Menzel, J.; Abraham, K.; Stangl, G.I.; Ueland, P.M.; Obeid, R.; Schulze, M.B.; Herter-Aeberli, I.; Schwerdtle, T.; Weikert, C. Vegan Diet and Bone Health-Results from the Cross-Sectional RBVD Study. Nutrients 2021, 13, 685. [Google Scholar] [CrossRef]
- Kohrle, J.; Jakob, F.; Contempre, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef]
- Schomburg, L.; Kohrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2011, 8, 160–171. [Google Scholar] [CrossRef]
- Chiu-Ugalde, J.; Wirth, E.K.; Klein, M.O.; Sapin, R.; Fradejas-Villar, N.; Renko, K.; Schomburg, L.; Kohrle, J.; Schweizer, U. Thyroid function is maintained despite increased oxidative stress in mice lacking selenoprotein biosynthesis in thyroid epithelial cells. Antioxid. Redox. Signal. 2012, 17, 902–913. [Google Scholar] [CrossRef]
- Goyens, P.; Golstein, J.; Nsombola, B.; Vis, H.; Dumont, J.E. Selenium deficiency as a possible factor in the pathogenesis of myxoedematous endemic cretinism. Acta Endocrinol. 1987, 114, 497–502. [Google Scholar] [CrossRef]
- Contempre, B.; Duale, N.L.; Dumont, J.E.; Ngo, B.; Diplock, A.T.; Vanderpas, J. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin. Endocrinol. 1992, 36, 579–583. [Google Scholar] [CrossRef]
- Yao, Y.; Pei, F.; Kang, P. Selenium, iodine, and the relation with Kashin-Beck disease. Nutrition 2011, 27, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Reyes, R.; Egrise, D.; Boelaert, M.; Goldman, S.; Meuris, S. Iodine deficiency mitigates growth retardation and osteopenia in selenium-deficient rats. J. Nutr. 2006, 136, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B. The influence of iron status on iodine utilization and thyroid function. Annu. Rev. Nutr. 2006, 26, 367–389. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Kohrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef]
- Yu, X.; Shan, Z.; Li, C.; Mao, J.; Wang, W.; Xie, X.; Liu, A.; Teng, X.; Zhou, W.; Li, C.; et al. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J. Clin. Endocrinol. Metab. 2015, 100, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Indo, Y.; Higashi, A.; Matsuda, I.; Kashiwabara, N.; Nakashima, I. Conversion of thyroxine into tri-iodothyronine in zinc deficient rat liver. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 799–805. [Google Scholar] [CrossRef]
- Chen, M.D.; Lin, P.Y.; Lin, W.H. Zinc supplementation on serum levels and hepatic conversion of thyroid hormones in obese (ob/ob) mice. Biol. Trace Elem. Res. 1998, 61, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.; Futagoishi-Suginohara, Y.; Matsukura, M.; Nakamura, T.; Higashi, A.; Shinohara, M.; Matsuda, I. Zinc supplementation alters thyroid hormone metabolism in disabled patients with zinc deficiency. J. Am. Coll. Nutr. 1994, 13, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Pekary, A.E.; Lukaski, H.C.; Mena, I.; Hershman, J.M. Processing of TRH precursor peptides in rat brain and pituitary is zinc dependent. Peptides 1991, 12, 1025–1032. [Google Scholar] [CrossRef]
- Martin, N.P.; Marron Fernandez de Velasco, E.; Mizuno, F.; Scappini, E.L.; Gloss, B.; Erxleben, C.; Williams, J.G.; Stapleton, H.M.; Gentile, S.; Armstrong, D.L. A rapid cytoplasmic mechanism for PI3 kinase regulation by the nuclear thyroid hormone receptor, TRbeta, and genetic evidence for its role in the maturation of mouse hippocampal synapses in vivo. Endocrinology 2014, 155, 3713–3724. [Google Scholar] [CrossRef] [PubMed]
- Civitareale, D.; Saiardi, A.; Falasca, P. Purification and characterization of thyroid transcription factor 2. Biochem. J. 1994, 304 Pt 3, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, T.; Sakurai, A.; DeGroot, L.J. Effects of zinc and other divalent metals on deoxyribonucleic acid binding and hormone-binding activity of human alpha 1 thyroid hormone receptor expressed in Escherichia coli. Endocrinology 1991, 129, 3027–3033. [Google Scholar] [CrossRef]
- Mittag, J.; Behrends, T.; Nordstrom, K.; Anselmo, J.; Vennstrom, B.; Schomburg, L. Serum copper as a novel biomarker for resistance to thyroid hormone. Biochem. J. 2012, 443, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Hedrich, H.J. The Laboratory Mouse; Academic Press: Cambridge, MA, USA, 2012; p. 1. Available online: https://www.elsevier.com/books/the-laboratory-mouse/hedrich/978-0-12-336425-8 (accessed on 22 October 2021).
- Renko, K.; Hoefig, C.S.; Hiller, F.; Schomburg, L.; Kohrle, J. Identification of iopanoic acid as substrate of type 1 deiodinase by a novel nonradioactive iodide-release assay. Endocrinology 2012, 153, 2506–2513. [Google Scholar] [CrossRef] [Green Version]
- Sandell, E.B.; Kolthoff, I.M. Micro determination of iodine by a catalytic method. Microchim. Acta 1937, 1, 9–25. [Google Scholar] [CrossRef]
- Renko, K.; Hoefig, C.S.; Dupuy, C.; Harder, L.; Schwiebert, C.; Kohrle, J.; Schomburg, L. A Nonradioactive DEHAL Assay for Testing Substrates, Inhibitors, and Monitoring Endogenous Activity. Endocrinology 2016, 157, 4516–4525. [Google Scholar] [CrossRef]
- Paul Friedman, K.; Watt, E.D.; Hornung, M.W.; Hedge, J.M.; Judson, R.S.; Crofton, K.M.; Houck, K.A.; Simmons, S.O. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol. Sci. 2016, 151, 160–180. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, M.; Lossow, K.; Kopp, J.F.; Schwerdtle, T.; Kipp, A.P. Crosstalk of Nrf2 with the Trace Elements Selenium, Iron, Zinc, and Copper. Nutrients 2019, 11, 2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, J.F.; Muller, S.M.; Pohl, G.; Lossow, K.; Kipp, A.P.; Schwerdtle, T. A quick and simple method for the determination of six trace elements in mammalian serum samples using ICP-MS/MS. J. Trace Elem. Med. Biol. 2019, 54, 221–225. [Google Scholar] [CrossRef]
- Meyer, S.; Markova, M.; Pohl, G.; Marschall, T.A.; Pivovarova, O.; Pfeiffer, A.F.H.; Schwerdtle, T. Development, validation and application of an ICP-MS/MS method to quantify minerals and (ultra-)trace elements in human serum. J. Trace Elem. Med. Biol. 2018, 49, 157–163. [Google Scholar] [CrossRef]
- Wandt, V.K.; Winkelbeiner, N.; Lossow, K.; Kopp, J.F.; Schwarz, M.; Alker, W.; Nicolai, M.M.; Simon, L.; Dietzel, C.; Hertel, B.; et al. Ageing-associated effects of a long-term dietary modulation of four trace elements in mice. Redox Biol. 2021, 46, 102083. [Google Scholar] [CrossRef]
- Florian, S.; Krehl, S.; Loewinger, M.; Kipp, A.; Banning, A.; Esworthy, S.; Chu, F.F.; Brigelius-Flohe, R. Loss of GPx2 increases apoptosis, mitosis, and GPx1 expression in the intestine of mice. Free Radic. Biol. Med. 2010, 49, 1694–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krehl, S.; Loewinger, M.; Florian, S.; Kipp, A.P.; Banning, A.; Wessjohann, L.A.; Brauer, M.N.; Iori, R.; Esworthy, R.S.; Chu, F.F.; et al. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis 2012, 33, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.; Schweizer, U. Thyroid Hormone Transport and Transporters. Vitam. Horm. 2018, 106, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef]
- Chakera, A.J.; Pearce, S.H.; Vaidya, B. Treatment for primary hypothyroidism: Current approaches and future possibilities. Drug Des. Devel. Ther. 2012, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.A.; American Association Of Clinical Endocrinologists. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012, 22, 1200–1235. [Google Scholar] [CrossRef]
- Obregon, M.J.; Escobar del Rey, F.; Morreale de Escobar, G. The effects of iodine deficiency on thyroid hormone deiodination. Thyroid 2005, 15, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Solis, S.J.; Villalobos, P.; Orozco, A.; Delgado, G.; Quintanar-Stephano, A.; Garcia-Solis, P.; Hernandez-Montiel, H.L.; Robles-Osorio, L.; Valverde, R.C. Inhibition of intrathyroidal dehalogenation by iodide. J. Endocrinol. 2011, 208, 89–96. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Jiang, Y.; Bao, S.; Shan, Z.; Teng, W. Expression of Iodotyrosine Deiodinase in Thyroid and Other Organs in Iodine-Deficient and Iodine-Excess Rats. Biol. Trace Elem. Res. 2015, 167, 272–279. [Google Scholar] [CrossRef]
- Yoshihara, A.; Luo, Y.; Ishido, Y.; Usukura, K.; Oda, K.; Sue, M.; Kawashima, A.; Hiroi, N.; Suzuki, K. Inhibitory effects of methimazole and propylthiouracil on iodotyrosine deiodinase 1 in thyrocytes. Endocr. J. 2019, 66, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Larsen, P.R.; Zavacki, A.M. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur. Thyroid J. 2012, 1, 232–242. [Google Scholar] [CrossRef]
- Bianco, A.C.; da Conceicao, R.R. The Deiodinase Trio and Thyroid Hormone Signaling. Methods Mol. Biol. 2018, 1801, 67–83. [Google Scholar] [CrossRef]
- Malik, R.; Hodgson, H. The relationship between the thyroid gland and the liver. QJM 2002, 95, 559–569. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef]
- Akahoshi, N.; Anan, Y.; Hashimoto, Y.; Tokoro, N.; Mizuno, R.; Hayashi, S.; Yamamoto, S.; Shimada, K.I.; Kamata, S.; Ishii, I. Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice. J. Nutr. Biochem. 2019, 69, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Contempre, B.; Le Moine, O.; Dumont, J.E.; Denef, J.F.; Many, M.C. Selenium deficiency and thyroid fibrosis. A key role for macrophages and transforming growth factor beta (TGF-beta). Mol. Cell. Endocrinol. 1996, 124, 7–15. [Google Scholar] [CrossRef]
- Meinhold, H.; Campos-Barros, A.; Behne, D. Effects of selenium and iodine deficiency on iodothyronine deiodinases in brain, thyroid and peripheral tissue. Acta Med. Austriaca 1992, 19 (Suppl. 1), 8–12. [Google Scholar] [PubMed]
- Zavacki, A.M.; Ying, H.; Christoffolete, M.A.; Aerts, G.; So, E.; Harney, J.W.; Cheng, S.Y.; Larsen, P.R.; Bianco, A.C. Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology 2005, 146, 1568–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Morales, A.; Gullberg, H.; Fernandez, L.; Stahlberg, N.; Lee, N.H.; Vennstrom, B.; Norstedt, G. Patterns of liver gene expression governed by TRbeta. Mol. Endocrinol. 2002, 16, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- Nock, S.; Johann, K.; Harder, L.; Wirth, E.K.; Renko, K.; Hoefig, C.S.; Kracke, V.; Hackler, J.; Engelmann, B.; Rauner, M.; et al. CD5L Constitutes a Novel Biomarker for Integrated Hepatic Thyroid Hormone Action. Thyroid 2020, 30, 908–923. [Google Scholar] [CrossRef]
- Ramadoss, P.; Abraham, B.J.; Tsai, L.; Zhou, Y.; Costa-e-Sousa, R.H.; Ye, F.; Bilban, M.; Zhao, K.; Hollenberg, A.N. Novel mechanism of positive versus negative regulation by thyroid hormone receptor beta1 (TRbeta1) identified by genome-wide profiling of binding sites in mouse liver. J. Biol. Chem. 2014, 289, 1313–1328. [Google Scholar] [CrossRef] [Green Version]
- LaFave, L.T.; Augustin, L.B.; Mariash, C.N. S14: Insights from knockout mice. Endocrinology 2006, 147, 4044–4047. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Manchon, C.; Butta, N.; Ferrer, M.; Ayuso, M.S.; Parrilla, R. Molecular cloning and functional characterization of the human cytosolic malic enzyme promoter: Thyroid hormone responsiveness. DNA Cell Biol. 1997, 16, 533–544. [Google Scholar] [CrossRef]
- Gonzalez-Manchon, C.; Ayuso, M.S.; Parrilla, R. AP-1 and T3RE cis elements operate as a functional unit in the transcriptional control of the human malic enzyme gene. Gene 1999, 226, 111–119. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.B. Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol. Trace Elem. Res. 2014, 159, 87–98. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.C.; Chung, S.; Kim, S.; Yoon, J.W.; Park, Y.J. Exploring the role of copper and selenium in the maintenance of normal thyroid function among healthy Koreans. J. Trace Elem. Med. Biol. 2020, 61, 126558. [Google Scholar] [CrossRef]
- Rasic-Milutinovic, Z.; Jovanovic, D.; Bogdanovic, G.; Trifunovic, J.; Mutic, J. Potential Influence of Selenium, Copper, Zinc and Cadmium on L-Thyroxine Substitution in Patients with Hashimoto Thyroiditis and Hypothyroidism. Exp. Clin. Endocrinol. Diabetes 2017, 125, 79–85. [Google Scholar] [CrossRef]
- Talebi, S.; Ghaedi, E.; Sadeghi, E.; Mohammadi, H.; Hadi, A.; Clark, C.C.T.; Askari, G. Trace Element Status and Hypothyroidism: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2020, 197, 1–14. [Google Scholar] [CrossRef]
- Mehl, S.; Sun, Q.; Gorlich, C.L.; Hackler, J.; Kopp, J.F.; Renko, K.; Mittag, J.; Schwerdtle, T.; Schomburg, L. Cross-sectional analysis of trace element status in thyroid disease. J. Trace Elem. Med. Biol. 2020, 58, 126430. [Google Scholar] [CrossRef]

| Elements | Feeding Recommen-dations [mg/kg] | TE Content of Feed [mg/kg] | TE in Drinking Water [mg/kg] | Total Supply [mg/kg] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| +Se/+I | +Se/−I | −Se/+I | −Se/−I | +Se/+I | +Se/−I | −Se/+I | −Se/−I | |||
| Se | 0.15 | 0.02 | 0.13 | 0.13 | - | - | 0.15 | 0.15 | 0.02 | 0.02 |
| I | 0.15 | 0.03 | 0.15 | - | 0.15 | 0.18 | 0.03 | 0.18 | 0.03 | |
| Cu | 6.00 | 3.65 | - | - | - | - | 3.65 * | 3.65 * | 3.65 * | 3.65 * |
| Fe | 35.0 | 17.2 | 17.8 | 17.8 | 17.8 | 17.8 | 35.0 | 35.0 | 35.0 | 35.0 |
| Mn | 10.0 | 9.65 | - | - | - | - | 9.65 | 9.65 | 9.65 | 9.65 |
| Zn | 30.0 | 46.2 | - | - | - | - | 46.2 | 46.2 | 46.2 | 46.2 |
| Tissue | Element [Unit] | +Se/+I | +Se/−I | −Se/+I | −Se/−I | Se Effect | I Effect |
|---|---|---|---|---|---|---|---|
| serum | Fe [µg/L] | 1916 ± 572 | 2277 ± 1093 | 2049 ± 341 | 2260 ± 409 | n.s. | n.s. |
| transferrin [mg/mL] | 2.1 ± 0.2 | 2.4 ± 0.6 | 2.2 ± 0.2 | 2.3 ± 0.3 | n.s. | n.s. | |
| Zn [µg/L] | 711 ± 126 | 680 ± 158 | 689 ± 138 | 773 ± 85.1 | n.s. | n.s. | |
| Cu [µg/L] | 428 ± 92.7 | 351 ± 58.7 | 353 ± 95.1 | 433 ± 127 | n.s. | n.s. | |
| liver | Fe [mg/kg] | 72.3 ± 10.5 | 67.6 ± 17.5 | 70.8 ± 8.5 | 66.7 ± 9.3 | n.s. | n.s. |
| Zn [mg/kg] | 27.1 ± 4.1 | 27.8 ± 2.8 | 27.1 ± 3.1 | 25.8 ± 1.7 | n.s. | n.s. | |
| Cu [mg/kg] | 4.6 ± 0.3 | 4.8 ± 0.3 | 4.6 ± 0.4 | 4.9 ± 0.3 | n.s. | 0.088 | |
| kidney | Fe [mg/kg] | 84.2 ± 4.7 | 77.0 ± 8.5 | 87.7 ± 8.1 | 89.5 ± 12.9 | * | n.s. |
| Zn [mg/kg] | 21.5 ± 1.0 | 22.3 ± 0.7 | 22.2 ± 0.5 | 22.9 ± 1.5 | n.s. | 0.053 | |
| Cu [mg/kg] | 5.1 ± 0.3 | 4.9 ± 0.3 | 4.9 ± 0.3 | 5.1 ± 0.4 | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lossow, K.; Renko, K.; Schwarz, M.; Schomburg, L.; Schwerdtle, T.; Kipp, A.P. The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice. Nutrients 2021, 13, 3773. https://doi.org/10.3390/nu13113773
Lossow K, Renko K, Schwarz M, Schomburg L, Schwerdtle T, Kipp AP. The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice. Nutrients. 2021; 13(11):3773. https://doi.org/10.3390/nu13113773
Chicago/Turabian StyleLossow, Kristina, Kostja Renko, Maria Schwarz, Lutz Schomburg, Tanja Schwerdtle, and Anna Patricia Kipp. 2021. "The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice" Nutrients 13, no. 11: 3773. https://doi.org/10.3390/nu13113773
APA StyleLossow, K., Renko, K., Schwarz, M., Schomburg, L., Schwerdtle, T., & Kipp, A. P. (2021). The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice. Nutrients, 13(11), 3773. https://doi.org/10.3390/nu13113773








