Abstract
Growing evidence demonstrates human milk’s protective effect against necrotizing enterocolitis (NEC). Human milk derives these properties from biologically active compounds that influence intestinal growth, barrier function, microvascular development, and immunological maturation. Among these protective compounds are growth factors that are secreted into milk with relatively high concentrations during the early postnatal period, when newborns are most susceptible to NEC. This paper reviews the current knowledge on human milk growth factors and their mechanisms of action relevant to NEC prevention. It will also discuss the stability of these growth factors with human milk pasteurization and their potential for use as supplements to infant formulas with the goal of preventing NEC.
Keywords:
necrotizing enterocolitis; human milk; growth factors; Epidermal Growth Factor (EGF); Heparin-Binding EGF-like Growth Factor (HB-EGF); Insulin-like Growth Factor 1 (IGF-1) Insulin-like Growth Factor 2 (IGF-2); Vascular Endothelial Growth Factor (VEGF); Erythropoetin (EPO); Granulocyte Colony Stimulating Factor (G-CSF); Holder pasteurization 1. Introduction
Necrotizing enterocolitis (NEC) is the most frequently encountered disease affecting the premature infant intestine with an estimated incidence of 5 to 10% among very low birth weight (<1500 g) neonates [1]. NEC carries a mortality rate of about 20–30% [2]. Of babies who develop NEC, approximately 30% will require surgery. Additionally, long-term complications are associated with this disease including parenteral nutrition-associated cholestasis and liver dysfunction, poor growth/malnutrition, metabolic bone disease, short bowel syndrome, sepsis/severe infection, and neurocognitive impairment [3].
The incidence of NEC has been reported to be lower in premature infants fed human milk compared with those fed infant formula [4,5]. Human milk provides immunologic, nutritional and developmental benefits to the growing newborn via multiple molecular and cellular mechanisms. It is thought that growth factors (GFs) contained in human milk may mediate some of these mechanisms.
NEC is characterized by different degrees of intestinal necrosis likely resulting as the end point of several mechanistic pathways where factors such as abnormal bacterial colonization, immunologic immaturity, immature intestinal barrier function, and microvascular under-development play a role. This review discusses the current knowledge on the protective properties conferred by human milk GFs on the intestines after birth and when known, offers perspective on GFs present in the amniotic fluid prenatally. We review the mechanism of action (Table 1) and reported levels of GFs in breast milk (Table 2) and discuss the potential therapeutic applications for reducing NEC in high-risk neonates.

Table 1.
A summary of mechanisms by which each growth factor offers protection from NEC in the neonatal intestines. Additionally, information regarding stability of the compound orally (gastric secretions) and following Holder pasteurization is shown.

Table 2.
Human milk growth factor concentrations with comparisons by preterm birth status and stage of lactation.
Question: In the clinical context of necrotizing enterocolitis, what are the developmental benefits elicited by human milk growth factors and how should these best be utilized from a therapeutic perspective?
Objectives:
- List the growth factors contained within human milk that are shown to have clinical importance in preventing necrotizing enterocolitis.
- For each growth factor, summarize levels in human milk and describe how levels change over time.
- Describe each growth factor’s biochemical and cellular mechanism for augmenting intestinal health.
- Summarize therapeutic trials of growth factors
- Describe donor milk processing and effects on human milk growth factors.
- Provide insight into the next steps required to establish therapeutic potential for each growth factor.
2. Methods
In a systematic search process, our team reviewed primary literature results written in English, from keyword queries in Ovid MEDLINE and Epub as well as Google Scholar. Texts with the following status: Ahead of Print, In-Process, In-Data-Review and Other Non-Indexed Citations, Daily and Versions from dates from 1946 to March 05, 2021 were assessed. From that search, 3683 articles matched search terms and 120 articles were included based on scientific rigor, impact and relevance to our topic.
Search Strategy:
- exp Milk, Human/ or exp Infant Formula/ or (breast adj2 (milk or feed)).ti,ab,kw. or ((donor or mother*) adj3 milk).ti,ab,kw. (n = 31,935)
- (intestin* adj5 (development or pathology)).ti,ab,kw. (n = 7381)
- growth factor.ti,ab,kw. (n = 328,920)
- exp “Intercellular Signaling Peptides and Proteins”/ (n = 1,078,972)
- 3 or 4 (n = 1,216,365)
- 1 and 5 (n = 1180)
- exp Infant, Premature/ (n = 57,394)
- exp Infant, Premature, Diseases/ or exp Infant, Low Birth Weight/ or exp Infant, Very Low Birth Weight/ (n = 73,576)
- exp Infant, Premature/ or exp Infant, Premature, Diseases/ or exp Infant, Low Birth Weight/ or exp Infant, Very Low Birth Weight/ or (premature or preterm).ti,ab,kw. (n = 239,737)
- 7 or 8 or 9 (n = 239,737)
- 6 and 10 (n = 178)
- limit 11 to english language (n = 174)
- exp Enterocolitis, Necrotizing/ (n = 3683)
- 1 and 5 and 13 (n = 67)
- 12 or 14 (n = 193)
- limit 15 to english language (n = 191)
3. Narrative
3.1. Epidermal Growth Factor (EGF)
Epidermal Growth Factor (EGF) is thought to decrease the susceptibility of infants to NEC via several mechanisms that are reviewed here. Data continue to emerge from both animal and human studies exploring the potential therapeutic use of EGF as an additive to pasteurized breast milk or to infant formulas [44,45]. Additionally, in utero, the fetal intestine is exposed to increasing levels of EGF in amniotic fluid as gestation progresses with the most rapid increase in concentration seen near term, a characteristic that suggests a role for EGF in the maturation of the intestines in late gestation [46,47].
EGF, a growth factor first discovered in saliva [48], exists at relatively high concentrations in human colostrum and decreases steadily in human milk throughout the first 2 months of lactation [9,38] to about half their initial levels in milk from women who delivered full term infants [38]. Concentrations of EGF in milk from women who delivered at 23–27 weeks of gestation were higher than in milk from mothers of term infants at similar stages of lactation [37]. Importantly, the Holder pasteurization method commonly used for donor breast milk processing does not reduce EGF concentrations [49].
EGF has been known since the early 1980s to play a role in intestinal epithelial growth, maturation, and development [50,51]. More specifically, EGF binds to EGF receptors (EGFR) and increases the proliferation rate of intestinal epithelial cells in adult mice within hours of exposure [6]. These early studies demonstrated increased intestinal size, weight, cell production, and digestive enzyme activity. In addition, EGF has recently been shown to promote the barrier function of intestinal epithelial cells [10]. In Caco-2 cells exposed to hydrogen peroxide, EGF prevents tight junction and actin cytoskeleton disruption, and reduces epithelial barrier permeability through MAPK (mitogen-activated protein kinase)-dependent mechanisms [8]. When EGF signaling is inhibited in dams, neonatal mice have increased bacterial translocation of pathogenic E. Coli in mesenteric lymph nodes, splenic tissue, or hepatic tissue samples. This further suggests a protective role for EGF/EGFR dependent signaling on intestinal barrier [9]. This translocation is thought to occur via goblet cell associated passages (GAP) which are suppressed by a functioning EGF pathway [9].
EGF also promotes intestinal epithelial repair and regeneration following injury [7]. In a neonatal rat model of NEC, EGF supplementation of rat milk substitute (RMS) decreased NEC incidence, decreased histologic NEC severity, reduced the ileal production of the pro-inflammatory cytokine IL18, and increased the production of interleukin (IL)-10 and of its transcription factor, Sp1, when compared with RMS alone [52]. More recently, EGF’s contribution to immunomodulation was further suggested by evidence that milk-derived EGF attenuates Toll-like Receptor4 (TLR4) signaling in the neonatal intestine [11], which is thought to contribute to the development of NEC [11,12].
In a step towards supplementing formula with EGF, genetically modified soybeans have been engineered to produce functional human EGF on an industrial scale [44]. In a rat model of NEC, soybean-derived EGF supplementation has been shown to improve intestinal barrier function, to reduce the expression of pro-inflammatory proteins Cyclooxygenase-2 (COX-2) and Inducible Nitric Oxide Synthase (iNOS), and to decrease the incidence of intestinal injury [44]. Given its protective effects in experimental models and its stability in the gastric environment [53], EGF wields potential as a preventative therapy among high-risk newborns.
3.2. Heparin-Binding EGF-like Growth Factor (HB-EGF)
Heparin-Binding EGF-like Growth Factor (HB-EGF) is a member of the EGFR family of ligands. First described in the 1990s as a product of human macrophages [54], HB-EGF is an autocrine signaling molecule secreted by many cell types, including macrophages, fibroblasts, smooth muscle cells and epithelial cells [39,55]. Its presence in both human milk and amniotic fluid suggests its importance in early development of the intestine.
While levels of HB-EGF are quantitatively less than those of EGF in human milk [39,55], HB-EGF has higher potency than EGF as it binds the EGF receptor with a much higher affinity than EGF. As opposed to EGF, previous studies did not show consistent differences in HB-EGF concentration between human milk from different post-conceptional age or post-natal age [39].
HB-EGF promotes epithelial cell proliferation, migration and wound healing [13], thus improving gut barrier function [17]. In addition to its ability to bind the EGFR, HB-EGF binds to N-arginine dibasic convertase to mediate cell migration via the EGF receptor ErbB1 [15]. Following ischemia/reperfusion injury, HB-EGF is upregulated and enhances intestinal epithelial cell restitution via Phosphoinositide 3-kinase (PI3K/Akt) and Mitogen-activated protein kinase/ Extracellular Signal-Regulated kinase (MEK/ERK1/2) [56]. Similar to EGF, HB-EGF also attenuates local inflammation [57] and intestinal epithelial cell apoptosis [22] in several models. Additionally, in rat NEC models, HB-EGF reduces production of reactive oxygen species (ROS) [18], promotes intestinal epithelial cell regeneration [14,16], attenuates apoptosis [22], and protects intestinal stem cells from injury [58].
In addition, HB-EGF appears to preserve intestinal microvillous blood flow in rats exposed to NEC stress [20]. HB-EGF induced angiogenesis in human umbilical vein endothelial cells [19] and enhanced vasodilation in ex vivo rat pup intestines [21]. While these studies support a protective effect of HB-EGF on the intestinal vasculature, more investigation is needed to better delineate the HB-EGF effect on the intestinal microcirculation in NEC. HB-EGF has not been trialed in humans.
3.3. Insulin-like Growth Factor (IGF-1 and IGF-2)
Insulin-like growth factors (IGF)-1 and IGF-2 are growth factors present in the serum mostly bound to IGFBPs which regulate their stability and bioavailability [59]. IGF-1 is produced by the placenta [60] and the liver [61]. It is a major regulator of fetal growth and development of most organ systems [62].
IGF-1 is present at high concentrations in the colostrum and its concentration in breast milk declines over the first 6 months of lactation [60,61,63,64]. Studies have reported either similar or higher concentrations of IGF-1 levels in preterm versus term human milk [40,41]. IGF-2 levels may increase during the first week of lactation [65], and subsequently slowly decline over several months. No significant differences in IGF-2 levels were noted between term and preterm human milk. [41]
When bound to IGF Binding Protein 2 (IGFBP-2), IGF-1 and IGF-2 are largely stable when isolated and incubated in gastric fluid obtained from human neonates [41]. Without this chaperone binding interaction, IGF-1, IGF-2, and IGFBP are rapidly cleaved by gastric enzymes [41]. IGF-1 and IGF-2 were significantly reduced following Holder pasteurization by 39.4% and 9.9%, respectively [66].
IGF-1 promotes cell survival similarly to HB-EGF, primarily by inhibiting cell apoptosis through the PI3K/Akt signaling pathway [28]. In the intestine, IGF-1 has been shown to stimulate proliferation of intestinal stem cells [67] and to promote the survival of crypt cells following murine models of radiation injury [68,69] and oxidative injury [70]. IGF-1 administered enterally protects against NEC in rats by protecting the intestinal mucosal barrier [24] and by reducing the inflammatory response [24]. In the same model, enterally administered IGF-1 suppresses TLR4, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and Interleukin (IL)-6 mRNA levels [24]. Systemic administration of IGF-1-BP3 complex protects against NEC in pigs [71] and in neonatal mice (unpublished data). Additionally, IGF-1, when administered intraperitoneally in conjunction with erythropoietin, protects the murine intestine against injury and cellular apoptosis in a model of hypoxia/reperfusion [27]. IGF was also shown to enhance intestinal absorption of nutrients such as D-glucose, L-alanine, and ions in a healthy piglets [23]. The summation of these effects is reduced apoptosis and an attenuated inflammatory response in models of intestinal tissue injury.
In the mouse neonatal intestine, IGF-1 secreted by resident macrophages promotes endothelial cell proliferation and microvascular development and protects against NEC (our lab—unpublished data). Further, systemic exogenous administration of IGF-1 to neonatal mice protects against NEC and maintains mucosal microvasculature integrity (our lab—unpublished data). Both IGF-1 and IGF-2 have been shown to independently promote cell migration of human umbilical cord vascular endothelial cells (HUVEC) in vitro and formation of their tubular structures (primitive vessels) [25,26]. In summary, growing evidence derived from animal studies indicates that the IGFs protect the integrity of the developing intestinal epithelium through proliferative, antiapoptotic and proangiogenic influences.
In a randomized control trial involving 60 very low birth weight (range: 750 to 1250g) neonates, IGF-1, when added to formula at twice the dose present in colostrum, showed improved gut barrier function at day 14 as evidenced by lower lactulose/mannitol excretion ratios in babies receiving IGF-1. [72]. However, this effect was not sustained when evaluated at later post-natal days. Additionally, no effect of enterally administered IGF-1 was noted on feeding tolerance nor weight gain [72]. Intravenous administration of human recombinant IGF-1 appears to be well tolerated in short term follow up in phase-II clinical trials [73,74]. Larger studies are needed to determine whether enterally administered IGF-1 may protect against NEC in humans.
3.4. Vascular Endothelial Growth Factor (VEGF)
Vascular Endothelial Growth Factor is a growth factor present in breast milk at much higher concentrations than found in human serum [42]. It is a member of a superfamily of related growth factors that include VEGF-A, B, C, D, E and placental derived growth factor (PlGF). VEGF-A, commonly referred to as VEGF, plays the most prominent role in regulating vascular angiogenesis during homeostasis and disease [75,76].
VEGF is present at relatively high concentrations in the human milk [40,77,78,79]. In a cohort of 43 mother-baby dyads, mothers who delivered infants at term demonstrated VEGF concentrations (>75 ng/mL) that were greater than milk from mothers who delivered before 37 weeks (30–40 ng/mL). Yet, findings are inconsistent between studies as to whether VEGF is higher in term or preterm milk [78,79]. In a cohort of term infants, VEGF concentrations in human milk were found to decrease as lactation progressed [77]
Mechanistically, cellular hypoxia allows for stabilization of hypoxia inducible factor (HIF) which in turn serves as a transcription factor for upregulation of VEGF [80]. The VEGF family of proteins binds to tyrosine kinase receptors located predominantly on the surface of vascular endothelial cells [81]. Upon binding to VEGFR2, VEGF induces intracellular signal transduction via the notch pathway and activation of the phosphatidylinositol 3-kinase/Akt pathway, to promote endothelial cell proliferation, migration and survival [29].
VEGF promotes vascular development (angiogenesis) in most organs. In very low birth weight infants, VEGF dysregulation may be associated with impaired microvascular development leading to organ dysfunction and increased morbidity. VEGF has a well demonstrated role in the pathogenesis of ROP, but there is mounting literature showing that it may contribute to pulmonary hypertension commonly associated with bronchopulmonary dysplasia [82]. Similarly, there is increasing evidence that defective VEGFR2 signaling may also play a role in NEC [83,84] and restoring VEGF production has been shown to preserve intestinal endothelial cell proliferation and to decrease the incidence of severe NEC in neonatal mice [85]. Further examination is needed to determine whether human milk-derived VEGF protects the neonatal intestines from NEC. In summary, VEGF dysfunction may contribute to several complications of prematurity.3.5. Erythropoetin (EPO):
Erythropoetin (EPO) is a glycoprotein produced in the liver and the kidneys that stimulates erythropoiesis primarily in response to cellular hypoxia. Functional EPO receptors are present on fetal and neonatal intestinal cells and regulate EPO’s function [86].
Both human milk and amniotic fluid contain EPO [87]. Amniotic fluid EPO levels increase in response to fetal hypoxia, as seen with exposure to maternal hypertension or preeclampsia, suggesting that EPO from swallowed amniotic fluid may offer fetal intestinal protection from these hypoxic conditions in utero [88]. In Human milk, EPO levels increase as lactation duration increases [89]. EPO is present in similar levels in both term and preterm human milk [43] (Pre/Term: 11.7 mU/mL) as measured in the first 4 months of life.
Much like EGF, HB-EGF and IGF-1, EPO promotes intestinal villous integrity via stimulation of intestinal epithelial cellular proliferation and migration. In the postnatal period, suckling rats whose mothers were supplemented with recombinant EPO showed increasing villous number and small bowel length [30]. A lesser but significant effect was seen for neonatal rats who received parenteral EPO [90]. This effect was mediated in part by EPO-induced migration of intestinal epithelial cells and resistance to Tumor Necrosis Factor (TNF)-induced apoptosis. In experimental NEC, EPO protects the intestinal epithelium by diminishing excessive autophagy via the Akt/ mechanistic target of rapamycin (mTOR) signaling pathway and by upregulating B-Cell Lymphoma gene 2 (Bcl-2) via the MAPK/ERK pathway to reduce apoptosis [90].
EPO contributes to intestinal barrier integrity by preserving tight junctions. Specifically, EPO increases the expression of zona occludens-1 (ZO-1) via the PI3K/Akt pathway. ZO-1 binds multiple tight junction-associated proteins and the peri-junctional actin ring and is vulnerable to proinflammatory cytokines including interferon-gamma (IFN-γ) [31]. In vitro, EPO was shown to reverse IFN-γ-induced downregulation of ZO-1 and alteration of the intestinal barrier [31]. Additionally, EPO attenuated the secretion of stimulated IL-8 from intestinal epithelial cells stimulated with TNF-α and IL-1β in vitro [32]. In vivo, oral administration of EPO decreased the incidence of experimental NEC in neonatal rats and prevented the loss of ZO-1 at tight junctions [31]. Thus, EPO appears to attenuate the inflammatory cascade, promote healthy villous epithelial regeneration, and optimize intestinal epithelial barrier function. Investigations of whether EPO prevents or mitigates NEC in preterm infants reveal mixed results. In a randomized control trial of infants <32 weeks gestational age, repeated dosing of intramuscular EPO decreased the incidence of Bell stage II and III NEC by 36 weeks postmenstrual age [91]. The trial’s limitations included lack of blinding to the study groups and a high reported incidence of NEC in the study cohort (17.1%), although notably, this included babies with Bell Stage I NEC, but is substantially higher than the incidence seen in the Vermont-Oxford Network of NICUs (7.6%) [92]. In a multicenter, randomized, double blind trial, intravenous EPO had no impact on the incidence of NEC in infants born at less than 28 weeks [93]. Another smaller trial administered enteral EPO in an artificial amniotic fluid solution to infants born at less than 28 weeks gestational age. Here, EPO had no effect on the incidence of NEC [94]. In a small randomized control trial, infants given enteral EPO demonstrated earlier tolerance of full feeding volumes with improved weight gain [95]. While EPO appears to be well tolerated when administered to human neonates, large-size trials are needed to conclusively determine whether exogenous EPO confers a preventative or therapeutic effect against NEC.
3.5. Granulocyte Colony Stimulating Growth Factor (G-CSF)
Granulocyte colony stimulating factor (G-CSF) is produced by the developing fetus and placenta both for maintenance of hematopoiesis and in response to inflammation [96,97,98]. As a GF, G-CSF elicits wide ranging effects apart from those for which it was named (leukopoiesis). Its therapeutic role has been evaluated in both humans and animal models and its clinical use among neonates remains to be established.
G-CSF is present in relatively high amounts in amniotic fluid and human colostrum, though it is notably decreased in preterm milk [96]. Measured on day of life 2, term levels are reported in the range of 0.0156 µg/100mL, while preterm are lower at 0.0080 µg/100mL. No data are available on the levels of G-CSF in milk produced at advanced lactational stages.
G-CSF is a glycoprotein that promotes proliferation and differentiation of granulocytes and neutrophils via the Janus Tyrosine Kinase/ signal transducers and activators of transcription (Jak/STAT) and Mek/Erk pathways but also has a variety of nonhematopoietic effects much like EPO [99]. Enteral G-CSF is not systemically absorbed, but rather binds to enteral villus receptors and stimulates growth and development of the fetal bowel [35,36,100]. Interestingly, both human and recombinant G-CSF resist gastric degradation in human milk, but are degraded when added to infant formula [101].
G-CSF promotes gut barrier integrity and epithelial cell health in the neonatal intestine. In a rat model of hypoxia-reoxygenation, enterally administered G-CSF reduced histopathologic evidence of mucosal damage in the small and large intestines [33]. In rodent models of chemotherapy-induced intestinal injury and permeability, G-CSF partially prevented bacterial translocation by reducing apoptosis and restoring villus height [102]. In contrast, in a mouse model of NEC, subcutaneous G-CSF during NEC induction increased the severity of intestinal inflammation and tissue damage [103]. This effect was dependent on neutrophil activity, as transgenic mice lacking neutrophil elastase, a serine protease essential for neutrophil activity, did not develop NEC even if given subcutaneous G-CSF [103].
G-CSF has been studied as a potential therapy for infants with neutropenia and sepsis. Neutropenia may be a risk factor for the development of NEC and has been associated with worsened prognosis [104,105]. Using G-CSF to prevent or improve neutropenia could in theory offer some protection from the development of NEC. Administration of G-CSF improves recovery of absolute neutrophil count while decreasing all-cause mortality in preterm infants with sepsis and neutropenia [106,107]. Beyond augmenting neutrophil counts, recombinant G-CSF may improve neutrophil function by increasing phagocytosis and oxidative burst activity [106]. However, in a large cohort of neutropenic infants, exogenous G-CSF was associated with increased incidence of secondary sepsis despite improvement in neutropenia [108]. In a multicenter, randomized controlled trial, prophylactic treatment of neutropenic premature infants was not associated with significant differences in survival free of infection [109]. Given these conflicting results, G-CSF is not routinely given to neutropenic infants, and its role in preventing NEC requires further investigation. In a small pilot study of Bell stage 1 (suspected) NEC, infants who received enteral G-CSF for 5 days in addition to standard treatment of withheld enteral nutrition, antibiotics, and gastric decompression had reduced incidence of progression to stage II or III NEC and faster clinical and radiologic resolution resulting in reduced duration of systemic therapy and reduced length of hospital stay [110]. In another randomized control trial, enteral G-CSF administered to preterm infants was, similarly to EPO, associated with improved feeding tolerance and decreased incidence of NEC despite no increase in serum G-CSF levels [95]. While these studies suggest that G-CSF could have therapeutic potential when administered enterally, results need to be replicated in additional randomized trials and further study is required to elucidate its mechanism of action in human neonates.
3.6. Donor Milk and Holder Pasteurization’s Effects on Growth Hormone Levels in Human Milk
Pasteurized human donor milk is recommended for feeding preterm infants when maternal breast milk is unavailable [111]. Donor milk is the product of pooled human milk from mothers at various stages of lactation, having delivered at wide ranges of pregnancy duration. Holder pasteurization, one of the most common methods used for pasteurization by donor milk banks, heats the human milk to 62.5 degrees Celsius for 30 min. The objective of the pasteurization method is to reduce the potential for transmission of infectious particles via the donor milk [49,66]. Yet, this method reduces concentrations of some human milk components known to be beneficial to the preterm neonate.
As an unintended consequence of heating, growth factor concentrations may be reduced via protein denaturation and degradation and the pasteurization method variably affects concentrations of different growth factors [112,113,114]. Of the growth factors reviewed in this article, EGF and HB-EGF concentrations do not significantly change with pasteurization. In contrast, IGF-1, IGF-2, and EPO were all reduced by pasteurization, and insufficient data are available for G-CSF [49,66,115]. It is possible that lower temperature or reduced exposure time to heat may help preserve growth factor biologic activity while providing adequate protection against infection [116].
Donated milk that is pooled and processed for infant feedings is often milk expressed at later stages of lactation. As established in this review, many GF vital to the premature neonate’s intestinal development decrease in concentration as lactation progresses. [112,113]. Therefore, the conditions that ultimately result in batches of donor milk utilized by NICUs likely lead to providing preterm infants milk that has relatively lower concentrations of GF [113]. Further studies are required to examine the net effect of donor milk processing on GFs.
Donor milk reduces risk of NEC [117,118,119,120]. In a retrospective cohort analysis of 319 neonates of very low birth weight (VLBWs), infants received either their mother’s own milk and donor human milk, or their mother’s own milk and formula. Feedings consisting in mother’s own milk and donor milk were associated with a significant reduction of the incidence of NEC when compared with the group receiving their mother’s own milk and formula (1.8% vs. 6.0%, p = 0.048). Further, a meta-analysis of five randomized control trials showed that donor milk when compared with preterm formula reduces the risk of NEC by up to 79% in the combined analysis. This suggests that the protective effects remain despite the reduced concentrations of growth factors resulting from pasteurization, and/or other components preserved in pasteurization offset the reduction in concentrations of protective growth factors [121].
4. Limitations
This narrative review is meant to provide an overview to the reader of both what is known and knowledge gaps requiring further study. It attempts to summarize decades of growth factor research and put it in the context of necrotizing enterocolitis. We do not provide additional data nor attempt to reanalyze the data presented. Despite critical and rigorous evaluation of each study included here, narrative reviews are generally subjected to authors’ experiential biases.
5. Conclusions
NEC carries severe risks including sepsis, need for bowel resection, intestinal failure, and death. Even milder cases compromise infant growth, increase antibiotic exposure, delay time to full enteral feeds, increase cost burden, and prolong length of stay in the NICU. Human milk feedings, particularly their mother’s own milk, remain a potent preventative strategy against the development of NEC. However, even infants fed exclusively their mother’s own milk may develop NEC. Our extensive literature search draws important similarities among the effects imparted by various growth factors contained within breast milk. Common mechanisms include augmented intestinal restitution, prolonged intestinal stem cell survival, improved intestinal perfusion, and enhanced intestinal cellular repair and migration (Figure 1).
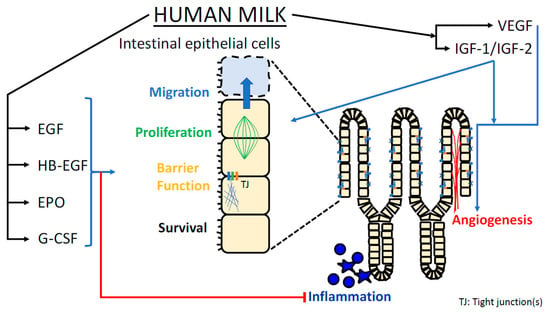
Figure 1.
Graphical representation of mechanisms by which growth factors present in breast milk may protect the neonatal intestine against NEC.
Understanding the components of human milk that confer protection and the influences of their concentrations in milk may offer opportunities for the augmentation of human milk’s preventative and therapeutic effects in infants at risk of NEC. This may include the addition of growth factors to the milk of both term and preterm neonates. It is important to note that milk concentration does not necessarily imply bioactivity and bioactivity is not necessarily a linear correlation with concentration. Thus, any preclinical or phase I trials examining the effects of supplementary GFs, must evaluate a dose response curve. Alternative methods for the pasteurization of donor milk should be explored with the goal of preserving all immunomodulators within breast milk. Increased understanding of how human milk growth factors function in concert with other nutrients in milk may best reveal opportunities to enhance the protective effects of these human milk proteins.
With the establishment of large-scale production of human growth factors (such as that achieved with EGF), the possibility of growth factor supplementation in breast milk is materializing. From a translational perspective, safety data from animal models must continue to demonstrate that GFs produced from other organisms (soybeans) is biologically safe and stable. Ensuing phase I clinical trials would likely need to establish safety in healthy neonates prior to the introduction to fragile preterm infants. While GF supplementation may help intestinal development, if supplemented at levels seen in breast milk, at best, GFs should be expected to decrease NEC only to the levels seen in babies exclusively fed breast milk. Future studies should continue to explore the mechanisms of intestinal development and the pathophysiology of NEC to elucidate early biomarkers of the disease.
Author Contributions
Conceptualization, D.J.Y., A.L.S., D.T.R., I.G.D.P.; methodology, D.J.Y., A.L.S., D.T.R., I.G.D.P.; resources, D.J.Y., A.L.S.; writing—original draft preparation, D.J.Y., A.L.S., D.T.R., I.G.D.P.; writing—review and editing, D.J.Y., D.T.R., I.G.D.P.; visualization, D.J.Y., I.G.D.P.; supervision, D.T.R., I.G.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Institute of Health Grant R01 DK116568 (I.G. De Plaen) and the Ann & Robert H. Lurie Children’s Hospital of Chicago (Division of Neonatology).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; EPO: erythropoietin; G-CSF: granulocyte colony-stimulating factor; GAP: goblet cell associated passages; GF: growth factor; HB-EGF: heparin-binding epidermal growth factor; HIF: hypoxia inducible factor; HUVEC: human umbilical cord vascular endothelial cells; IFN-γ: interferon gamma; IGF-1 and 2: insulin-like growth factor 1 and 2; IGFBP: Insulin-like growth factor binding protein; IL: interleukin; MAPK: mitogen-activated protein kinase; NEC: necrotizing enterocolitis; PIGF: placental derived growth factor; ROP: retinopathy of prematurity; ROS: reactive oxygen species, TLR: Toll-like receptor, TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor; VLBW: very low birth weight; ZO-1: zona occludens-1, PI3K/Akt: Phosphoinositide 3-kinase, MEK/ERK1/2: Mitogen-activated protein kinase/ Extracellular Signal-Regulated kinase, COX2: Cyclooxygenase-2, iNOS: Inducible Nitric Oxide Synthase, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, mTOR: mechanistic Target of Rapamycin, Bcl2: B-cell Lymphoma gene 2 JAK/STAT: Janus Tyrosine Kinase/ signal transducers and activators of transcription
References
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of neonatal necrotising enterocolitis in high-income countries: A systematic review. Arch. Dis. Child. - Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef] [PubMed]
- Bazacliu, C.; Neu, J. Necrotizing enterocolitis: Long term complications. Curr. Pediatr. Rev. 2019, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chowning, R.; Radmacher, P.; Lewis, S.L.; Serke, L.; Pettit, N.; Adamkin, D.H. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth. J. Perinatol. 2016, 36, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Carroll, K. An Exclusively Human Milk Diet Reduces Necrotizing Enterocolitis. Breastfeed. Med. 2014, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Al-Nafussi, A.I.; Wright, N.A. The effect of epidermal growth factor (EGF) on cell proliferation of the gastrointestinal mu-cosa in rodents. Virchows Archiv B. 1982, 40, 63. [Google Scholar] [CrossRef]
- Zhang, X.; Bandyopadhyay, S.; Araujo, L.P.; Tong, K.; Flores, J.; Laubitz, D.; Zhao, Y.; Yap, G.; Wang, J.; Zou, Q.; et al. Elevating EGFR-MAPK program by a nonconventional Cdc42 enhances intestinal epithelial survival and regeneration. JCI Insight 2020, 5, 5. [Google Scholar] [CrossRef]
- Basuroy, S.; Seth, A.; Elias, B.; Naren, A.P.; Rao, R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J. 2005, 393, 69–77. [Google Scholar] [CrossRef]
- Knoop, K.A.; Coughlin, P.E.; Floyd, A.N.; Ndao, I.M.; Hall-Moore, C.; Shaikh, N.; Gasparrini, A.J.; Rusconi, B.; Escobedo, M.; Good, M. Maternal activation of the EGFR prevents translocation of gut-residing pathogenic Escherichia coli in a model of late-onset neonatal sepsis. Proc. Natl. Acad. Sci. USA 2020. 117, 7941–7949. [CrossRef]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal Growth Factor and Intestinal Barrier Function. Mediat. Inflamm. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Good, M.; Sodhi, C.P.; Egan, C.E.; Afrazi, A.; Jia, H.; Yamaguchi, Y.; Lu, P.; Branca, M.F.; Ma, C.; Prindle, T.; et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015, 8, 1166–1179. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Neal, M.D.; Siggers, R.; Sho, S.; Ma, C.; Branca, M.F.; Prindle, T., Jr.; Russo, A.M.; Afrazi, A.; Good, M.; et al. Intestinal Epithelial Toll-Like Receptor 4 Regulates Goblet Cell Development and Is Required for Necrotizing Enterocolitis in Mice. Gastroenterology 2012, 143, 708–718. [Google Scholar] [CrossRef]
- Dao, D.T.; Anez-Bustillos, L.; Adam, R.M.; Puder, M.; Bielenberg, D.R. Heparin-Binding Epidermal Growth Factor–Like Growth Factor as a Critical Mediator of Tissue Repair and Regeneration. Am. J. Pathol. 2018, 188, 2446–2456. [Google Scholar] [CrossRef]
- Feng, J.; Besner, G.E. Heparin-binding epidermal growth factor–like growth factor promotes enterocyte migration and prolif-eration in neonatal rats with necrotizing enterocolitis. J. Pediatric Surg. 2007, 42, 214–220. [Google Scholar] [CrossRef]
- Nishi, E.; Prat, A.; Hospital, V.; Elenius, K.; Klagsbrun, M. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J. 2001, 20, 3342–3350. [Google Scholar] [CrossRef]
- Xia, G.; Rachfal, A.W.; Martin, A.E.; Besner, G.E. Upregulation of endogenous heparin-binding EGF-like growth factor (HB-EGF) expression after intestinal ischemia/reperfusion injury. J. Investig. Surg. 2003, 16, 57–63. [Google Scholar] [CrossRef]
- Yang, J.; Radulescu, A.; Chen, C.L.; Zhang, H.Y.; James, I.O.; Besner, G.E. Heparin-binding epidermal growth factor-like growth factor improves intestinal barrier function and reduces mortality in a murine model of peritonitis. Surg. 2012, 153, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.A.; Xia, G.; Mehta, V.B.; Glenn, S.; Michalsky, M.; Besner, G.E. Heparin-Binding EGF-Like Growth Factor (HB-EGF) Decreases Oxygen Free Radical Production In Vitro and In Vivo. Antioxidants Redox Signal. 2002, 4, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.B.; Besner, G.E. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 2007, 25, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Radulescu, A.; Zorko, N.; Besner, G.E. Heparin-Binding EGF-Like Growth Factor Increases Intestinal Microvascular Blood Flow in Necrotizing Enterocolitis. Gastroenterol. 2009, 137, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Brigstock, D.; Besner, G.E. Heparin-Binding EGF-Like Growth Factor Is a Potent Vasodilator of Terminal Mesenteric Arterioles. J. Surg. Res. 2008, 144, 201. [Google Scholar] [CrossRef]
- Feng, J.; El-Assal, O.N.; Besner, G.E. Heparin-binding epidermal growth factor–like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J. Pediatr. Surg. 2006, 41, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.N.; Carey, H.V. Oral IGF-I enhances nutrient and electrolyte absorption in neonatal piglet intestine. Am. J. Physiol. Liver Physiol. 1999, 277, G619–G625. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Liu, G.R.; Li, N.; Yuan, G. Insulin-like growth factor I reduces the occurrence of necrotizing enterocolitis by reducing inflammatory response and protecting intestinal mucosal barrier in neonatal rats model. Eur. Rev. Med Pharmacol. Sci. 2017, 21, 4711–4719. [Google Scholar] [PubMed]
- Lee, O.H.; Bae, S.K.; Bae, M.H.; Lee, Y.M.; Moon, E.J.; Cha, H.J.; Kwon, Y.G.; Kim, K.W. Identification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis models. Br. J. Cancer 2000, 82, 385–391. [Google Scholar] [CrossRef]
- Shigematsu, S.; Yamauchi, K.; Nakajima, K.; Iijima, S.; Aizawa, T.; Hashizume, K. IGF-1 Regulates Migration and Angiogenesis of Human Endothelial Cells. Endocr. J. 1999, 46, S59–S62. [Google Scholar] [CrossRef] [PubMed]
- Akisu, M.; Durmaz, B.; Koroglu, O.A.; Unlubay, S.; Yalaz, M.; Akin, H.; Ates, U.; Baka, M.; Ozkinay, F.; Cogulu, O.; et al. The effects of IGF-1 and erythropoietin on apoptosis and telomerase activity in necrotizing enterocolitis model. Pediatr. Res. 2021, 90, 559–564. [Google Scholar] [CrossRef]
- Povsic, T.J.; Kohout, T.A.; Lefkowitz, R.J. β-Arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phos-phatidylinositol 3-kinase (PI3K) and anti-apoptosis. J. Biol. Chem. 2003, 278, 51334–51339. [Google Scholar] [CrossRef]
- Takeshita, K.; Satoh, M.; Ii, M.; Silver, M.; Limbourg, F.; Mukai, Y.; Rikitake, Y.; Radtke, F.; Gridley, T.; Losordo, D.; et al. Critical Role of Endothelial Notch1 Signaling in Postnatal Angiogenesis. Circ. Res. 2007, 100, 70–78. [Google Scholar] [CrossRef]
- Juul, S.E.; Ledbetter, D.J.; Joyce, A.E.; Dame, C.; Christensen, R.D.; Zhao, Y.; DeMarco, V. Erythropoietin acts as a trophic factor in neonatal rat intestine. Gut 2001, 49, 182–189. [Google Scholar] [CrossRef]
- Shiou, S.R.; Yu, Y.; Chen, S.; Ciancio, M.J.; Petrof, E.O.; Sun, J.; Claud, E.C. Erythropoietin Protects Intestinal Epithelial Barrier Function and Lowers the Incidence of Experimental Neonatal Necrotizing Enterocolitis. J. Biol. Chem. 2011, 286, 12123–12132. [Google Scholar] [CrossRef]
- Claud, E.C.; Savidge, T.; Walker, W.A. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res. 2003, 53, 419–425. [Google Scholar] [CrossRef]
- Canpolat, F.E.; Yurdakök, M.; Özsoy, Ş.; Hazıroğlu, R.; Korkmaz, A. Protective effects of recombinant human granulocyte colony stimulating factor in a rat model of necrotizing enterocolitis. Pediatr. Surg. Int. 2006, 22, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A.; Lunøe, M.; Du, Y.; Christensen, R.D. Granulocyte colony-stimulating factor is present in human milk and its receptor is present in human fetal intestine. Pediatr. 2000, 105, e7. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Maheshwari, A.; Christensen, R.D. Recombinant granulocyte colony-stimulating factor administered enterally to neonates is not absorbed. Pediatr. 2003, 112, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Gersting, J.A.; Christensen, R.D.; Calhoun, D.A. Effects of Enterally Administering Granulocyte Colony-Stimulating Factor to Suckling Mice. Pediatr. Res. 2004, 55, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, B.; Fituch, C.C.; Williams, C.S.; Hurst, N.M.; Schanler, R.J. Increased Epidermal Growth Factor Levels in Human Milk of Mothers with Extremely Premature Infants. Pediatr. Res. 2003, 54, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Idota, T. The concentration of epidermal growth factor in Japanese mother’s milk. J. Nutr. Sci. Vitaminol. 1995, 41, 241–251. [Google Scholar] [CrossRef]
- Michalsky, M.; Lara-Marquez, M.L.; Chun, L.; Besner, G. Heparin-binding EGF-like growth factor is present in human amniotic fluid and breast milk. J. Pediatr. Surg. 2002, 37, 1–6. [Google Scholar] [CrossRef]
- Ozgurtas, T.; Aydin, I.; Turan, O.; Koc, E.; Hirfanoglu, I.M.; Acikel, C.H.; Akyol, M.; Erbil, M.K. Vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor-I and platelet-derived growth factor levels in human milk of mothers with term and preterm neonates. Cytokine 2010, 50, 192–194. [Google Scholar] [CrossRef]
- Elmlinger, M.W.; Hochhaus, F.; Loui, A.; Frommer, K.W.; Obladen, M.; Ranke, M.B. Insulin-Like Growth Factors and Binding Proteins in Early Milk from Mothers of Preterm and Term Infants. Horm. Res. Paediatr. 2007, 68, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Siafakas, C.G.; Anatolitou, F.; Fusunyan, R.D.; Walker, W.A.; Sanderson, I.R. Vascular Endothelial Growth Factor (VEGF) Is Present in Human Breast Milk and Its Receptor Is Present on Intestinal Epithelial Cells. Pediatr. Res. 1999, 45, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kling, P.J.; Sullivan, T.M.; Roberts, R.A.; Philipps, A.F.; Koldovsky, O. Human Milk as a Potential Enteral Source of Erythropoietin. Pediatr. Res. 1998, 43, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Isani, M.; Illingworth, L.; Herman, E.; Schmidt, M.; Barron, L.; Bowling, J.; Elizee, M.; Bai, I.; Gayer, C.; Grishin, A.; et al. Soybean-derived recombinant human epidermal growth factor protects against experimental necrotizing enterocolitis. J. Pediatr. Surg. 2018, 53, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Su, H.H.; Chen, J.C.; Chen, P.T. Production of recombinant human epidermal growth factor in Bacillus subtilis. J. Taiwan Inst. Chem. Eng. 2020, 106, 86–91. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Abramowicz, J.S. Epidermal Growth Factor (Egf) Concentrations in Amniotic Fluid and Maternal Urine During Pregnancy. Acta Obstet. et Gynecol. Scand. 1990, 69, 217–221. [Google Scholar] [CrossRef]
- Varner, M.; Dildy, G.; Hunter, C.; Dudley, D.; Clark, S.; Mitchell, M. Amniotic fluid epidermal growth factor levels in normal and abnormal pregnancies. J. Soc. Gynecol. Investig. JSGI. 1996, 3, 17–19. [Google Scholar] [CrossRef]
- Olsen, P.S.; Poulsen, S.S.; Kirkegaard, P.; Nexø, E. Role of submandibular saliva and epidermal growth factor in gastric cytoprotection. Gastroenterol. 1984, 87, 103–108. [Google Scholar] [CrossRef]
- Untalan, P.B.; Keeney, S.E.; Palkowetz, K.H.; Rivera, A.; Goldman, A. Heat Susceptibility of Interleukin-10 and Other Cytokines in Donor Human Milk. Breastfeed. Med. 2009, 4, 137–144. [Google Scholar] [CrossRef]
- Dembinski, A.B.; Johnson, L.R. Effect of Epidermal Growth Factor on the Development of Rat Gastric Mucosa. Endocrinol. 1985, 116, 90–94. [Google Scholar] [CrossRef]
- Malo, C.; Ménard, D. Influence of Epidermal Growth Factor on the Development of Suckling Mouse Intestinal Mucosa. Gastroenterol. 1982, 83, 28–35. [Google Scholar] [CrossRef]
- Halpern, M.D.; Dominguez, J.A.; Dvorakova, K.; Holubec, H.; Williams, C.S.; Meza, Y.G.; Ruth, M.C.; Dvorak, B. Ileal Cytokine Dysregulation in Experimental Necrotizing Enterocolitis Is Reduced by Epidermal Growth Factor. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Xu, R. Stability and distribution of orally administered epidermal growth factor in neonatal pigs. Life Sci. 1998, 63, 809–820. [Google Scholar] [CrossRef]
- Besner, G.; Higashiyama, S.; Klagsbrun, M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990, 1, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, B. Milk Epidermal Growth Factor and Gut Protection. J. Pediatr. 2010, 156, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- El-Assal, O.N.; Besner, G.E. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 2005, 129, 609–625. [Google Scholar] [CrossRef]
- Rocourt, D.V.; Mehta, V.B.; Besner, G.E. Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. J. Surg. Res. 2007, 139, 269–273. [Google Scholar] [CrossRef][Green Version]
- Chen, C.L.; Yu, X.; James, I.O.-A.; Zhang, H.Y.; Yang, J.; Radulescu, A.; Zhou, Y.; Besner, G.E. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab. Investig. 2011, 92, 331–344. [Google Scholar] [CrossRef]
- Sara, V.R.; Hall, K. Insulin-like growth factors and their binding proteins. Physiol. Rev. 1990, 70, 591–614. [Google Scholar] [CrossRef]
- He, B.; Zhang, N.; Jia, Y.; Sun, Q.; Zhao, R. Glucocorticoid receptor-mediated insulin-like growth factor-I transcriptional regulation in BeWo trophoblast cells before and after syncytialisation. Steroids 2016, 115, 26–33. [Google Scholar] [CrossRef]
- Lund, P.K.; Moats-Staats, B.M.; Hynes, M.A.; Simmons, J.G.; Jansen, M.; D’Ercole, A.J.; Van Wyk, J.J. Somatomedin-C/insulin-like growth factor-I and insulin-like growth factor-II mRNAs in rat fetal and adult tissues. J. Biol. Chem. 1986, 261, 14539–14544. [Google Scholar] [CrossRef]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Ramenghi, L.A.; Löfqvist, C.; Smith, L.E.H.; Hård, A.L. Role of insulinlike growth factor 1 in fetal development and in the early postnatal life of premature infants. Am. J. Perinatol. 2016, 33, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Itoh, K.; Kuroume, T. Levels of insulin-like growth factor I in full- and preterm human milk in comparison to levels in cow’s milk and in milk formulas. Biol Neonate. 1990, 58, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Milsom, S.R.; Blum, W.F.; Gunn, A. Temporal Changes in Insulin-Like Growth Factors I and II and in Insulin-Like Growth Factor Binding Proteins 1, 2, and 3 in Human Milk. Horm. Res. 2008, 69, 307–311. [Google Scholar] [CrossRef]
- Prosser, C.G. Insulin-like growth factors in milk and mammary gland. J. Mammary Gland. Biol. Neoplasia 1996, 1, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Goelz, R.; Hihn, E.; Hamprecht, K.; Dietz, K.; Jahn, G.; Poets, C.; Elmlinger, M. Effects of Different CMV-Heat-Inactivation-Methods on Growth Factors in Human Breast Milk. Pediatr. Res. 2009, 65, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Van Landeghem, L.; Santoro, M.A.; Mah, A.T.; Krebs, A.E.; Dehmer, J.J.; McNaughton, K.K.; Helmrath, M.A.; Magness, S.T.; Lund, P.K. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J. 2015, 29, 2828–2842. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.R.; Ohneda, K.; Keku, T.O.; D’Ercole, A.J.; Fuller, C.R.; Williams, K.L.; Lund, P.K. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am. J. Physiol. Liver Physiol. 2002, 283, G457–G464. [Google Scholar] [CrossRef]
- Qiu, W.; Leibowitz, B.; Zhang, L.; Yu, J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 2009, 29, 1622–1632. [Google Scholar] [CrossRef]
- Baregamian, N.; Song, J.; Jeschke, M.G.; Evers, B.M.; Chung, D.H. IGF-1 Protects Intestinal Epithelial Cells From Oxidative Stress-Induced Apoptosis. J. Surg. Res. 2006, 136, 31–37. [Google Scholar] [CrossRef]
- Holgersen, K.; Gao, X.; Narayanan, R.; Gaur, T.; Carey, G.; Barton, N.; Pan, X.; Muk, T.; Thymann, T.; Sangild, P.T. Supplemental Insulin-Like Growth Factor-1 and Necrotizing Enterocolitis in Preterm Pigs. Front. Pediatr. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, W.E.; van Vliet, I.; de Gast-Bakker, D.-A.H.; van der Schoor, S.R.; Alles, M.S.; Hoijer, M.; Tibboel, D.; van Goudoever, J.B. Effect of Enteral IGF-1 Supplementation on Feeding Tolerance, Growth, and Gut Permeability in Enterally Fed Premature Neonates. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Ley, D.; Hallberg, B.; Löfqvist, C.; Hansen-Pupp, I.; Ramenghi, L.A.; Borg, J.; Smith, L.E.H.; Hard, A.-L. IGF-1 as a Drug for Preterm Infants: A Step-Wise Clinical Development. Curr. Pharm. Des. 2018, 23, 5964–5970. [Google Scholar] [CrossRef] [PubMed]
- Ley, D.; Hallberg, B.; Hansen-Pupp, I.; Dani, C.; Ramenghi, L.A.; Marlow, N.; Beardsall, K.; Bhatti, F.; Dunger, D.; Higginson, J.D.; et al. rhIGF-1/rhIGFBP-3 in Preterm Infants: A Phase 2 Randomized Controlled Trial. J. Pediatr. 2019, 206, 56–65.e8. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Kobata, R.; Tsukahara, H.; Ohshima, Y.; Ohta, N.; Tokuriki, S.; Tamura, S.; Mayumi, M. High levels of growth factors in human breast milk. Early Hum. Dev. 2008, 84, 67–69. [Google Scholar] [CrossRef]
- Loui, A.; Eilers, E.; Strauss, E.; Pohl-Schickinger, A.; Obladen, M.; Koehne, P. Vascular Endothelial Growth Factor (VEGF) and Soluble VEGF Receptor 1 (sFlt-1) Levels in Early and Mature Human Milk from Mothers of Preterm versus Term Infants. J. Hum. Lact. 2012, 28, 522–528. [Google Scholar] [CrossRef]
- Ozgurtas, T.; Aydin, I.; Turan, O.; Koç, E.; Hirfanoglu, I.M.; Acikel, C.H.; Akyol, M.; Serdar, M.; Erbil, K.M. Soluble Vascular Endothelial Growth Factor Receptor 1 in Human Breast Milk. Horm. Res. Paediatr. 2011, 76, 17–21. [Google Scholar] [CrossRef]
- Oladipupo, S.; Hu, S.; Kovalski, J.; Yao, J.; Santeford, A.; Sohn, R.E.; Shohet, R.; Maslov, K.; Wang, L.; Arbeit, J.M. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. 2011, 108, 13264–13269. [Google Scholar] [CrossRef]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangio-genesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef]
- Stark, A.; Dammann, C.; Nielsen, H.C.; Volpe, M.V. A Pathogenic Relationship of Bronchopulmonary Dysplasia and Retinopathy of Prematurity? A Review of Angiogenic Mediators in Both Diseases. Front. Pediatr. 2018, 6, 125. [Google Scholar] [CrossRef]
- Yan, X.; Managlia, E.; Liu, S.X.; Tan, X.-D.; Wang, X.; Marek, C.; De Plaen, I.G. Lack of VEGFR2 signaling causes maldevel-opment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G716–G725. [Google Scholar] [CrossRef]
- Yan, X.; Managlia, E.; Tan, X.D.; De Plaen, I.G. Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis. Am. J. Physiol. Liver Physiol. 2019, 317, G57–G66. [Google Scholar] [CrossRef]
- Bowker, R.M.; Yan, X.; Managlia, E.; Liu, S.X.; Marek, C.; Tan, X.D.; De Plaen, I.G. Dimethyloxalylglycine preserves the intestinal microvasculature and protects against intestinal injury in a neonatal mouse NEC model: Role of VEGF signaling. Pediatr. Res. 2018, 83, 545–553. [Google Scholar] [CrossRef]
- Juul, S.E.; Yachnis, A.T.; Christensen, R.D. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum. Dev. 1998, 52, 235–249. [Google Scholar] [CrossRef]
- Halvorsen, S.; Finne, P.H. Erythropoietin production in the human fetus and newborn. Ann. N. Y. Acad. Sci. 1968, 149, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Teramo, K.A.; Widness, J.A. Increased fetal plasma and amniotic fluid erythropoietin concentrations: Markers of intrauterine hypoxia. Neonatology 2009, 95, 105–116. [Google Scholar] [CrossRef]
- Juul, S.E.; Zhao, Y.; Dame, J.B.; Du, Y.; Hutson, A.D.; Christensen, R.D. Origin and Fate of Erythropoietin in Human Milk. Pediatr. Res. 2000, 48, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shiou, S.R.; Guo, Y.; Lü, L.; Westerhoff, M.; Sun, J.; Petrof, E.O.; Claud, E.C. Erythropoietin Protects Epithelial Cells from Excessive Autophagy and Apoptosis in Experimental Neonatal Necrotizing Enterocolitis. PLOS ONE 2013, 8, e69620. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Sun, H.; Xu, F.; Li, K.; Nie, C.; Zhang, X.; Peng, X.; Xia, L.; Shen, Z.; et al. Erythropoietin prevents necrotizing enterocolitis in very preterm infants: A randomized controlled trial. J. Transl. Med. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Han, S.M.; Hong, C.R.; Knell, J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J. Pediatr. Surg. 2020, 55, 998–1001. [Google Scholar] [CrossRef]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.A.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef]
- Hosseini, M.; Azampour, H.; Raeisi, S.; Behtari, M.; Valizadeh, H.; Saboohi, R. The effects of enteral artificial amniotic fluid-containing erythropoietin on short term outcomes of preterm infants. Turk. J. Pediatr. 2019, 61, 392–398. [Google Scholar] [CrossRef]
- El-Ganzoury, M.M.; Awad, H.A.; El-Farrash, R.A.; El-Gammasy, T.M.; Ismail, E.A.; Mohamed, H.E.; Suliman, S.M. Enteral Granulocyte-Colony Stimulating Factor and Erythropoietin Early in Life Improves Feeding Tolerance in Preterm Infants: A Randomized Controlled Trial. J. Pediatr. 2014, 165, 1140–1145.e1. [Google Scholar] [CrossRef]
- Calhoun, D.A. Granulocyte colony-stimulating factor in preterm and term pregnancy, parturition, and intra-amniotic infection. Obstet. Gynecol. 2001, 97, 229–234. [Google Scholar] [CrossRef]
- Bailie, K.E.M.; Irvine, A.E.; Bridges, J.M.; McClure, B.G. Granulocyte and Granulocyte-Macrophage Colony-Stimulating Factors in Cord and Maternal Serum at Delivery. Pediatr. Res. 1994, 35, 164–168. [Google Scholar] [CrossRef]
- Saito, S.; Kasahara, T.; Kato, Y.; Ishihara, Y.; Ichijo, M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993, 5, 81–88. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; Watowich, S.S. Granulocyte colony-stimulating factor: Molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 2008, 42, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A.; Donnelly, W.H.; Du, Y.; Dame, J.B.; Li, Y.; Christensen, R.D. Distribution of Granulocyte Colony-Stimulating Factor (G-CSF) and G-CSF-Receptor mRNA and Protein in the Human Fetus. Pediatr. Res. 1999, 46, 333–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calhoun, D.A.; Lunøe, M.; Du, Y.; Staba, S.L.; Christensen, R.D. Concentrations of Granulocyte Colony-Stimulating Factor in Human Milk after in Vitro Simulations of Digestion. Pediatr. Res. 1999, 46, 767. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.G.; Duan, X.N.; Liu, Y.H.; Zhao, J.X.; Xu, L.; Ye, J.M. Role of granulocyte colony-stimulating factor in paclitaxel-induced intestinal barrier breakdown and bacterial translocation in rats. Chin. Med J. 2011, 124, 1870–1875. [Google Scholar]
- Klinke, M.; Vincent, D.; Trochimiuk, M.; Appl, B.; Tiemann, B.; Reinshagen, K.; Raluy, L.P.; Boettcher, M. Development of an improved murine model of necrotizing enterocolitis shows the importance of neutrophils in NEC pathogenesis. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Yoder, B.A.; Baer, V.L.; Snow, G.L.; Butler, A. Early-Onset Neutropenia in Small-for-Gestational-Age Infants. Pediatr. 2015, 136, 136. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, J.M.; Liu, S.; Olaloye, O.O.; Prochaska, E.C.; Yanowitz, T.; Riley, M.M.; Buland, J.R.; Brozanski, B.S.; Good, M.; Konnikova, L. Gestational Age-Specific Complete Blood Count Signatures in Necrotizing Enterocolitis. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Fleit, H.B.; Golightly, M.G.; La Gamma, E.F. In vivo Effect of Recombinant Human Granulocyte Colony-Stimulating Factor on Phagocytic Function and Oxidative Burst Activity in Septic Neutropenic Neonates. Neonatol. 2004, 86, 48–54. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Mitra, S.; Mukhopadhyay, D.; Chakraborty, S.; Chatterjee, S. Granulocyte colony-stimulating factor for preterms with sepsis and neutropenia: A randomized controlled trial. J. Clin. Neonatol. 2012, 1, 202–206. [Google Scholar] [CrossRef]
- Lee, J.A.; Sauer, B.; Tuminski, W.; Cheong, J.; Fitz-Henley, J.; Mayers, M.; Ezuma-Igwe, C.; Arnold, C.; Hornik, C.P.; Clark, R.H.; et al. Effectiveness of Granulocyte Colony-Stimulating Factor in Hospitalized Infants with Neutropenia. Am. J. Perinatol. 2016, 34, 458–464. [Google Scholar] [CrossRef]
- Kuhn, P.; Messer, J.; Paupe, A.; Espagne, S.; Kacet, N.; Mouchnino, G.; Klosowski, S.; Krim, G.; Lescure, S.; Le Bouedec, S.; et al. A Multicenter, Randomized, Placebo-Controlled Trial of Prophylactic Recombinant Granulocyte-Colony Stimulating Factor in Preterm Neonates with Neutropenia. J. Pediatr. 2009, 155, 324–330.e1. [Google Scholar] [CrossRef]
- Canpolat, F.E.; Yurdakök, M.; Korkmaz, A.; Yiğit, Ş.; Tekinalp, G. Enteral granulocyte colony-stimulating factor for the treatment of mild (stage I) necrotizing enterocolitis: A placebo-controlled pilot study. J. Pediatr. Surg. 2006, 41, 1134–1138. [Google Scholar] [CrossRef]
- Committee on Nutrition; Section on Breast Feeding; Committee on Fetus and Newborn. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef]
- Meier, P.; Patel, A.; Esquerra-Zwiers, A. Donor human milk update: Evidence, mechanisms, and priorities for research and practice. J. Pediatrics 2017, 180, 15–21. [Google Scholar] [CrossRef]
- Valentine, C.J.; Morrow, G.; Reisinger, A.; Dingess, K.A.; Morrow, A.L.; Rogers, L.K. Lactational Stage of Pasteurized Human Donor Milk Contributes to Nutrient Limitations for Infants. Nutr. 2017, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The Effect of Holder Pasteurization on Nutrients and Biologically-Active Components in Donor Human Milk: A Review. Nutr. 2016, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Unger, S.; O’Connor, D.; Stone, D.L.; Harvey, S.; Clandinin, M.T.; Field, C. Effect of pasteurization on selected immune components of donated human breast milk. J. Perinatol. 2011, 31, 593–598. [Google Scholar] [CrossRef] [PubMed]
- A Pitino, M.; Unger, S.; Doyen, A.; Pouliot, Y.; Aufreiter, S.; Stone, D.; Kiss, A.; O’Connor, D.L. High Hydrostatic Pressure Processing Better Preserves the Nutrient and Bioactive Compound Composition of Human Donor Milk. J. Nutr. 2019, 149, 497–504. [Google Scholar] [CrossRef]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An Exclusively Human Milk-Based Diet Is Associated with a Lower Rate of Necrotizing Enterocolitis than a Diet of Human Milk and Bovine Milk-Based Products. J. Pediatr. 2010, 156, 562–567. [Google Scholar] [CrossRef]
- Lucas, A.; Cole, T. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
- Miyake, H.; Lee, C.; Chusilp, S.; Bhalla, M.; Li, B.; Pitino, M.; Seo, S.; O’Connor, D.; Pierro, A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2020, 36, 155–163. [Google Scholar] [CrossRef]
- Johnson, T.J.; Berenz, A.; Wicks, J.; Esquerra-Zwiers, A.; Sulo, K.S.; Gross, M.E.; Szotek, J.; Meier, P.; Patel, A.L. The Economic Impact of Donor Milk in the Neonatal Intensive Care Unit. J. Pediatr. 2020, 224, 57–65. [Google Scholar] [CrossRef]
- Boyd, C.A.; Quigley, M.A.; Brocklehurst, P. Donor breast milk versus infant formula for preterm infants: Systematic review and meta-analysis. Arch. Dis. Child. - Fetal Neonatal Ed. 2007, 92, F169–F175. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).