Untargeted Metabolomic Analysis of Human Milk from Mothers of Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Samples
2.2. Sample Preparation
2.3. QC
2.4. Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS)
2.5. Data Extraction, Compound Identification and Metabolite Quantification
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Cohort
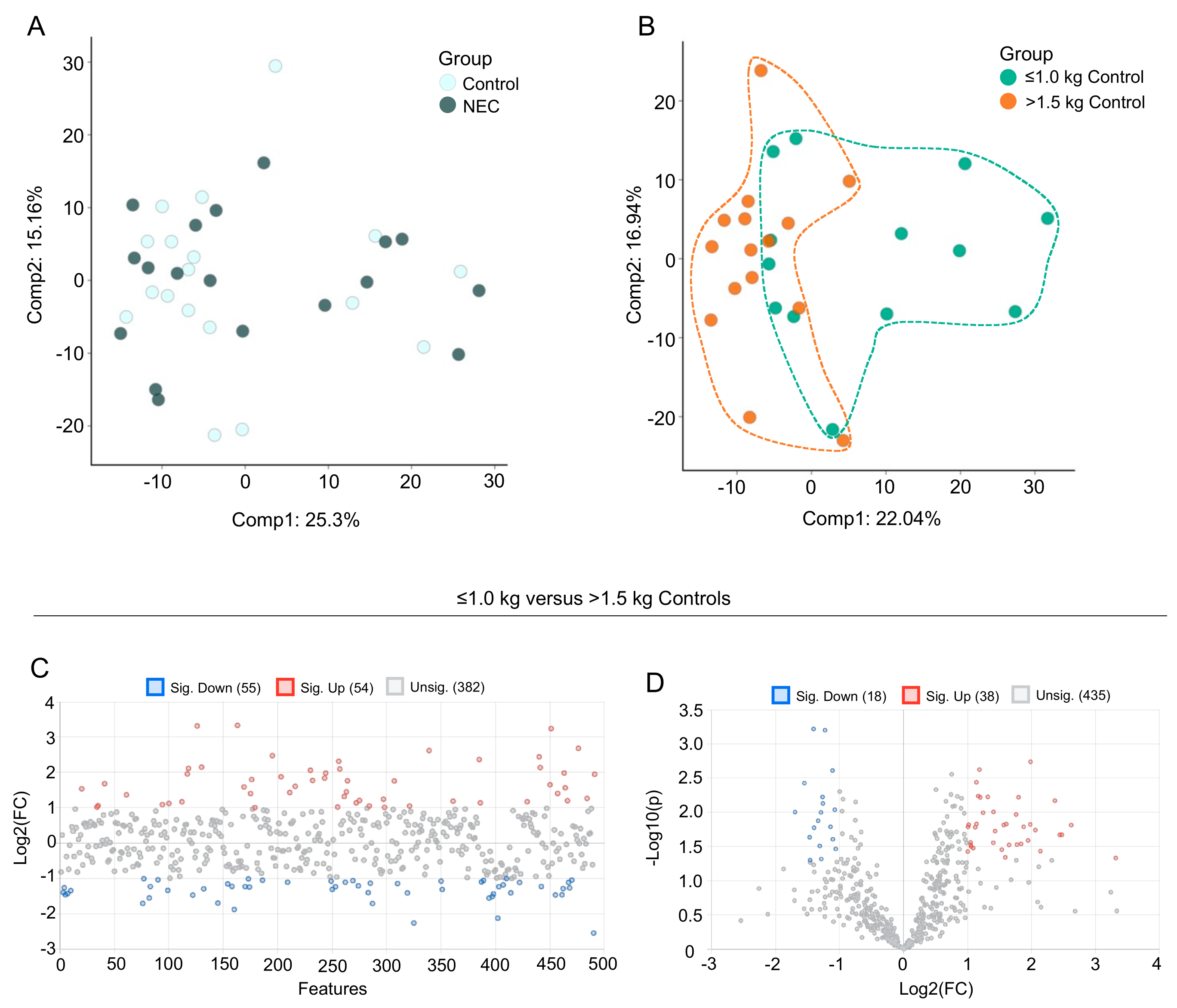
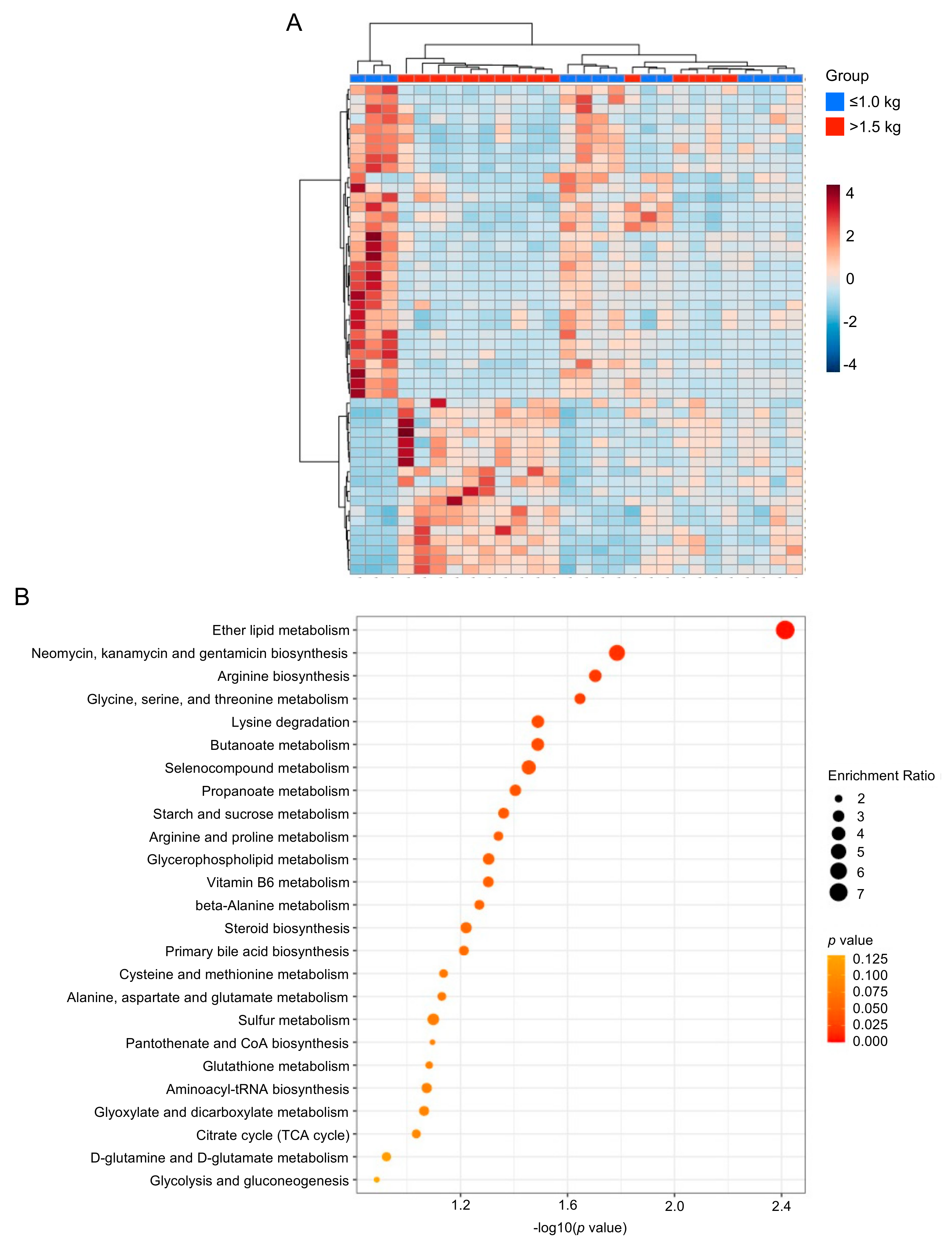
3.2. Multivariate Analysis of Milk Metabolic Profiles
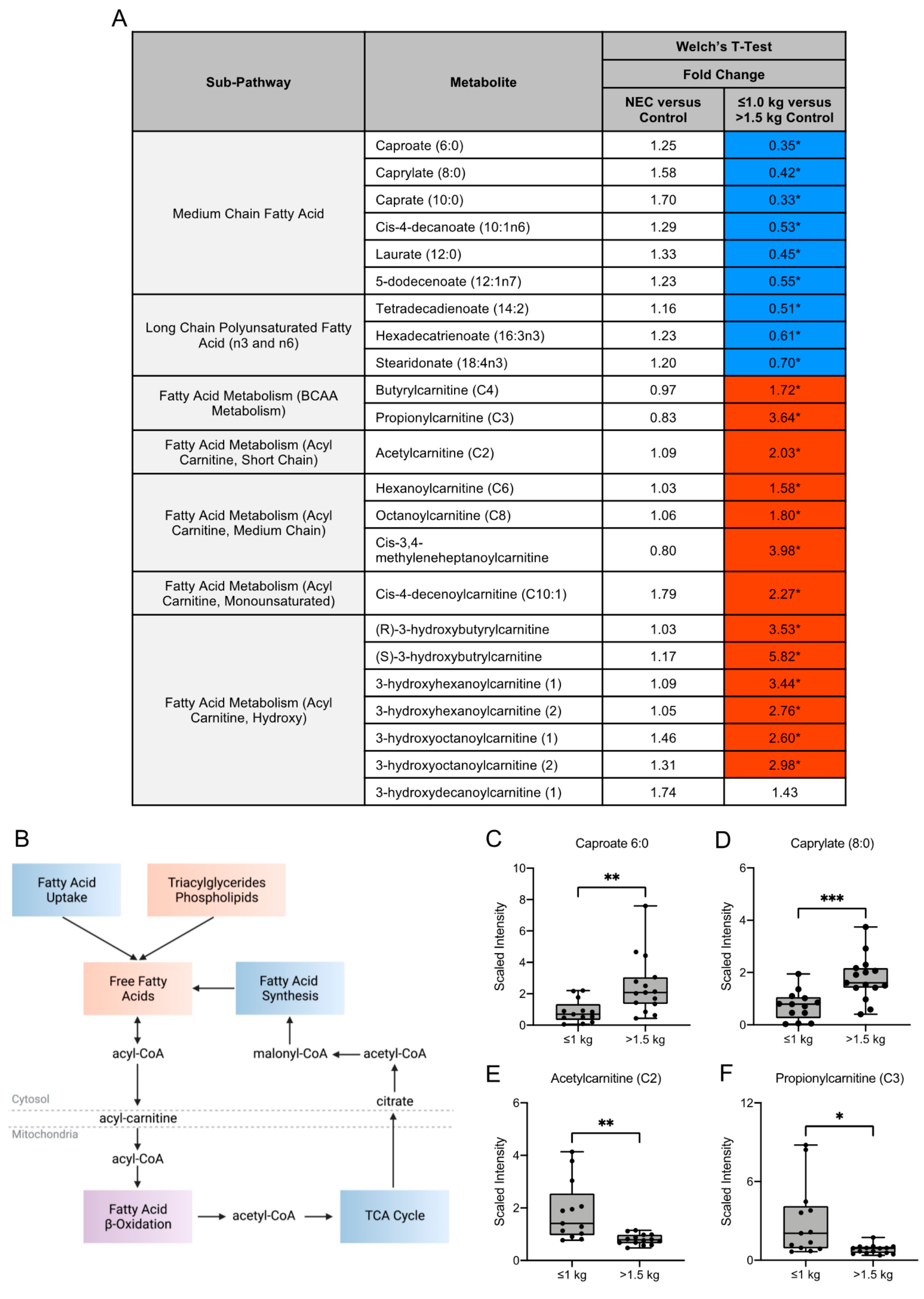
3.3. Fatty Acid Composition of Maternal Milk Varies by Infant Birth Weight
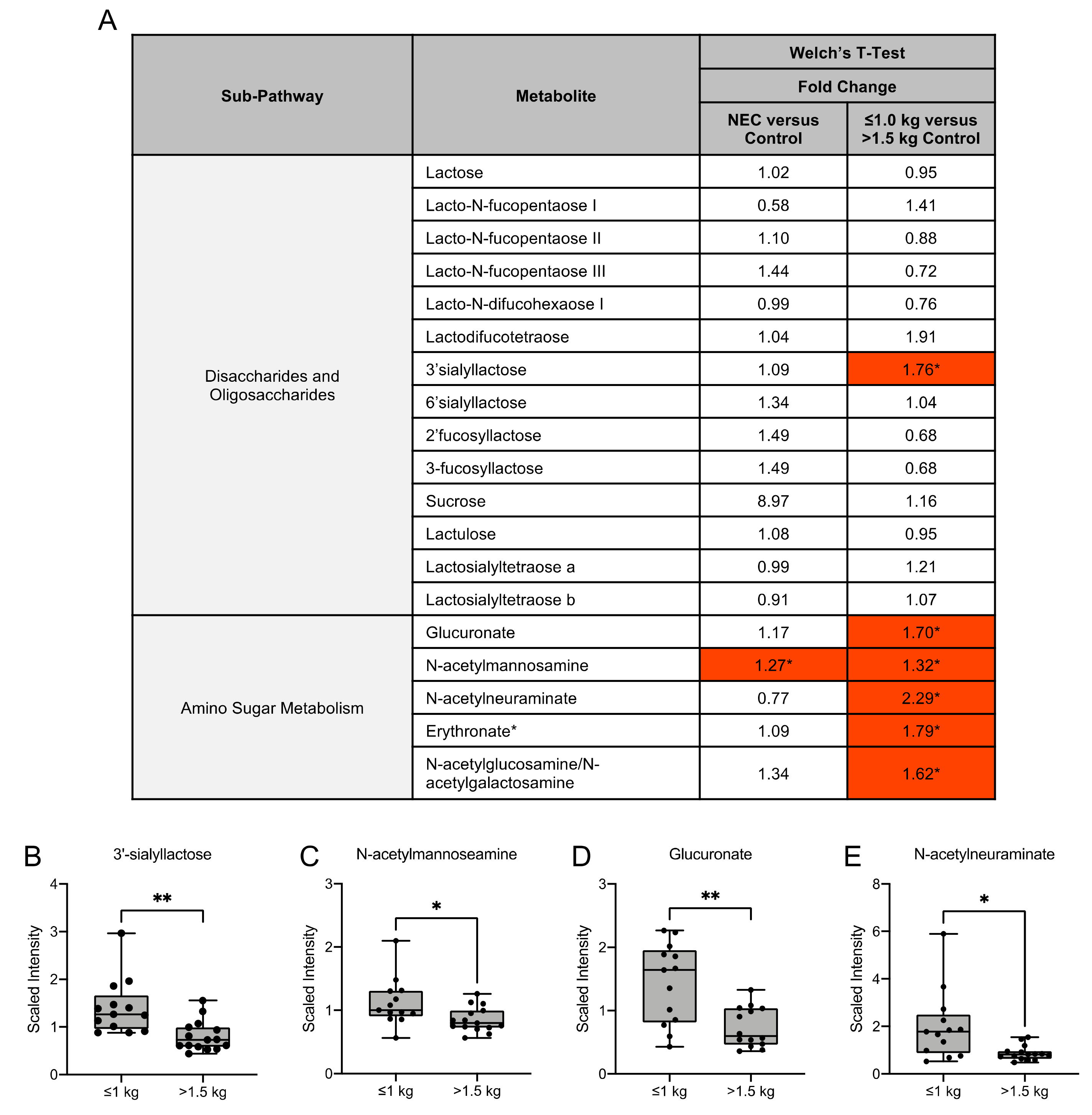
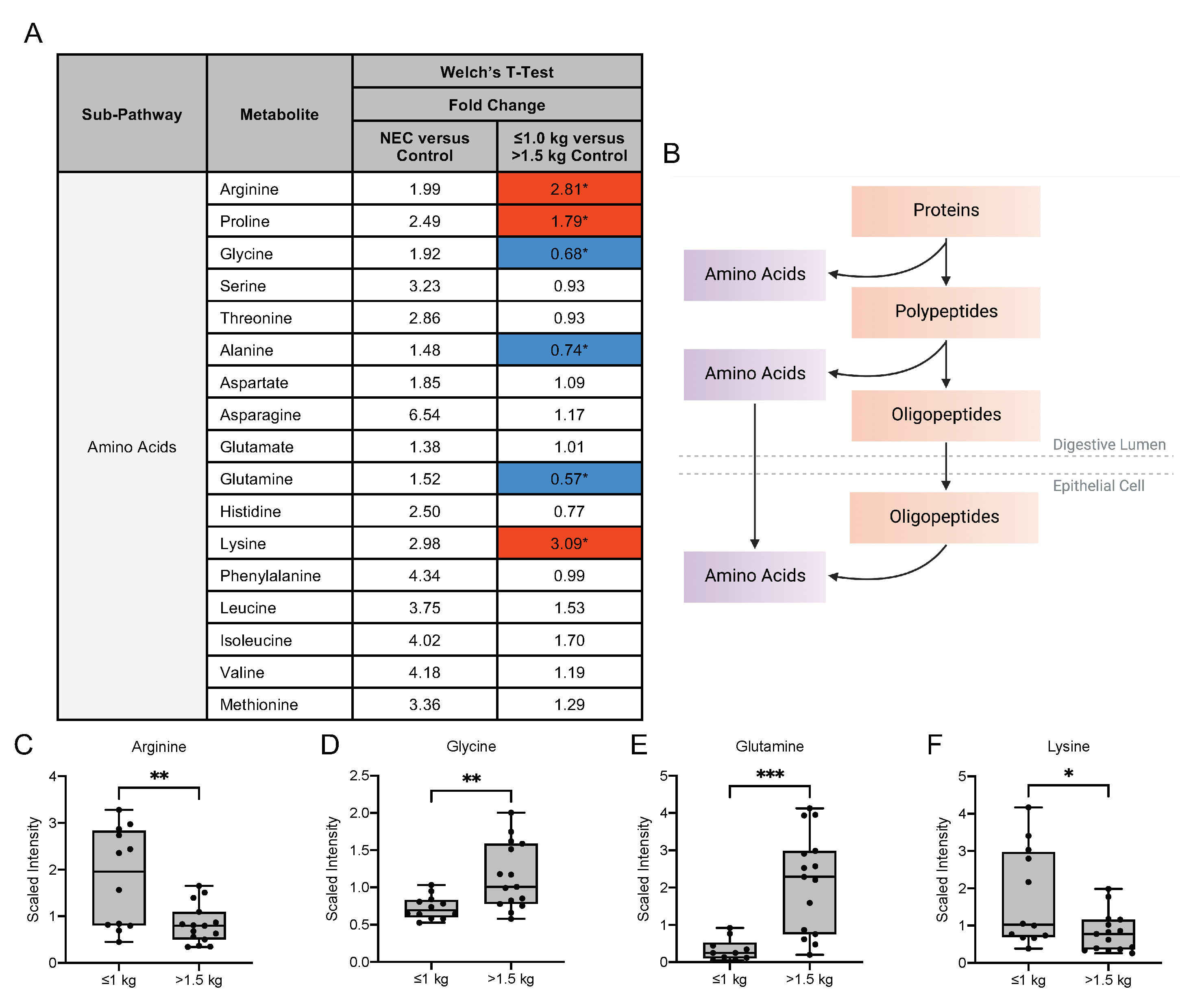
3.4. Human Milk Oligosaccharide, Amino Sugar and Amino Acid Components in Maternal Milk Differ by Infant Birth Weight
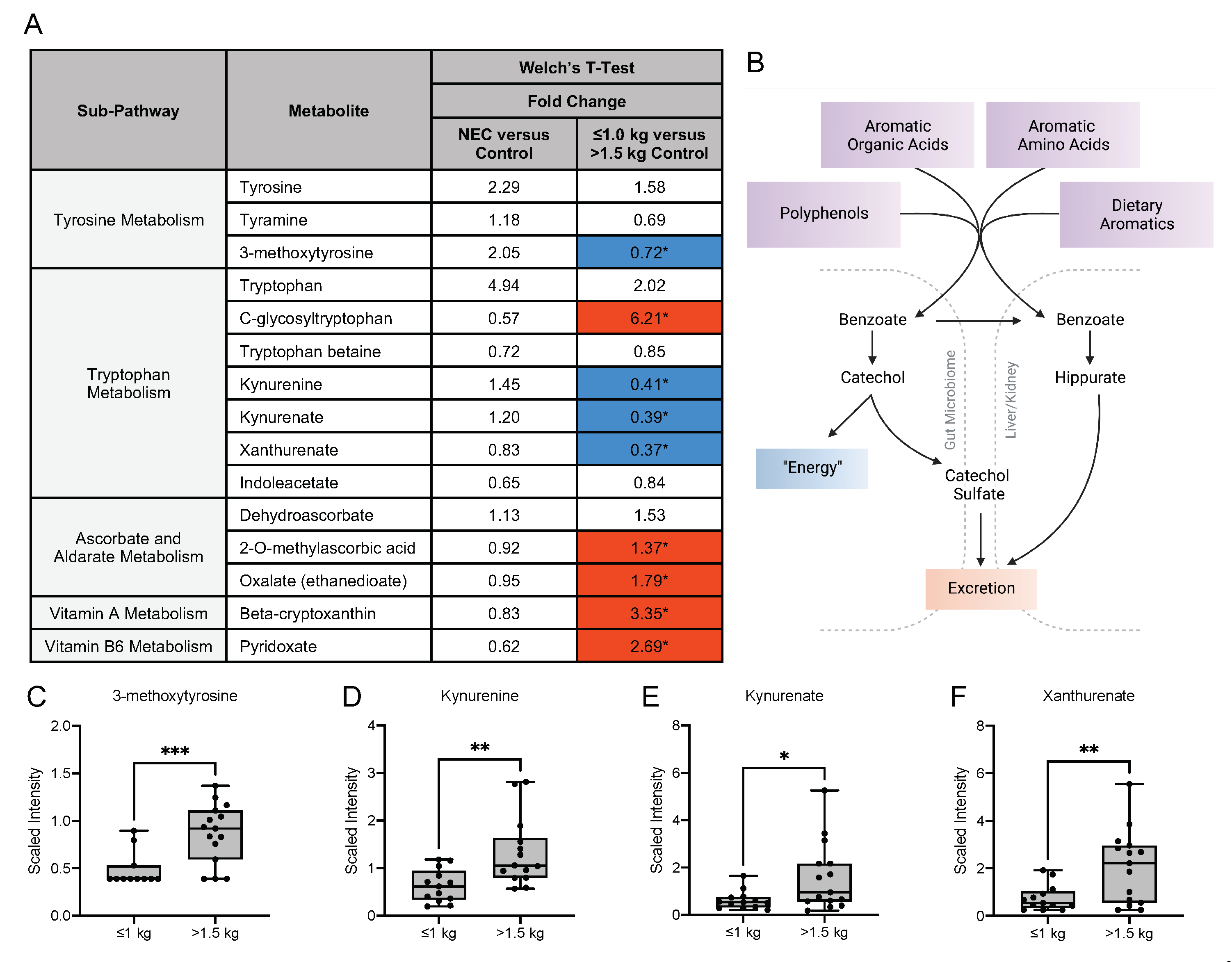
3.5. Metabolic Differences in the Microbiome-Derived and Vitamin Metabolites in Maternal Milk
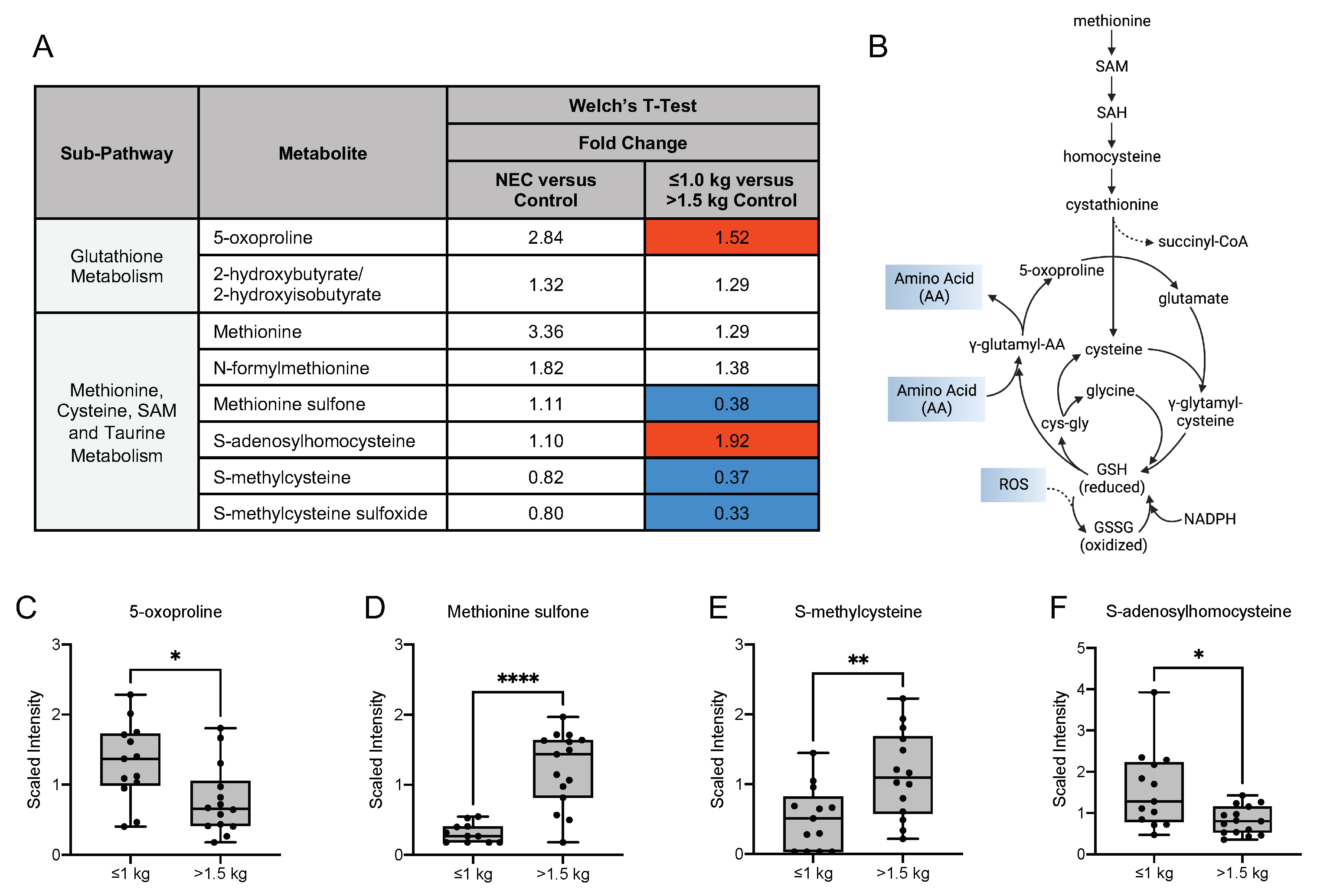
3.6. Differences in Oxidative Stress-Related, Glycolytic and Energy Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O.; Ellis, K.J. Infant feeding mode affects early growth and body composition. Pediatrics 2000, 106, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.L.; Sims, C.R.; Piccolo, B.D.; Andres, A. Maternal adiposity alters the human milk metabolome: Associations between nonglucose monosaccharides and infant adiposity. Am. J. Clin. Nutr. 2020, 112, 1228–1239. [Google Scholar] [CrossRef]
- Menni, C.; Kastenmüller, G.; Petersen, A.K.; Bell, J.T.; Psatha, M.; Tsai, P.-C.; Gieger, C.; Schulz, H.; Erte, I.; John, S.; et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int. J. Epidemiol. 2013, 42, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Jackson, R.T.; Khan, S.A.; Ahuja, J.; Pehrsson, P.R. Human Milk Nutrient Composition in the United States: Current Knowledge, Challenges, and Research Needs. Curr. Dev. Nutr. 2018, 2, nzy025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rourke, L.; Clarke, G.; Nolan, A.; Watkins, C.; Dinan, T.G.; Stanton, C.; Ross, R.P.; Ryan, C.A. Tryptophan metabolic profile in term and preterm breast milk: Implications for health. J. Nutr. Sci. 2018, 7, e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, L.S.; Mihi, B.; Agrawal, P.; Gong, Q.; Rimer, J.M.; Bidani, S.S.; Gale, S.E.; Goree, M.; Hu, E.; Lanik, W.E.; et al. Indole-3-Carbinol-Dependent Aryl Hydrocarbon Receptor Signaling Attenuates the Inflammatory Response in Experimental Necrotizing Enterocolitis. ImmunoHorizons 2021, 5, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yamaguchi, Y.; Fulton, W.B.; Wang, S.; Zhou, Q.; Jia, H.; Kovler, M.L.; Salazar, A.G.; Sampah, M.; Prindle, T.; et al. Maternal aryl hydrocarbon receptor activation protects newborns against necrotizing enterocolitis. Nat. Commun. 2021, 12, 1042. [Google Scholar] [CrossRef]
- Nolan, L.S.; Parks, O.B.; Good, M. A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis. Nutrients 2019, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.C.; Carter, E.B.; Raghuraman, N.; Nolan, L.S.; Gong, Q.; Lewis, A.N.; Good, M. Anti-severe acute respiratory syndrome coronavirus 2 antibodies induced in breast milk after Pfizer-BioNTech/BNT162b2 vaccination. Am. J. Obstet. Gynecol. 2021, 225, 101–103. [Google Scholar] [CrossRef]
- Marincola, F.C.; Noto, A.; Caboni, P.; Reali, A.; Barberini, L.; Lussu, M.; Murgia, F.; Santoru, M.L.; Atzori, L.; Fanos, V. A metabolomic study of preterm human and formula milk by high resolution NMR and GC/MS analysis: Preliminary results. J. Matern. Fetal. Neonatal Med. 2012, 25, 62–67. [Google Scholar] [CrossRef]
- Cesare Marincola, F.; Dessì, A.; Corbu, S.; Reali, A.; Fanos, V. Clinical impact of human breast milk metabolomics. Clin. Chim. Acta 2015, 451, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.W.; Stoll, B.J. Necrotising enterocolitis. Lancet 2006, 368, 1271–1283. [Google Scholar] [CrossRef]
- Sisk, P.M.; Lovelady, C.A.; Dillard, R.G.; Gruber, K.J.; O’Shea, T.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 2007, 27, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Patel, A.L.; Bigger, H.R.; Engstrom, J.L.; Meier, P.P. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology 2015, 107, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Lawson, M.A.E.; O’Neill, I.J.; Kujawska, M.; Gowrinadh Javvadi, S.; Wijeyesekera, A.; Flegg, Z.; Chalklen, L.; Hall, L.J. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2020, 14, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Carratù, B.; Boniglia, C.; Lammardo, A.M.; Riva, E.; Sanzini, E. Free glutamine and glutamic acid increase in human milk through a three-month lactation period. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 508–512. [Google Scholar] [CrossRef]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef] [Green Version]
- Blaut, M.; Clavel, T. Metabolic diversity of the intestinal microbiota: Implications for health and disease. J. Nutr. 2007, 137, 751S–755S. [Google Scholar] [CrossRef] [PubMed]
- Lawley, T.D.; Walker, A.W. Intestinal colonization resistance. Immunology 2013, 138, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Illana, Á.; Parra-Llorca, A.; Escuder-Vieco, D.; Pallás-Alonso, C.R.; Cernada, M.; Gormaz, M.; Vento, M.; Kuligowski, J. Biomarkers of oxidative stress derived damage to proteins and DNA in human breast milk. Anal. Chim. Acta 2018, 1016, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Gruca, L.L.; Bennett, C.; Parimi, P.S.; Hanson, R.W.; Kalhan, S.C. Metabolism of methionine in the newborn infant: Response to the parenteral and enteral administration of nutrients. Pediatr. Res. 2008, 64, 381–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Hogewind-Schoonenboom, J.E.; van Dongen, M.J.A.; de Groof, F.; Voortman, G.J.; Schierbeek, H.; Twisk, J.W.R.; Vermes, A.; Chen, C.; Huang, Y.; et al. Methionine requirement of the enterally fed term infant in the first month of life in the presence of cysteine. Am. J. Clin. Nutr. 2012, 95, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casavale, K.O.; Ahuja, J.K.C.; Wu, X.; Li, Y.; Quam, J.; Olson, R.; Pehrsson, P.; Allen, L.; Balentine, D.; Hanspal, M.; et al. NIH workshop on human milk composition: Summary and visions. Am. J. Clin. Nutr. 2019, 110, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Ramiro-Cortijo, D.; Singh, P.; Liu, Y.; Medina-Morales, E.; Yakah, W.; Freedman, S.D.; Martin, C.R. Breast Milk Lipids and Fatty Acids in Regulating Neonatal Intestinal Development and Protecting against Intestinal Injury. Nutrients 2020, 12, 534. [Google Scholar] [CrossRef] [Green Version]
- Bardanzellu, F.; Puddu, M.; Peroni, D.G.; Fanos, V. The Human Breast Milk Metabolome in Overweight and Obese Mothers. Front. Immunol. 2020, 11, 1533. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [Green Version]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. European Childhood Obesity Project Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enstad, S.; Cheema, S.; Thomas, R.; Fichorova, R.N.; Martin, C.R.; O’Tierney-Ginn, P.; Wagner, C.L.; Sen, S. The impact of maternal obesity and breast milk inflammation on developmental programming of infant growth. Eur. J. Clin. Nutr. 2021, 75, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Caplan, M.S.; Jilling, T. The role of polyunsaturated fatty acid supplementation in intestinal inflammation and neonatal necrotizing enterocolitis. Lipids 2001, 36, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Caplan, M.S.; Russell, T.; Xiao, Y.; Amer, M.; Kaup, S.; Jilling, T. Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr. Res. 2001, 49, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Moossavi, S.; Atakora, F.; Miliku, K.; Sepehri, S.; Robertson, B.; Duan, Q.L.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Integrated Analysis of Human Milk Microbiota With Oligosaccharides and Fatty Acids in the CHILD Cohort. Front. Nutr. 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef]
- Garrido, D.; Ruiz-Moyano, S.; Mills, D.A. Release and utilization of N-acetyl-D-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe 2012, 18, 430–435. [Google Scholar] [CrossRef]
- Bering, S.B. Human Milk Oligosaccharides to Prevent Gut Dysfunction and Necrotizing Enterocolitis in Preterm Neonates. Nutrients 2018, 10, 1461. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lönnerdal, B.; Slupsky, C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef] [Green Version]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef]
- Holscher, H.D.; Bode, L.; Tappenden, K.A. Human Milk Oligosaccharides Influence Intestinal Epithelial Cell Maturation In Vitro. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 296–301. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef]
- Rasmussen, S.O.; Martin, L.; Østergaard, M.V.; Rudloff, S.; Roggenbuck, M.; Nguyen, D.N.; Sangild, P.T.; Bering, S.B. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J. Nutr. Biochem. 2017, 40, 141–154. [Google Scholar] [CrossRef]
- Rudloff, S.; Kuntz, S.; Ostenfeldt Rasmussen, S.; Roggenbuck, M.; Sprenger, N.; Kunz, C.; Sangild, P.T.; Brandt Bering, S. Metabolism of Milk Oligosaccharides in Preterm Pigs Sensitive to Necrotizing Enterocolitis. Front. Nutr. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Skarbek, K.; Milewska, M.J. Biosynthetic and synthetic access to amino sugars. Carbohydr. Res. 2016, 434, 44–71. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.M.; Wu, G.; Galanko, J.A.; Chen, W.; Maynor, A.R.; Bose, C.L.; Rhoads, J.M. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J. Pediatr. 2000, 137, 785–793. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Zheng, X.-H.; Rong, X.; Huang, L.-G. Glutamine downregulates TLR-2 and TLR-4 expression and protects intestinal tract in preterm neonatal rats with necrotizing enterocolitis. J. Pediatr. Surg. 2014, 49, 1057–1063. [Google Scholar] [CrossRef]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mihi, B.; Gong, Q.; Nolan, L.S.; Gale, S.E.; Goree, M.; Hu, E.; Lanik, W.E.; Rimer, J.M.; Liu, V.; Parks, O.B.; et al. Interleukin-22 signaling attenuates necrotizing enterocolitis by promoting epithelial cell regeneration. Cell Rep. Med. 2021, 2, 100320. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, M.; Mangelis, A.; Eisenhofer, G.; Huebner, A. Age-specific pediatric reference intervals for plasma free normetanephrine, metanephrine, 3-methoxytyramine and 3-O-methyldopa: Particular importance for early infancy. Clin. Chim. Acta 2019, 494, 100–105. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Chen, P.-W.; Lee, N.-C.; Hsieh, W.-S.; Chiu, P.-C.; Hwu, W.-L.; Tsai, F.-J.; Lin, S.-P.; Chu, S.-Y.; Jong, Y.-J.; et al. 3-O-methyldopa levels in newborns: Result of newborn screening for aromatic l-amino-acid decarboxylase deficiency. Mol. Genet. Metab. 2016, 118, 259–263. [Google Scholar] [CrossRef]
- Moore, T.A.; Ahmad, I.M.; Schmid, K.K.; Berger, A.M.; Ruiz, R.J.; Pickler, R.H.; Zimmerman, M.C. Oxidative Stress Levels Throughout Pregnancy, at Birth, and in the Neonate. Biol. Res. Nurs. 2019, 21, 485–494. [Google Scholar] [CrossRef]
- Gay, M.C.L.; Koleva, P.T.; Slupsky, C.M.; du Toit, E.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; Campbell, D.E.; Prescott, S.L.; et al. Worldwide Variation in Human Milk Metabolome: Indicators of Breast Physiology and Maternal Lifestyle? Nutrients 2018, 10, 646–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamao, M.; Tsugawa, N.; Suhara, Y.; Wada, A.; Mori, T.; Murata, K.; Nishino, R.; Ukita, T.; Uenishi, K.; Tanaka, K.; et al. Quantification of fat-soluble vitamins in human breast milk by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2007, 859, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Redeuil, K.; Lévêques, A.; Oberson, J.-M.; Bénet, S.; Tissot, E.; Longet, K.; de Castro, A.; Romagny, C.; Beauport, L.; Fischer Fumeaux, C.J.; et al. Vitamins and carotenoids in human milk delivering preterm and term infants: Implications for preterm nutrient requirements and human milk fortification strategies. Clin. Nutr. 2021, 40, 222–228. [Google Scholar] [CrossRef] [PubMed]
| NEC | Control | p Value | ≤1.0 kg | >1.5 kg | p Value | |
|---|---|---|---|---|---|---|
| n = 18 | n = 18 | n = 13 | n = 15 | |||
| Male sex | 11 (48%) | 12 (52%) | 0.58 a | 6 (46%) | 12 (80%) | 0.06 a |
| Cesarean section delivery | 13 (72%) | 11 (61%) | 0.48 a | 10 (77%) | 8 (53%) | 0.19 a |
| Histologic chorioamnionitis | 2 (11%) | 2 (11%) | >0.99 a | 0 (0%) | 1 (7%) | 0.34 a |
| Race | 0.29 a | 0.03 a | ||||
| White | 16 (89%) | 12 (75%) | 7 (54%) | 13 (89%) | ||
| Black/African American/Other | 2 (11%) | 4 (25%) | 6 (46%) | 2 (11%) | ||
| Gestational Age (weeks) | 28 (25, 33) | 28 (26, 33) | 0.94 b | 27 (25, 28) | 33 (32, 34) | <0.0001 a |
| Birth Weight (grams) | 1020 (705, 1948) | 1560 (840, 2015) | 0.45 b | 805 (595, 947.5) | 2140 (1583, 2335) | <0.0001 b |
| Small for gestational age | 2 (10%) | 3 (12%) | 0.79 a | 3 (19%) | 0 (0%) | 0.03 a |
| Maternal Age (years) | 29 (24, 33) | 29 (27, 31) | 0.88 b | 29 (23, 34) | 29 (28, 33) | 0.69 b |
| Maternal Gravida | 2 (1, 4) | 2 (1, 4) | 0.64 b | 2 (1, 2) | 2 (1, 4) | 0.45 b |
| Maternal Betamethasone | 14 (82%) | 14 (82%) | >0.99 a | 11 (92%) | 12 (80%) | 0.40 a |
| DOL of maternal milk collection | 15 (7, 24) | 15 (7, 23) | 0.63 b | 9 (5, 21) | 14 (13, 20) | 0.24 b |
| DOL of NEC development | 17 (9, 21) | -- | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolan, L.S.; Lewis, A.N.; Gong, Q.; Sollome, J.J.; DeWitt, O.N.; Williams, R.D.; Good, M. Untargeted Metabolomic Analysis of Human Milk from Mothers of Preterm Infants. Nutrients 2021, 13, 3604. https://doi.org/10.3390/nu13103604
Nolan LS, Lewis AN, Gong Q, Sollome JJ, DeWitt ON, Williams RD, Good M. Untargeted Metabolomic Analysis of Human Milk from Mothers of Preterm Infants. Nutrients. 2021; 13(10):3604. https://doi.org/10.3390/nu13103604
Chicago/Turabian StyleNolan, Lila S., Angela N. Lewis, Qingqing Gong, James J. Sollome, Olivia N. DeWitt, Robert D. Williams, and Misty Good. 2021. "Untargeted Metabolomic Analysis of Human Milk from Mothers of Preterm Infants" Nutrients 13, no. 10: 3604. https://doi.org/10.3390/nu13103604
APA StyleNolan, L. S., Lewis, A. N., Gong, Q., Sollome, J. J., DeWitt, O. N., Williams, R. D., & Good, M. (2021). Untargeted Metabolomic Analysis of Human Milk from Mothers of Preterm Infants. Nutrients, 13(10), 3604. https://doi.org/10.3390/nu13103604






