The Effects of Specific Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins on Gait and Functional Capacity Parameters in Patients with Relapsing-Remitting Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
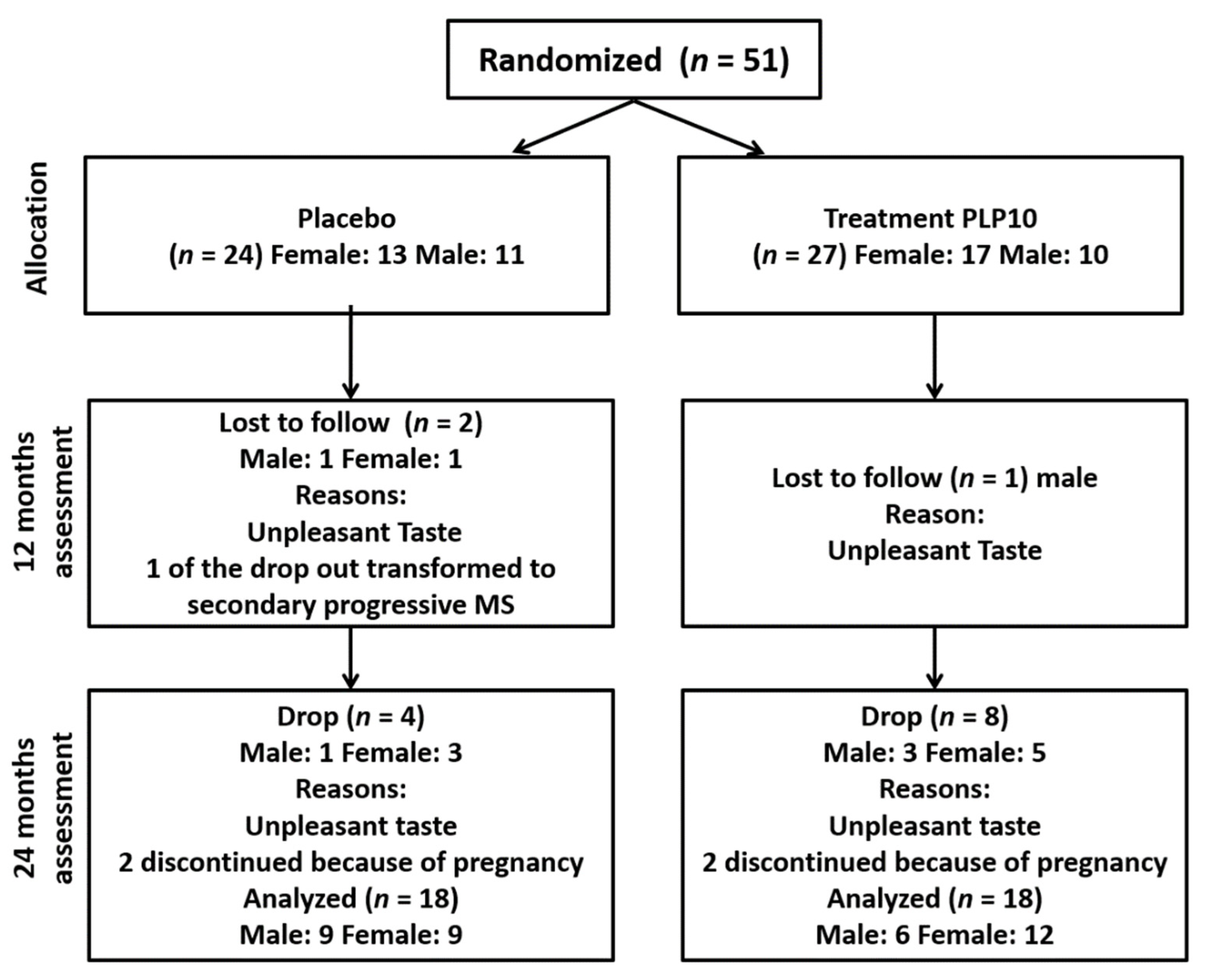
2.2. Randomization and Masking
2.3. Procedures and Endpoints
2.4. Anthropometry and Body Composition Assessment
2.5. Gait Analysis
2.6. Functional Capacity Assessment
2.7. Handgrip Strength Assessment
2.8. Isokinetic Dynamometry
2.9. Statistical Analysis
3. Results
3.1. Spatiotemporal Gait Parameters
3.2. Functional Capacity Assessment
3.3. Handgrip Strength
3.4. Isokinetic Knee Strength
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilokthornsakul, P.; Valuck, R.J.; Nair, K.V.; Corboy, J.R.; Allen, R.R.; Campbell, J.D. Multiple sclerosis prevalence in the United States commercially insured population. Neurology 2016, 86, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [Green Version]
- Zwibel, H.L.; Smrtka, J. Improving quality of life in multiple sclerosis: An unmet need. Am. J. Manag. Care 2011, 17 (Suppl. S5), S139–S145. [Google Scholar]
- Naci, H.; Fleurence, R.; Birt, J.; Duhig, A. Economic burden of multiple sclerosis: A systematic review of the literature. Pharmacoeconomics 2010, 28, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.D.; Gandevia, S.C.; Herbert, R.D. Prevalence of joint contractures and muscle weakness in people with multiple sclerosis. Disabil. Rehabil. 2014, 36, 1588–1593. [Google Scholar] [CrossRef]
- Hartung, H.P.; Aktas, O. Evolution of multiple sclerosis treatment: Next generation therapies meet next generation efficacy criteria. Lancet. Neurol. 2011, 10, 293–295. [Google Scholar] [CrossRef]
- Pike, J.; Jones, E.; Rajagopalan, K.; Piercy, J.; Anderson, P. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurol. 2012, 12, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinetti, M.E.; Inouye, S.K.; Gill, T.M.; Doucette, J.T. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA 1995, 273, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Larocca, N.G. Impact of walking impairment in multiple sclerosis: Perspectives of patients and care partners. Patient 2011, 4, 189–201. [Google Scholar] [CrossRef]
- Coleman, C.I.; Sidovar, M.F.; Roberts, M.S.; Kohn, C. Impact of mobility impairment on indirect costs and health-related quality of life in multiple sclerosis. PLoS ONE 2013, 8, e54756. [Google Scholar] [CrossRef] [PubMed]
- Feys, P.; Duportail, M.; Kos, D.; Van Asch, P.; Ketelaer, P. Validity of the TEMPA for the measurement of upper limb function in multiple sclerosis. Clin. Rehabil. 2002, 16, 166–173. [Google Scholar] [CrossRef]
- Yozbatiran, N.; Baskurt, F.; Baskurt, Z.; Ozakbas, S.; Idiman, E. Motor assessment of upper extremity function and its relation with fatigue, cognitive function and quality of life in multiple sclerosis patients. J. Neurol. Sci. 2006, 246, 117–122. [Google Scholar] [CrossRef]
- Nowak, D.A.; Hermsdorfer, J. Analysis of grip force during object manipulation. Method for the objective measurement of physiological normal and impaired hand function. Nervenarzt 2004, 75, 725–733. [Google Scholar]
- Chung, L.H.; Remelius, J.G.; Van Emmerik, R.E.; Kent-Braun, J.A. Leg power asymmetry and postural control in women with multiple sclerosis. Med. Sci. Sports Exerc. 2008, 40, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Thoumie, P.; Lamotte, D.; Cantalloube, S.; Faucher, M.; Amarenco, G. Motor determinants of gait in 100 ambulatory patients with multiple sclerosis. Mult. Scler. 2005, 11, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Minden, S.L.; Frankel, D.; Hadden, L.; Perloffp, J.; Srinath, K.P.; Hoaglin, D.C. The Sonya Slifka Longitudinal Multiple Sclerosis Study: Methods and sample characteristics. Mult. Scler. 2006, 12, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Comber, L.; Galvin, R.; Coote, S. Gait deficits in people with multiple sclerosis: A systematic review and meta-analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P. The molecular basis of nutritional intervention in multiple sclerosis: A narrative review. Complement. Med. 2011, 19, 228–237. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Nutrition facts in multiple sclerosis. ASN Neuro 2015, 7, 1759091414568185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, H.R.; Ferreira, G.D.C.; Trevenzoli, I.H.; Oliveira, K.J.; de Melo Reis, R.A. Fatty Acids, Antioxidants and Physical Activity in Brain Aging. Nutrients 2017, 9, 1263. [Google Scholar] [CrossRef] [Green Version]
- Zirpoli, H.; Chang, C.L.; Carpentier, Y.A.; Michael-Titus, A.T.; Ten, V.S.; Deckelbaum, R.J. Novel Approaches for Omega-3 Fatty Acid Therapeutics: Chronic Versus Acute Administration to Protect Heart, Brain, and Spinal Cord. Annu. Rev. Nutr. 2020, 40, 161–187. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael-Titus, A.T. Neurological benefits of omega-3 fatty acids. Neuromolecular Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- Pantzaris, M.C.; Loukaides, G.N.; Ntzani, E.E.; Patrikios, I.S. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: A randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 2013, 3, e002170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavrinou, P.S.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.S.; Giannaki, C.D. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aristotelous, P.; Stefanakis, M.; Pantzaris, M.; Pattichis, C.; Hadjigeorgiou, G.M.; Giannaki, C.D. Associations between functional capacity, isokinetic leg strength, sleep quality and cognitive function in multiple sclerosis patients: A cross-sectional study. Postgrad. Med. 2019, 131, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Committee for Medicinal Products for Human Use. Guideline on Clinical Trials in Small Populations European Medicines; Agency: London, UK, 2006; pp. 1–10. [Google Scholar]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.L. Scaling gait data to body size. Gait Posture 1996, 3, 222–223. [Google Scholar] [CrossRef]
- Pinzone, O.; Schwartz, M.H.; Baker, R. Comprehensive non-dimensional normalization of gait data. Gait Posture 2016, 44, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.H.; Rozumalski, A. The Gait Deviation Index: A new comprehensive index of gait pathology. Gait Posture 2008, 28, 351–357. [Google Scholar] [CrossRef]
- Moller, A.B.; Bibby, B.M.; Skjerbaek, A.G.; Jensen, E.; Sorensen, H.; Stenager, E.; Dalgas, U. Validity and variability of the 5-repetition sit-to-stand test in patients with multiple sclerosis. Disabil. Rehabil. 2012, 34, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Gijbels, D.; Eijnde, B.O.; Feys, P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult. Scler. 2011, 17, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Broekmans, T.; Gijbels, D.; Eijnde, B.O.; Alders, G.; Lamers, I.; Roelants, M.; Feys, P. The relationship between upper leg muscle strength and walking capacity in persons with multiple sclerosis. Mult. Scler. 2013, 19, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Osternig, L.R. Isokinetic dynamometry: Implications for muscle testing and rehabilitation. Exerc. Sport Sci. Rev. 1986, 14, 45–80. [Google Scholar] [CrossRef]
- Baltzopoulos, V.; Williams, J.G.; Brodie, D.A. Sources of error in isokinetic dynamometry: Effects of visual feedback on maximum torque. J. Orthop. Sports Phys. Ther. 1991, 13, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.E.; Cantwell, C.; Vowels, L.; Dodd, K. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2002, 72, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Givon, U.; Zeilig, G.; Achiron, A. Gait analysis in multiple sclerosis: Characterization of temporal-spatial parameters using GAITRite functional ambulation system. Gait Posture 2009, 29, 138–142. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Sandroff, B.M.; Motl, R.W. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012, 36, 154–156. [Google Scholar] [CrossRef]
- Jordan, K.; Challis, J.H.; Newell, K.M. Walking speed influences on gait cycle variability. Gait Posture 2007, 26, 128–134. [Google Scholar] [CrossRef]
- Winiarski, S.; Pietraszewska, J.; Pietraszewski, B. Three-Dimensional Human Gait Pattern: Reference Data for Young, Active Women Walking with Low, Preferred, and High Speeds. Biomed. Res. Int. 2019, 2019, 9232430. [Google Scholar] [CrossRef] [PubMed]
- Jeng, B.; Sandroff, B.M.; Motl, R.W. Energetic cost of walking and spasticity in persons with multiple sclerosis with moderate disability. NeuroRehabilitation 2018, 43, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Gunn, H.; Creanor, S.; Haas, B.; Marsden, J.; Freeman, J. Risk factors for falls in multiple sclerosis: An observational study. Mult. Scler. 2013, 19, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Bazelier, M.T.; van Staa, T.P.; Uitdehaag, B.M.; Cooper, C.; Leufkens, H.G.; Vestergaard, P.; Herings, R.M.; de Vries, F. Risk of fractures in patients with multiple sclerosis: A population-based cohort study. Neurology 2012, 78, 1967–1973. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.W.; Cho, C.C.; Finlayson, M.L. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult. Scler. 2007, 13, 1168–1175. [Google Scholar] [CrossRef]
- Finlayson, M.L.; Peterson, E.W. Falls, aging, and disability. Phys. Med. Rehabil. Clin. 2010, 21, 357–373. [Google Scholar] [CrossRef]
- Kieseier, B.C.; Pozzilli, C. Assessing walking disability in multiple sclerosis. Mult. Scler. 2012, 18, 914–924. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Dlugonski, D.; Sandroff, B.M.; Suh, Y.; Pula, J.H.; Sosnoff, J.J.; Motl, R.W. Gait and six-minute walk performance in persons with multiple sclerosis. J. Neurol. Sci. 2013, 334, 72–76. [Google Scholar] [CrossRef]
- Baert, I.; Freeman, J.; Smedal, T.; Dalgas, U.; Romberg, A.; Kalron, A.; Conyers, H.; Elorriaga, I.; Gebara, B.; Gumse, J.; et al. Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: A European multicenter study. Neurorehabilit. Neural Repair 2014, 28, 621–631. [Google Scholar] [CrossRef]
- Cowley, P.M.; Ploutz-Snyder, L.L.; Baynard, T.; Heffernan, K.; Jae, S.Y.; Hsu, S.; Lee, M.; Pitetti, K.H.; Reiman, M.P.; Fernhall, B. Physical fitness predicts functional tasks in individuals with Down syndrome. Med. Sci. Sports Exerc. 2010, 42, 388–393. [Google Scholar] [CrossRef]
- Dunlop, D.D.; Hughes, S.L.; Manheim, L.M. Disability in activities of daily living: Patterns of change and a hierarchy of disability. Am. J. Public Health 1997, 87, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Baer, G.D.; Ashburn, A.M. Trunk movements in older subjects during sit-to-stand. Arch. Phys. Med. Rehabil. 1995, 76, 844–849. [Google Scholar] [CrossRef]
- Kralj, A.; Jaeger, R.J.; Munih, M. Analysis of standing up and sitting down in humans: Definitions and normative data presentation. J. Biomech. 1990, 23, 1123–1138. [Google Scholar] [CrossRef]
- Riley, P.O.; Schenkman, M.L.; Mann, R.W.; Hodge, W.A. Mechanics of a constrained chair-rise. J. Biomech. 1991, 24, 77–85. [Google Scholar] [CrossRef]
- Seven, Y.B.; Akalan, N.E.; Yucesoy, C.A. Effects of back loading on the biomechanics of sit-to-stand motion in healthy children. Hum. Mov. Sci. 2008, 27, 65–79. [Google Scholar] [CrossRef]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Eng, J.J.; Whittaker, M.W. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: Relationship with muscle strength. Spinal Cord 2004, 42, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Reid, K.F.; Fielding, R.A. Skeletal muscle power: A critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev. 2012, 40, 4–12. [Google Scholar] [CrossRef]
- Lambert, C.P.; Archer, R.L.; Evans, W.J. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med. Sci. Sports Exerc. 2001, 33, 1613–1619. [Google Scholar] [CrossRef]
| Variables | Placebo Group | OMEGA Group | p Value |
|---|---|---|---|
| N | 18 | 18 | - |
| Age (years) | 38.1 ± 5.3 | 39.1 ± 8.7 | 0.665 |
| Weight (kg) | 70.2 ± 17.2 | 70.1 ± 15.8 | 0.987 |
| Height (cm) | 166.7 ± 8.2 | 166.4 ± 8.9 | 0.923 |
| Body mass index | 25.1 ± 5.3 | 25.1 ± 4.3 | 0.997 |
| EDSS score | 2.36 ± 1.09 | 2.22 ± 1.08 | 0.705 |
| Variables | Placebo Group (n = 18) | OMEGA Group (n = 18) | Main Effects and Interactions (p Value) | ||
|---|---|---|---|---|---|
| Time | Time X Group | Group | |||
| Left Single Support (s) | |||||
| Baseline | 0.436 ± 0.04 | 0.440 ± 0.04 | 0.896 | 0.035 | 0.328 |
| 12 months | 0.443 ± 0.03 | 0.437 ± 0.04 | |||
| 24 months | 0.452 ± 0.04 | 0.421 ± 0.03 | |||
| Right Step Time (s) | |||||
| Baseline | 0.577 ± 0.06 | 0.555 ± 0.05 | 0.047 | 0.025 | 0.131 |
| 12 months | 0.586 ± 0.06 | 0.579 ± 0.08 | |||
| 24 months | 0.589 ± 0.08 | 0.526 ± 0.05 | |||
| Left Stride Time (s) | |||||
| Baseline | 1.16 ± 0.14 | 1.12 ± 0.09 | 0.148 | 0.051 | 0.073 |
| 12 months | 1.17 ± 0.1 | 1.14 ± 0.16 | |||
| 24 months | 1.18 ± 0.15 | 1.05 ± 0.12 | |||
| Right Stride Time (s) | |||||
| Baseline | 1.15 ± 0.14 | 1.11 ± 0.09 | 0.196 | 0.039 | 0.060 |
| 12 months | 1.16 ± 0.1 | 1.13 ± 0.13 | |||
| 24 months | 1.17 ± 0.16 | 1.04 ± 0.11 | |||
| Variables | Placebo Group (n = 18) | OMEGA Group (n =18) | Main Effects and Interactions (p Value) | ||
|---|---|---|---|---|---|
| Time | Time X Group | Group | |||
| Left Gait Deviation Index | |||||
| Baseline | 80.88 ± 12.61 | 78.38 ± 7.07 | 0.028 | 0.001 | 0.233 |
| 12 months | 74.70 ± 10.08 | 79.20 ± 6.82 | |||
| 24 months | 72.38 ± 11.83 | 81.04 ± 7.87 | |||
| Right Gait Deviation Index | |||||
| Baseline | 81.63 ± 9.92 | 81.80 ± 8.78 | 0.185 | 0.001 | 0.075 |
| 12 months | 78.56 ± 9.63 | 82.40 ± 7.37 | |||
| 24 months | 73.82 ± 11.67 | 85.34 ± 7.76 | |||
| Normalized left cadence | |||||
| Baseline | 31.15 ± 3.97 | 31.72 ± 2.64 | 0.259 | 0.099 | 0.154 |
| 12 months | 30.49 ± 2.81 | 31.80 ± 3.82 | |||
| 24 months | 30.59 ± 3.79 | 33.97 ± 4.47 | |||
| Normalized right cadence | |||||
| Baseline | 29.03 ± 0.84 | 29.03 ± 0.98 | 0.001 | 0.069 | 0.098 |
| 12 months | 30.80 ± 0.84 | 32.22 ± 0.98 | |||
| 24 months | 30.81 ± 2.77 | 33.51 ± 3.70 | |||
| Normalized left walking speed | |||||
| Baseline | 0.359 ± 0.61 | 0.402 ± 0.45 | 0.097 | 0.110 | 0.024 |
| 12 months | 0.376 ± 0.06 | 0.399 ± 0.05 | |||
| 24 months | 0.370 ± 0.06 | 0.428 ± 0.06 | |||
| Normalized right walking speed | |||||
| Baseline | 0.364 ± 0.06 | 0.402 ± 0.04 | 0.149 | 0.140 | 0.024 |
| 12 months | 0.38 ± 0.06 | 0.408 ± 0.05 | |||
| 24 months | 0.369 ± 0.06 | 0.431 ± 0.06 | |||
| Variables | Placebo Group (n = 18) | OMEGA Group (n = 18) | Main Effects and Interactions (p Value) | ||
|---|---|---|---|---|---|
| Time | Time X Group | Group | |||
| Six Minute Walk Test Normalized (m) | |||||
| Baseline | 504 ± 131 | 505 ± 99 | 0.137 | 0.186 | 0.337 |
| 12 months | 523 ± 90 | 546 ± 73 | |||
| 24 months | 494 ± 79 | 551 ± 101 | |||
| STS5 (s) | |||||
| Baseline | 12.57 ± 3.52 | 12.37 ± 2.83 | 0.001 | 0.078 | 0.359 |
| 12 months | 10.67 ± 2.75 | 10.33 ± 2.26 | |||
| 24 months | 11.52 ± 1.97 | 9.91 ± 1.72 | |||
| STS 30 (rep) | |||||
| Baseline | 12.55 ± 2.97 | 12.44 ± 2.63 | 0.001 | 0.040 | 0.297 |
| 12 months | 14.66 ± 3.1 | 15.16 ± 2.66 | |||
| 24 months | 13.22 ± 2.18 | 15.22 ± 2.39 | |||
| STS60 (rep) | |||||
| Baseline | 25 ± 6.10 | 25.16 ± 5.51 | 0.001 | 0.032 | 0.279 |
| 12 months | 29.55 ± 6.20 | 30.33 ± 5.12 | |||
| 24 months | 26.22 ± 4.27 | 30.38 ± 4.86 | |||
| TUG (s) | |||||
| Baseline | 8.90 ± 1.76 | 8.21 ± 1.47 | 0.003 | 0.411 | 0.097 |
| 12 months | 8.04 ± 1.94 | 7.44 ± 1.84 | |||
| 24 months | 8.80 ± 1.61 | 7.63 ± 1.09 | |||
| EDSS | |||||
| Baseline | 2.36 ± 1.09 | 2.22 ± 1.08 | 0.001 | 0.001 | 0.446 |
| 12 months | 2.41 ± 1.10 | 2.27 ± 1.04 | |||
| 24 months | 2.83 ± 1.20 | 2.27 ± 1.04 | |||
| Variables | Placebo Group (n = 18) | OMEGA Group (n = 18) | Main Effects and Interactions (p Value) | ||
|---|---|---|---|---|---|
| Time | Time X Group | Group | |||
| Body Fat (%) | |||||
| Baseline | 28.98 ± 9.16 | 29.36 ± 7.02 | 0.538 | 0.635 | 0.641 |
| 12 months | 27.65 ± 10.44 | 29.20 ± 8.70 | |||
| 24 months | 27.80 ± 8.40 | 29.26 ± 8.08 | |||
| Abdominal girth (cm) | |||||
| Baseline | 96.94 ± 18.93 | 93.61 ± 12.41 | 0.283 | 0.489 | 0.661 |
| 12 months | 95.16 ± 17.05 | 92.94 ± 13.07 | |||
| 24 months | 94.11 ± 15.72 | 93.16 ± 12.39 | |||
| Trunk fat (%) | |||||
| Baseline | 33.11 ± 12.94 | 32.07 ± 9.36 | 0.774 | 0.421 | 0.255 |
| 12 months | 33.06 ± 12.32 | 31.62 ± 8.67 | |||
| 24 months | 31.37 ± 11.73 | 31.76 ± 9.50 | |||
| Body mass index | |||||
| Baseline | 25.11 ± 5.37 | 25.11 ± 4.36 | 0.453 | 0.141 | 0.837 |
| 12 months | 24.76 ± 5.19 | 24.98 ± 4.74 | |||
| 24 months | 24.58 ± 5.11 | 24.98 ± 4.70 | |||
| Variables | Placebo Group (n = 18) | OMEGA Group (n = 18) | Main Effects and Interactions (p Value) | ||
|---|---|---|---|---|---|
| Time | Time X Group | Group | |||
| Right Handgrip Strength (kg) | |||||
| Baseline | 28.90 ± 9.60 | 28.24 ± 7.47 | 0.001 | 0.739 | 0.796 |
| 12 months | 30.81 ± 11.44 | 29.25 ± 7.87 | |||
| 24 months | 32.58 ± 9.83 | 32.22 ± 8.87 | |||
| Left Handgrip Strength (kg) | |||||
| Baseline | 29.33 ± 9.55 | 26.30 ± 6.69 | 0.012 | 0.415 | 0.560 |
| 12 months | 30.36 ± 11.16 | 28.42 ± 7.02 | |||
| 24 months | 30.91 ± 11.89 | 30.07 ± 7.60 | |||
| Max Isokinetic Strength of Right Knee Extensors at 60°/s Normalized by BW (N·m/kg) | |||||
| Baseline | 2.05 ± 0.79 | 2.19 ± 0.62 | 0.774 | 0.421 | 0.255 |
| 12 months | 1.96 ± 0.89 | 2.29 ± 0.68 | |||
| 24 months | 2.02 ± 0.74 | 2.31 ± 0.42 | |||
| Max Isokinetic Strength of Left Knee Extensors at 60°/s Normalized by BW (N·m/kg) | |||||
| Baseline | 1.95 ± 0.62 | 2.15 ± 0.45 | 0.037 | 0.618 | 0.284 |
| 12 months | 1.94 ± 0.82 | 2.22 ± 0.59 | |||
| 24 months | 2.16 ± 0.74 | 2.30 ± 0.40 | |||
| Max Isokinetic Strength of Right Knee Flexors at 60°/s Normalized by BW (N·m/kg) | |||||
| Baseline | 1.31 ± 0.45 | 1.42 ± 0.35 | 0.001 | 0.718 | 0.217 |
| 12 months | 1.30 ± 0.55 | 1.50 ± 0.31 | |||
| 24 months | 0.97 ± 0.39 | 1.12 ± 0.28 | |||
| Max Isokinetic Strength of Left Knee Flexors at 60°/s Normalized by BW(N·m/kg) | |||||
| Baseline | 1.31 ± 0.36 | 1.36 ± 0.31 | 0.025 | 0.373 | 0.484 |
| 12 months | 1.24 ± 0.50 | 1.39 ± 0.36 | |||
| 24 months | 1.0 ± 0.46 | 1.05 ± 0.26 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aristotelous, P.; Stefanakis, M.; Pantzaris, M.; Pattichis, C.S.; Calder, P.C.; Patrikios, I.S.; Sakkas, G.K.; Giannaki, C.D. The Effects of Specific Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins on Gait and Functional Capacity Parameters in Patients with Relapsing-Remitting Multiple Sclerosis. Nutrients 2021, 13, 3661. https://doi.org/10.3390/nu13103661
Aristotelous P, Stefanakis M, Pantzaris M, Pattichis CS, Calder PC, Patrikios IS, Sakkas GK, Giannaki CD. The Effects of Specific Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins on Gait and Functional Capacity Parameters in Patients with Relapsing-Remitting Multiple Sclerosis. Nutrients. 2021; 13(10):3661. https://doi.org/10.3390/nu13103661
Chicago/Turabian StyleAristotelous, Panayiotis, Manos Stefanakis, Marios Pantzaris, Constantinos S. Pattichis, Philip C. Calder, Ioannis S. Patrikios, Giorgos K. Sakkas, and Christoforos D. Giannaki. 2021. "The Effects of Specific Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins on Gait and Functional Capacity Parameters in Patients with Relapsing-Remitting Multiple Sclerosis" Nutrients 13, no. 10: 3661. https://doi.org/10.3390/nu13103661








