Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Mycelium Preparation
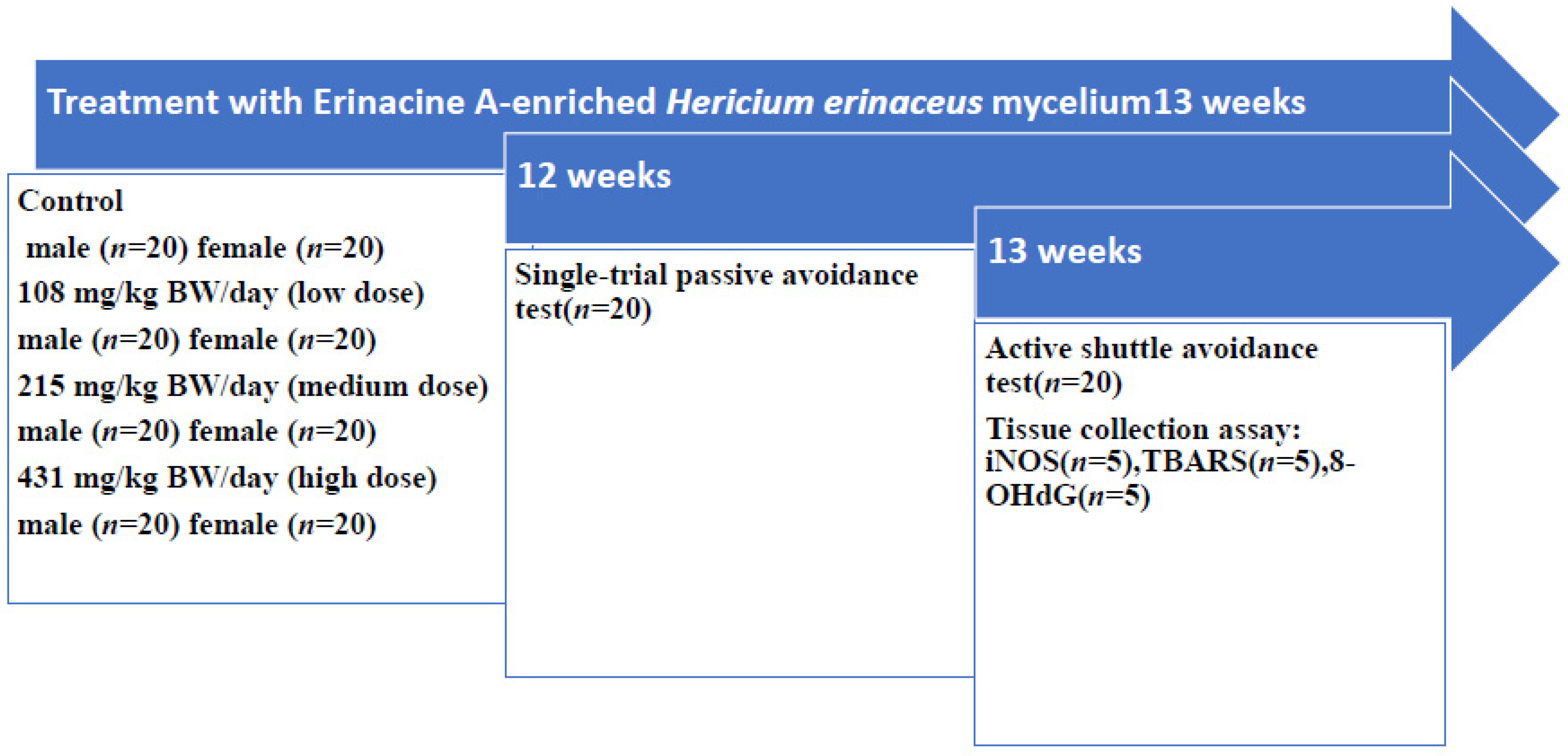
2.3. Experimental Design
2.4. Behavioral Tests
2.4.1. Passive Avoidance Task
2.4.2. Active Shuttle Avoidance Test
2.5. Tissue Preparation
2.6. Measurement of iNOS and TBARS
2.7. Data Analysis
3. Results
3.1. Behavioral Assays
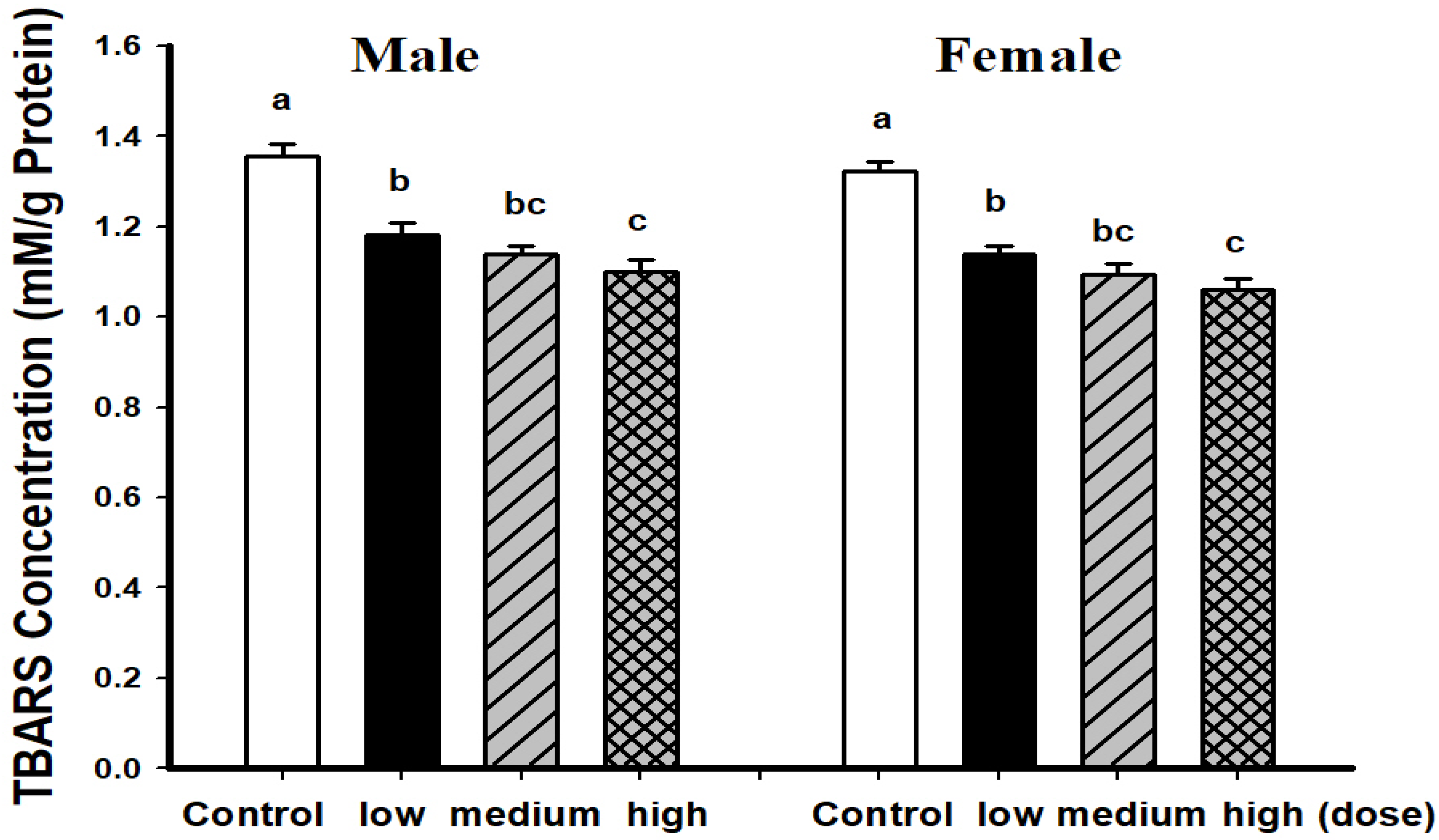
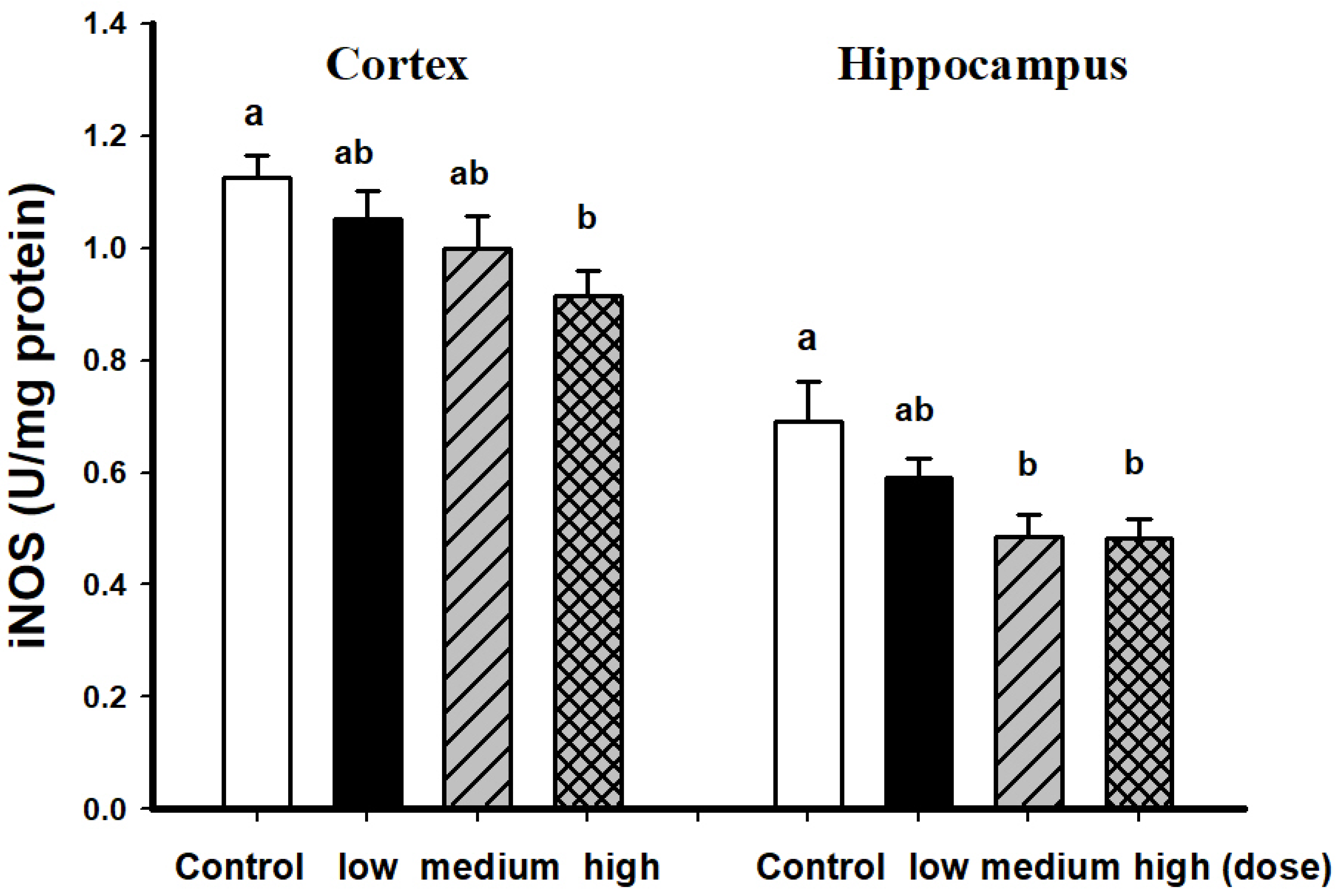
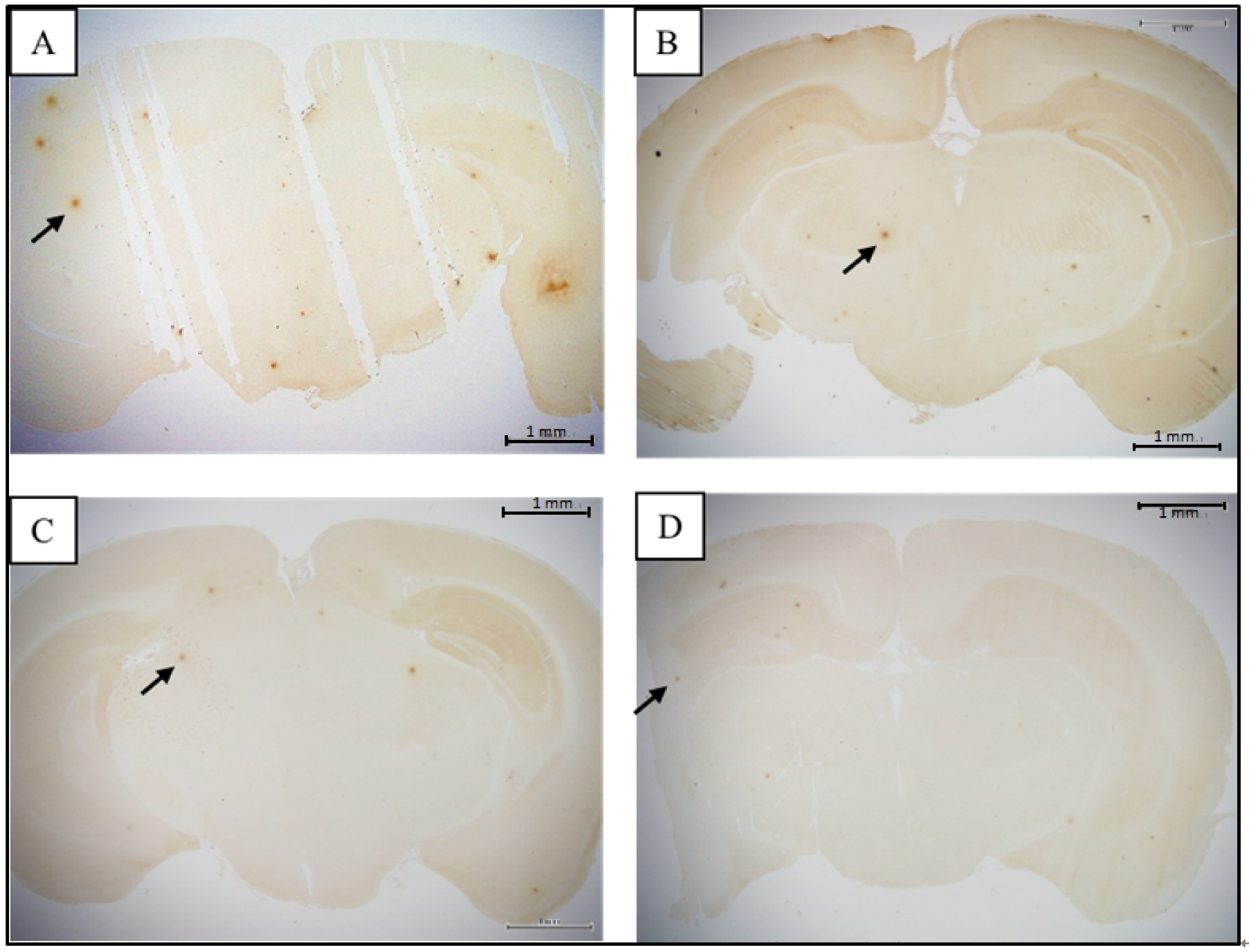
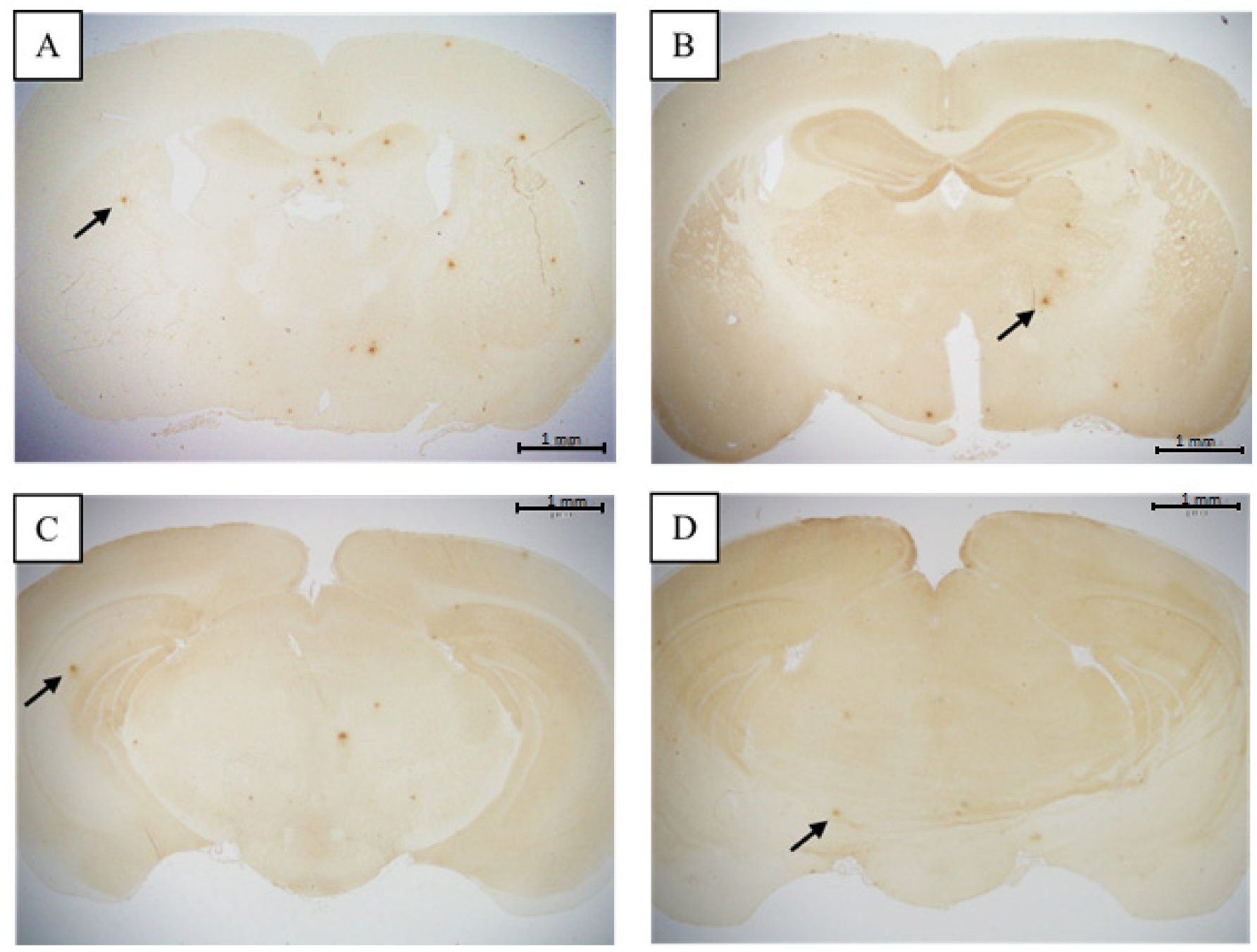
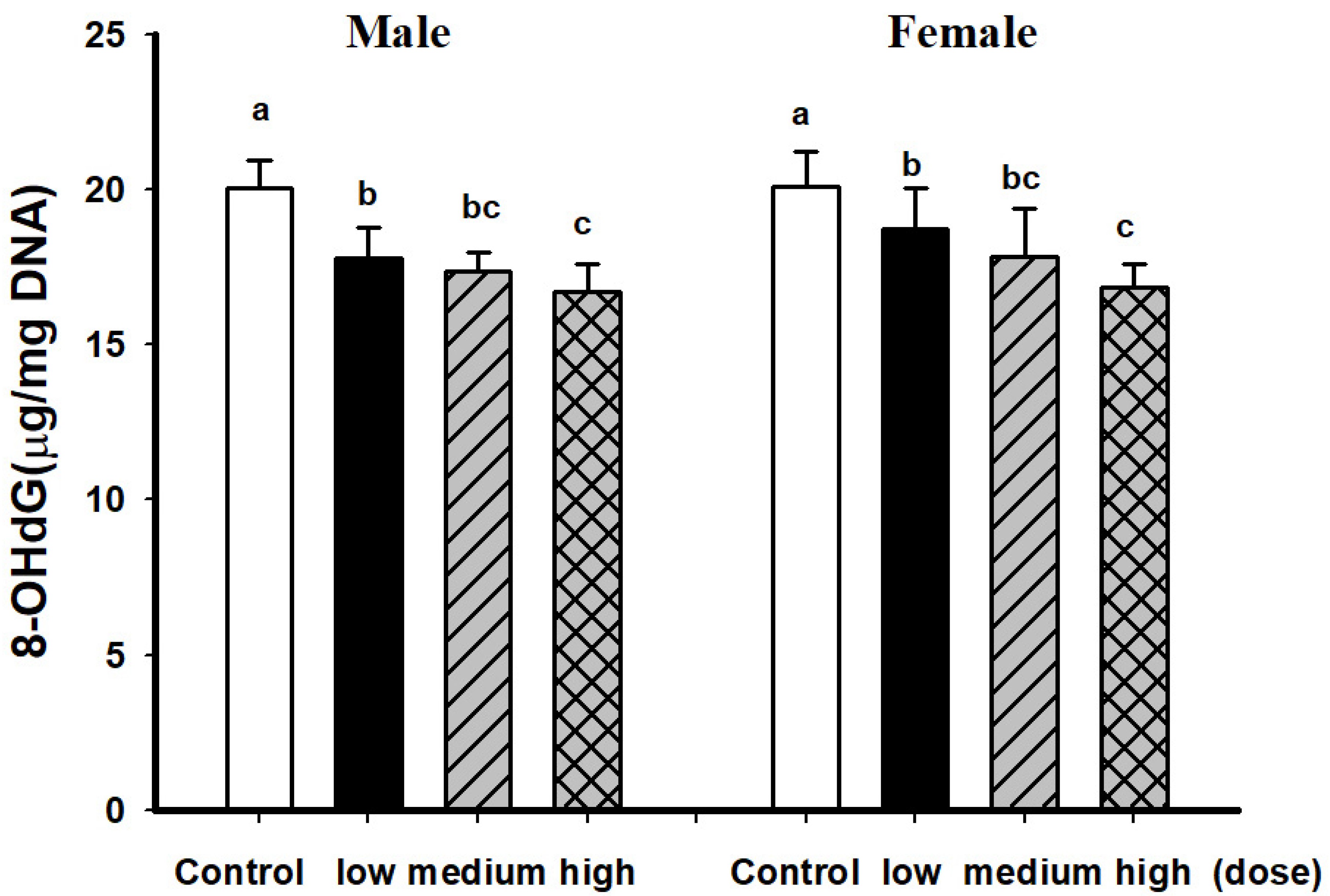
3.2. Comparison of Brain Pathological Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winslow, B.T.; Onysko, M.K.; Stob, C.M.; Hazlewood, K.A. Treatment of Alzheimer disease. Am. Fam. Physician 2011, 83, 1403–1412. [Google Scholar]
- Solomon, A.; Mangialasche, F.; Richard, E.; Andrieu, S.; Bennett, D.A.; Breteler, M.; Fratiglioni, L.; Hooshmand, B.; Khachaturian, A.S.; Schneider, L.S.; et al. Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 2011, 253, 229–250. [Google Scholar] [CrossRef] [Green Version]
- Kanasi, E.; Ayilavarapu, S.; Jones, J. The aging population: Demographics and the biology of aging. Periodontol. 2000 2016, 72, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Berger, S.L. Epigenetics of aging and aging-related disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 6, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.H.; Marioni, R.E.; Harris, S.E.; Deary, I.J. Brain age and other bodily ‘ages’: Implications for neuropsychiatry. Mol. Psychiatry 2019, 24, 266–281. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. The SAMP8 mouse: A model of Alzheimer disease? Biogerontology 2002, 3, 57–60. [Google Scholar] [CrossRef]
- Son, C.-G.; Shin, J.-W.; Cho, J.-H.; Cho, C.-K.; Yun, C.-H.; Han, S.-H. Induction of murine interleukin-1 beta expression by water-soluble components from Hericium erinaceum. Acta Pharmacol. Sin. 2006, 27, 1058–1064. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Xu, D.; Gao, Q. A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int. J. Biol. Macromol. 2015, 81, 656–661. [Google Scholar] [CrossRef]
- Yim, M.-H.; Shin, J.-W.; Son, J.-Y.; Oh, S.-M.; Han, S.-H.; Cho, J.-H.; Cho, C.-K.; Yoo, H.-S.; Lee, Y.-W.; Son, C.-G.; et al. Soluble components of Hericium erinaceum induce NK cell activation via production of interleukin-12 in mice splenocytes. Acta Pharmacol. Sin. 2007, 28, 901–907. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991, 32, 4561–4564. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Hosokawa, S.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1996, 37, 7399–7402. [Google Scholar] [CrossRef]
- Lee, E.W.; Shizuki, K.; Hosokawa, S.; Suzuki, M.; Suganuma, H.; Inakuma, T.; Li, J.; Ohnishi-Kameyama, M.; Nagata, T.; Furukawa, S.; et al. Two novel diterpenoids, erinacines H and I from the mycelia of Hericium erinaceum. Biosci. Biotechnol. Biochem. 2000, 64, 2402–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005, 25, 617–623. [Google Scholar] [CrossRef]
- Lee, K.-F.; Chen, J.-H.; Teng, C.-C.; Shen, C.-H.; Hsieh, M.-C.; Lu, C.-C.; Lee, K.-C.; Lee, L.-Y.; Chen, W.-P.; Chen, C.-C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.-C.; Lu, C.-C.; Shen, C.-H.; Tung, S.-Y.; Hsieh, M.-C.; Lee, K.-C.; Lee, L.-Y.; Chen, C.-C.; Teng, C.-C.; Huang, W.-S.; et al. Hericium erinaceus mycelium and its isolated erinacine A protection from MPTP-induced neurotoxicity through the ER stress, triggering an apoptosis cascade. J. Transl. Med. 2016, 14, 78. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, T.-T.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Lu, C.-K.; Shen, C.-C.; Huang, F.C.-Y.; Chen, C.-C.; Shiao, Y.-J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar]
- Li, I.-C.; Chen, Y.-L.; Lee, L.-Y.; Chen, W.-P.; Tsai, Y.-T.; Chen, C.-C. Evaluation of the toxicological safety of erinacine A-enriched Hericium erinaceus in a 28-day oral feeding study in Sprague–Dawley rats. Food Chem. Toxicol. 2014, 70, 61–67. [Google Scholar] [CrossRef]
- Shimada, A.; Hosokawa, M.; Ohta, A.; Akiguchi, I.; Takeda, T. Localization of atrophy-prone areas in the aging mouse brain: Comparison between the brain atrophy model SAM-P/10 and the normal control SAM-R/1. Neuroscience 1994, 59, 859–869. [Google Scholar] [CrossRef]
- Glowinski, J.; Iversen, L. Regional studies of catecholamines in the rat brain—III: Subcellullar distribution of endogenous and exogenous catecholamines in various brain regions. Biochem. Pharmacol. 1966, 15, 977–987. [Google Scholar] [CrossRef]
- DeBalsi, K.L.; Hoff, K.E.; Copeland, W.C. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res. Rev. 2017, 33, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.B.; Franko, M.W.; Farr, S.A.; Armbrecht, H.J.; Morley, J.E. Identification of age-dependent changes in expression of senescence-accelerated mouse (SAMP8) hippocampal proteins by expression array analysis. Biochem. Biophys. Res. Commun. 2000, 272, 657–661. [Google Scholar] [CrossRef]
- Flood, J.F.; Morley, J.E. Learning and memory in the SAMP8 mouse. Neurosci. Biobehav. Rev. 1997, 22, 1–20. [Google Scholar] [CrossRef]
- Tzeng, T.-T.; Chen, C.-C.; Chen, C.-C.; Tsay, H.-J.; Lee, L.-Y.; Chen, W.-P.; Shen, C.-C.; Shiao, Y.-J. The cyanthin diterpenoid and sesterterpene constituents of Hericium erinaceus mycelium ameliorate Alzheimer’s disease-related pathologies in APP/PS1 transgenic mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef] [Green Version]
- Malinski, T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J. Alzheimers Dis. 2007, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Poon, H.F.; Calabrese, V.; Scapagnini, G.; Butterfield, D.A. Free radicals and brain aging. Clin. Geriatr. Med. 2004, 20, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Prolongation of life: Role of free radical reactions in aging. J. Am. Geriatr. Soc. 1969, 17, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Ehmann, W.D.; Butler, S.M.; Markesbery, W.R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 1995, 45, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R.; Carney, J.M. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999, 9, 133–146. [Google Scholar] [CrossRef]
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s disease. Subcell. Biochem. 2012, 65, 329–352. [Google Scholar]
- Roberts, G.W.; Gentleman, S.M.; Lynch, A.; Murray, L.; Landon, M.; Graham, D.I. Beta amyloid protein deposition in the brain after severe head injury: Implications for the pathogenesis of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Blasco, S.; Valero, R.A.; Rodriguez-Crespo, I.; Villalobos, C.; Nunez, L. Mitochondrial Ca2+ overload underlies Aβ oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS ONE 2008, 3, e2718. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Lee, C.D.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T.M.; Collins, J.L. ApoE promotes the proteolytic degradation of Aβ. Neuron 2008, 58, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Kurochkin, I.V.; Guarnera, E.; Berezovsky, I.N. Insulin-degrading enzyme in the fight against Alzheimer’s disease. Trends Pharmacol. Sci. 2018, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Recuero, M.; Serrano, E.; Bullido, M.J.; Valdivieso, F. Aβ production as consequence of cellular death of a human neuroblastoma overexpressing APP. FEBS Lett. 2004, 570, 114–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-C.; Tzeng, T.-T.; Chen, C.-C.; Ni, C.-L.; Lee, L.-Y.; Chen, W.-P.; Shiao, Y.-J.; Shen, C.-C. Erinacine S, a rare sesterterpene from the mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Won, M.H.; Kang, T.-C.; Jeon, G.-S.; Lee, J.-C.; Kim, D.-Y.; Choi, E.-M.; Lee, K.H.; Choi, C.D.; Chung, M.-H.; Cho, S.S. Immunohistochemical detection of oxidative DNA damage induced by ischemia–reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999, 836, 70–78. [Google Scholar] [CrossRef]
- Luceri, C.; Bigagli, E.; Femia, A.P.; Caderni, G.; Giovannelli, L.; Lodovici, M. Aging related changes in circulating reactive oxygen species (ROS) and protein carbonyls are indicative of liver oxidative injury. Toxicol. Rep. 2018, 5, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Gmitterová, K.; Heinemann, U.; Gawinecka, J.; Varges, D.; Ciesielczyk, B.; Valkovic, P.; Benetin, J.; Zerr, I. 8-OHdG in cerebrospinal fluid as a marker of oxidative stress in various neurodegenerative diseases. Neurodegener. Dis. 2009, 6, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogami, M.; Shiga, J.; Inuzuka, N.; Takatsu, A. Age-associated decrease in 8-hydroxy-2′-deoxyguanosine (8-OHdG) immunoreactivity in the autopsied brain. Leg. Med. 2002, 1, 29–33. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chyau, C.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Liu, J.L.; Lin, W.H.; Mong, M.C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Trial | 1 Day | 2 Days | 3 Days | |
|---|---|---|---|---|
| Male | ||||
| Control | 48.20 ± 0.95 | 58.55 ± 1.18 a | 56.35 ± 1.22 a | 49.25 ± 0.86 |
| 108 mg/kgBW/day | 49.65 ± 0.94 | 67.98 ± 0.80 b | 61.00 ± 0.79 b | 51.50 ± 0.82 |
| 215 mg/kgBW/day | 47.20 ± 0.86 | 69.70 ± 1.11 b | 62.20 ± 0.83 b | 51.25 ± 1.34 |
| 431 mg/kgBW/day | 47.40 ± 0.91 | 70.80 ± 0.87 b | 63.35 ± 0.85 b | 52.60 ± 1.56 |
| Female | ||||
| Control | 47.60 ± 1.18 | 60.65 ± 0.86 a | 55.80 ± 1.18 a | 49.10 ± 1.01 |
| 108 mg/kgBW/day | 48.55 ± 1.42 | 70.00 ± 0.78 b | 64.40 ± 1.15 b | 51.85 ± 1.11 |
| 215 mg/kgBW/day | 44.35 ± 1.46 | 71.90 ± 0.82 b | 65.85 ± 0.67 b | 52.65 ± 2.07 |
| 431 mg/kgBW/day | 46.30 ± 1.57 | 72.20 ± 0.79 b | 64.85 ± 0.67 b | 48.45 ± 1.86 |
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| Male | ||||
| Control | 9.85 ± 0.34 | 12.25 ± 0.36 a | 11.55 ± 0.36 a | 12.75 ± 0.31 a |
| 108 mg/kgBW/day | 10.35 ± 0.44 | 14.50 ± 0.51 b | 13.85 ± 0.51 b | 14.30 ± 0.56 b |
| 215 mg/kgBW/day | 10.00 ± 0.43 | 14.90 ± 0.45 b | 14.70 ± 0.45 b | 15.05 ± 0.39 b |
| 431 mg/kgBW/day | 9.95 ± 0.32 | 14.05 ± 0.40 b | 14.70 ± 0.40 b | 15.00 ± 0.24 b |
| Female | ||||
| Control | 12.35 ± 0.57 | 11.05 ± 0.40 a | 12.30 ± 0.25 a | 13.70 ± 0.19 a |
| 108 mg/kgBW/day | 10.70 ± 0.44 | 12.85 ± 0.37 b | 14.55 ± 0.37 b | 15.35 ± 0.46 b |
| 215 mg/kgBW/day | 10.45 ± 0.32 | 13.85 ± 0.31 b | 15.55 ± 0.46 b | 15.40 ± 0.20 b |
| 431 mg/kgBW/day | 10.20 ± 0.19 | 13.50 ± 0.53 b | 15.10 ± 0.37 b | 15.35 ± 0.34 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.-Y.; Chou, W.; Chen, W.-P.; Wang, M.-F.; Chen, Y.-J.; Chen, C.-C.; Tung, K.-C. Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients 2021, 13, 3659. https://doi.org/10.3390/nu13103659
Lee L-Y, Chou W, Chen W-P, Wang M-F, Chen Y-J, Chen C-C, Tung K-C. Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients. 2021; 13(10):3659. https://doi.org/10.3390/nu13103659
Chicago/Turabian StyleLee, Li-Ya, Wayne Chou, Wan-Ping Chen, Ming-Fu Wang, Ying-Ju Chen, Chin-Chu Chen, and Kwong-Chung Tung. 2021. "Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice" Nutrients 13, no. 10: 3659. https://doi.org/10.3390/nu13103659
APA StyleLee, L.-Y., Chou, W., Chen, W.-P., Wang, M.-F., Chen, Y.-J., Chen, C.-C., & Tung, K.-C. (2021). Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients, 13(10), 3659. https://doi.org/10.3390/nu13103659





