Acute L-Citrulline Supplementation Increases Nitric Oxide Bioavailability but Not Inspiratory Muscle Oxygenation and Respiratory Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Inspiratory Pressure
2.4. Pulmonary Function
2.5. Fractional Exhaled Nitric Oxide
2.6. Near-Infrared Spectroscopy Measurement
2.7. Statistical Analysis
3. Results
3.1. Supplementation and Performance
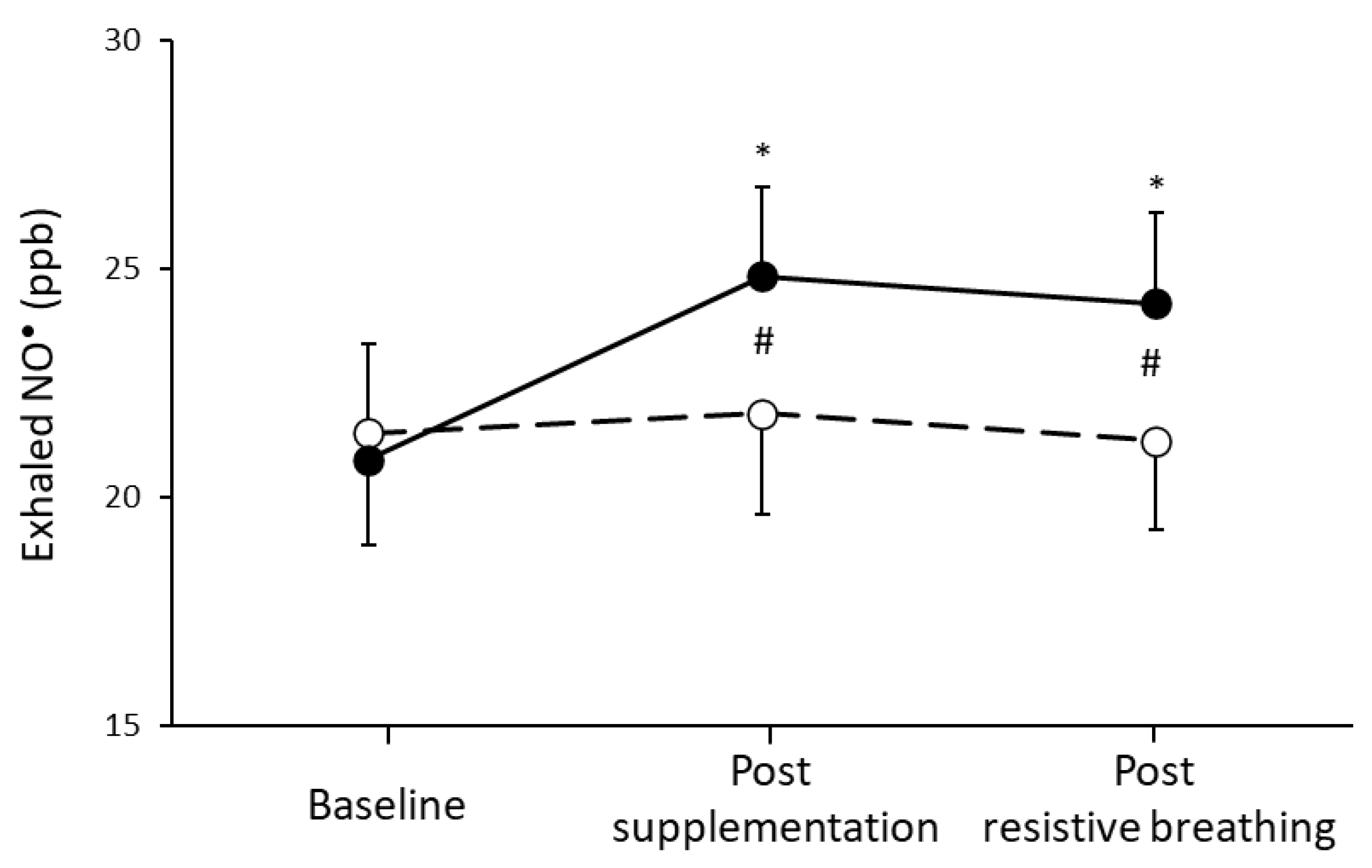
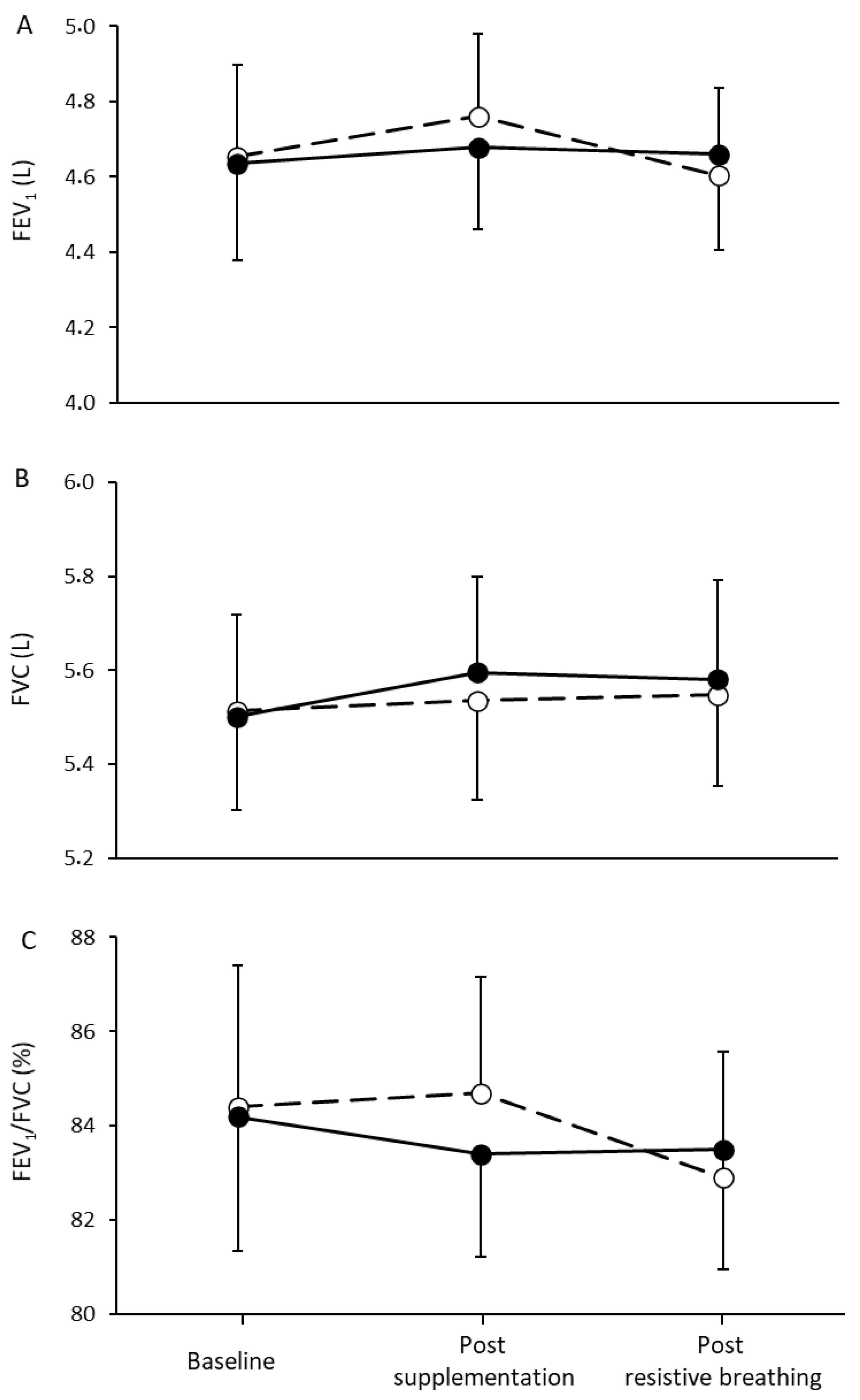
3.2. Respiratory Capacity and Muscle Oxygenation
4. Discussion
4.1. L-Citrulline Supplementation and NO• Bioavailability
4.2. Inspiratory Muscle Performance and Resistance to Fatigue
4.3. Sternocleidomastoid Muscle Oxygenation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Van De Poll, M.C.G.; Siroen, M.P.C.; van Leeuwen, P.A.; Soeters, P.B.; Melis, G.C.; Boelens, P.G.; Deutz, N.; DeJong, C.H.C. Interorgan amino acid exchange in humans: Consequences for arginine and citrulline metabolism. Am. J. Clin. Nutr. 2007, 85, 167–172. [Google Scholar] [CrossRef]
- Windmueller, H.G.; Spaeth, A.E. Source and fate of circulating citrulline. Am. J. Physiol. Metab. 1981, 241, E473–E480. [Google Scholar] [CrossRef]
- Marini, J.C.; Agarwal, U.; Didelija, I.C.; Azamian, M.; Stoll, B.; Nagamani, S.C. Plasma glutamine is a minor precursor for the synthesis of citrulline: A multispecies study. J. Nutr. 2017, 147, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Thompson, C.; Wylie, L.; Vanhatalo, A. Dietary nitrate and physical performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.; Paschalis, V.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Mülsch, A.; Böhme, E.; Busse, R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ. Res. 1986, 58, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giulivi, C.; Kato, K.; Cooper, C. Nitric oxide regulation of mitochondrial oxygen consumption I: Cellular physiology. Am. J Physiol. Cell Physiol. 2006, 291, C1225–C1231. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.; Jones, A.M.; Musch, T.I.; Poole, D.C. Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir. Physiol. Neurobiol. 2013, 187, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellsten, Y.; Nyberg, M.; Jensen, L.G.; Mortensen, S. Vasodilator interactions in skeletal muscle blood flow regulation. J. Physiol. 2012, 590, 6297–6305. [Google Scholar] [CrossRef]
- Bailey, S.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Jones, A.M. Acute l-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J. Appl. Physiol. 2010, 109, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Bescós, R.; Sureda, A.; Tur, J.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Camic, C.L.; Housh, T.J.; Zuniga, J.M.; Hendrix, R.C.; Mielke, M.; Johnson, G.O.; Schmidt, R.J. Effects of arginine-based supplements on the physical working capacity at the fatigue threshold. J. Strength Cond. Res. 2010, 24, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Trexler, E.T. Effects of citrulline supplementation on exercise performance in humans: A review of the current literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.; Jones, A.M. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levillain, O. Expression and function of arginine-producing and consuming-enzymes in the kidney. Amino Acids 2012, 42, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Rowell, L.B. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): Cycles of revision and new vision. J. Appl. Physiol. 2004, 97, 384–392. [Google Scholar] [CrossRef]
- Saltin, B. Exercise hyperaemia: Magnitude and aspects on regulation in humans. J. Physiol. 2007, 583, 819–823. [Google Scholar] [CrossRef]
- Dempsey, J.A.; La Gerche, A.; Hull, J.H. Is the healthy respiratory system built just right, overbuilt, or underbuilt to meet the demands imposed by exercise? J. Appl. Physiol. 2020, 129, 1235–1256. [Google Scholar] [CrossRef]
- Amann, M.; Calbet, J.A. Convective oxygen transport and fatigue. J. Appl. Physiol. 2008, 104, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Kent-Braun, J.A.; Fitts, R.H.; Christie, A. Skeletal muscle fatigue. Compr. Physiol. 2012, 2, 997–1044. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, P.B.; Archiza, B.; Ramsook, A.H.; Mitchell, R.; Peters, C.M.; Molgat-Seon, Y.; Henderson, W.R.; Koehle, M.; Boushel, R.; Sheel, A.W. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp. Physiol. 2017, 102, 1535–1547. [Google Scholar] [CrossRef] [Green Version]
- Harms, C.A.; Wetter, T.J.; Croix, C.M.S.; Pegelow, D.F.; Dempsey, J.A. Effects of respiratory muscle work on exercise performance. J. Appl. Physiol. 2000, 89, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Romer, L.M.; Lovering, A.; Haverkamp, H.C.; Pegelow, D.F.; Dempsey, J.A. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J. Physiol. 2006, 571, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.F.; Archiza, B.; Guenette, J.A.; West, C.R.; Sheel, A.W. Effect of diaphragm fatigue on subsequent exercise tolerance in healthy men and women. J. Appl. Physiol. 2018, 125, 1987–1996. [Google Scholar] [CrossRef]
- Cole, M.A.; Brown, M.D. Response of the human triceps surae muscle to electrical stimulation during varying levels of blood flow restriction. Eur. J. Appl. Physiol. 2000, 82, 39–44. [Google Scholar] [CrossRef]
- Pitcher, J.B.; Miles, T.S. Influence of muscle blood flow on fatigue during intermittent human hand-grip exercise and recovery. Clin. Exp. Pharmacol. Physiol. 1997, 24, 471–476. [Google Scholar] [CrossRef]
- Sheel, A.W.; Boushel, R.C.; Dempsey, J.A. Competition for blood flow distribution between respiratory and locomotor muscles: Implications for muscle fatigue. J. Appl. Physiol. 2018, 125, 820–831. [Google Scholar] [CrossRef]
- Tachi, M.; Kouzaki, M.; Kanehisa, H.; Fukunaga, T. The influence of circulatory difference on muscle oxygenation and fatigue during intermittent static dorsiflexion. Eur. J. Appl. Physiol. 2004, 91, 682–688. [Google Scholar] [CrossRef]
- Trinity, J.D.; Broxterman, R.M.; Richardson, R.S. Regulation of exercise blood flow: Role of free radicals. Free Radic. Biol. Med. 2016, 98, 90–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Doesl-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative functionin vivoin health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrey, S.; Ferrari, M. Muscle oximetry in sports science: A systematic review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef] [PubMed]
- De Troyer, A.; Boriek, A.M. Mechanics of the respiratory muscles. Compr. Physiol. 2011, 1, 1273–1300. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, M.; Ferrari, M.; Quaresima, V. Gastrocnemius medialis and vastus lateralis oxygenation during whole-body vibration exercise. Med. Sci. Sports Exerc. 2007, 39, 694–700. [Google Scholar] [CrossRef]
- Buchheit, M.; Ufland, P.; Haydar, B.; Laursen, P.B.; Ahmaidi, S. Reproducibility and sensitivity of muscle reoxygenation and oxygen uptake recovery kinetics following running exercise in the field. Clin. Physiol. Funct. Imaging 2011, 31, 337–346. [Google Scholar] [CrossRef]
- Niemeijer, V.M.; Spee, R.F.; Jansen, J.P.; Buskermolen, A.B.C.; Van Dijk, T.; Wijn, P.F.F.; Kemps, H.M.C. Test-retest reliability of skeletal muscle oxygenation measurements during submaximal cycling exercise in patients with chronic heart failure. Clin. Physiol. Funct. Imaging 2017, 37, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Scott, B.R.; Slattery, K.M.; Sculley, D.V.; Lockie, R.G.; Dascombe, B.J. Reliability of telemetric electromyography and near-infrared spectroscopy during high-intensity resistance exercise. J. Electromyogr. Kinesiol. 2014, 24, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Williams, S.A.; Canale, R.E.; Farney, T.M.; Kabir, M.M. Acute effect of nitric oxide supplement on blood nitrate/nitrite and hemodynamic variables in resistance trained men. J. Strength Cond. Res. 2010, 24, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2016, 115, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Sartori, C.; Lepori, M.; Busch, T.; Duplain, H.; Hildebrandt, W.; Bärtsch, P.; Nicod, P.; Falke, K.J.; Scherrer, U. Exhaled nitric oxide does not provide a marker of vascular endothelial function in healthy humans. Am. J. Respir. Crit. Care Med. 1999, 160, 879–882. [Google Scholar] [CrossRef]
- Barkhidarian, B.; Khorshidi, M.; Shab-Bidar, S.; Hashemi, B. Effects of L-citrulline supplementation on blood pressure: A systematic review and meta-analysis. Avicenna J. Phytomed. 2019, 9, 10–20. [Google Scholar]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole-body vibration training and l-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2016, 41, 292–297. [Google Scholar] [CrossRef]
- Wong, A.; Chernykh, O.; Figueroa, A. Chronic l-citrulline supplementation improves cardiac sympathovagal balance in obese postmenopausal women: A preliminary report. Auton. Neurosci. 2016, 198, 50–53. [Google Scholar] [CrossRef]
- Orozco-Gutiérrez, J.J.; Castillo-Martínez, L.; Orea-Tejeda, A.; Diaz, O.V.; Valdespino-Trejo, A.; Narváez-David, R.; Keirns-Davis, C.; Carrasco-Ortiz, O.; Navarro-Navarro, A.; Sánchez-Santillán, R. Effect of L-arginine or L-citrulline oral supplementation on blood pressure and right ventricular function in heart failure patients with preserved ejection fraction. Cardiol. J. 2010, 17, 612–618. [Google Scholar]
- Mador, M.J.; Acevedo, F.A. Effect of respiratory muscle fatigue on subsequent exercise performance. J. Appl. Physiol. 1991, 70, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Kohan, E.J.; Wirth, G.A. Anatomy of the neck. Clin. Plast. Surg. 2014, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.; Schneider, E.; Gevenois, P.-A.; De Troyer, A. Respiratory effects of the scalene and sternomastoid muscles in humans. J. Appl. Physiol. 2003, 94, 1467–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadgan, B.; Guenette, J.A.; Sheel, A.W.; Reid, W.D. Sternocleidomastoid muscle deoxygenation in response to incremental inspiratory threshold loading measured by near infrared spectroscopy. Respir. Physiol. Neurobiol. 2011, 178, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-Y.; Showalter, M.R.; Linderholm, A.L.; Franzi, L.M.; Kivler, C.; Li, Y.; Sa, M.R.; Kons, Z.A.; Fiehn, O.; Qi, L.; et al. l-Arginine supplementation in severe asthma. JCI Insight 2020, 5, e137777. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Kissoon, N.; Sandler, E.; Gauger, C.; Froyen, M.; Duckworth, L.; Brown, M.; Murphy, S. Effect of oral arginine supplementation on exhaled nitric oxide concentration in sickle cell anemia and acute chest syndrome. J. Pediatr. Hematol. Oncol. 2010, 32, e249–e258. [Google Scholar] [CrossRef] [PubMed]
- Fiz, J.A.; Romero, P.; Gomez, R.; Hernandez, M.; Ruiz, J.; Izquierdo, J.; Coll, R.; Morera, J. Indices of respiratory muscle endurance in healthy subjects. Respiration 1998, 65, 21–27. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Scheuermann, B.W. Gender differences in the fatigability of the inspiratory muscles. Med. Sci. Sports Exerc. 2006, 38, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, M.; Perret, C.; Kayser, B.; Boutellier, U.; Spengler, C.M. Task failure from inspiratory resistive loaded breathing: A role for inspiratory muscle fatigue? Eur. J. Appl. Physiol. 2003, 90, 405–410. [Google Scholar] [CrossRef]
- Johnson, B.D.; Babcock, M.A.; Suman, O.E.; Dempsey, J.A. Exercise-induced diaphragmatic fatigue in healthy humans. J. Physiol. 1993, 460, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Babcock, M.A.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Dempsey, J.A. Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol. 1997, 82, 1573–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.-Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.P.; Wolfe, R.R. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchward-Venne, T.A.; Cotie, L.; MacDonald, M.; Mitchell, C.; Prior, T.; Baker, S.K.; Phillips, S.M. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E71–E83. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorou, A.A.; Zinelis, P.T.; Malliou, V.J.; Chatzinikolaou, P.N.; Margaritelis, N.V.; Mandalidis, D.; Geladas, N.D.; Paschalis, V. Acute L-Citrulline Supplementation Increases Nitric Oxide Bioavailability but Not Inspiratory Muscle Oxygenation and Respiratory Performance. Nutrients 2021, 13, 3311. https://doi.org/10.3390/nu13103311
Theodorou AA, Zinelis PT, Malliou VJ, Chatzinikolaou PN, Margaritelis NV, Mandalidis D, Geladas ND, Paschalis V. Acute L-Citrulline Supplementation Increases Nitric Oxide Bioavailability but Not Inspiratory Muscle Oxygenation and Respiratory Performance. Nutrients. 2021; 13(10):3311. https://doi.org/10.3390/nu13103311
Chicago/Turabian StyleTheodorou, Anastasios A., Panagiotis T. Zinelis, Vassiliki J. Malliou, Panagiotis N. Chatzinikolaou, Nikos V. Margaritelis, Dimitris Mandalidis, Nickos D. Geladas, and Vassilis Paschalis. 2021. "Acute L-Citrulline Supplementation Increases Nitric Oxide Bioavailability but Not Inspiratory Muscle Oxygenation and Respiratory Performance" Nutrients 13, no. 10: 3311. https://doi.org/10.3390/nu13103311






