Malvidin Protects against and Repairs Peptic Ulcers in Mice by Alleviating Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemical Compounds
2.2. Animals
2.3. Gastric Ulcer Induced by Absolute Ethanol
2.4. Gastric Ulcer Induced by Non-Steroidal Anti-Inflammatory Drug
2.5. Gastric Ulcer Induced by Ischemia-Reperfusion
2.6. Gastric Ulcer Induced by Acetic Acid
2.7. Duodenal Ulcer Induced by Polypharmacy
2.8. Antioxidant and Inflammatory Parameters
2.9. Quantitative qPCR Analyses
2.10. Statistical Analyses
3. Results
3.1. Malvidin Protected the Gastric Mucosa from Lesions Induced by Absolute Ethanol and Ameliorated the Inflammatory Parameters in the Stomach
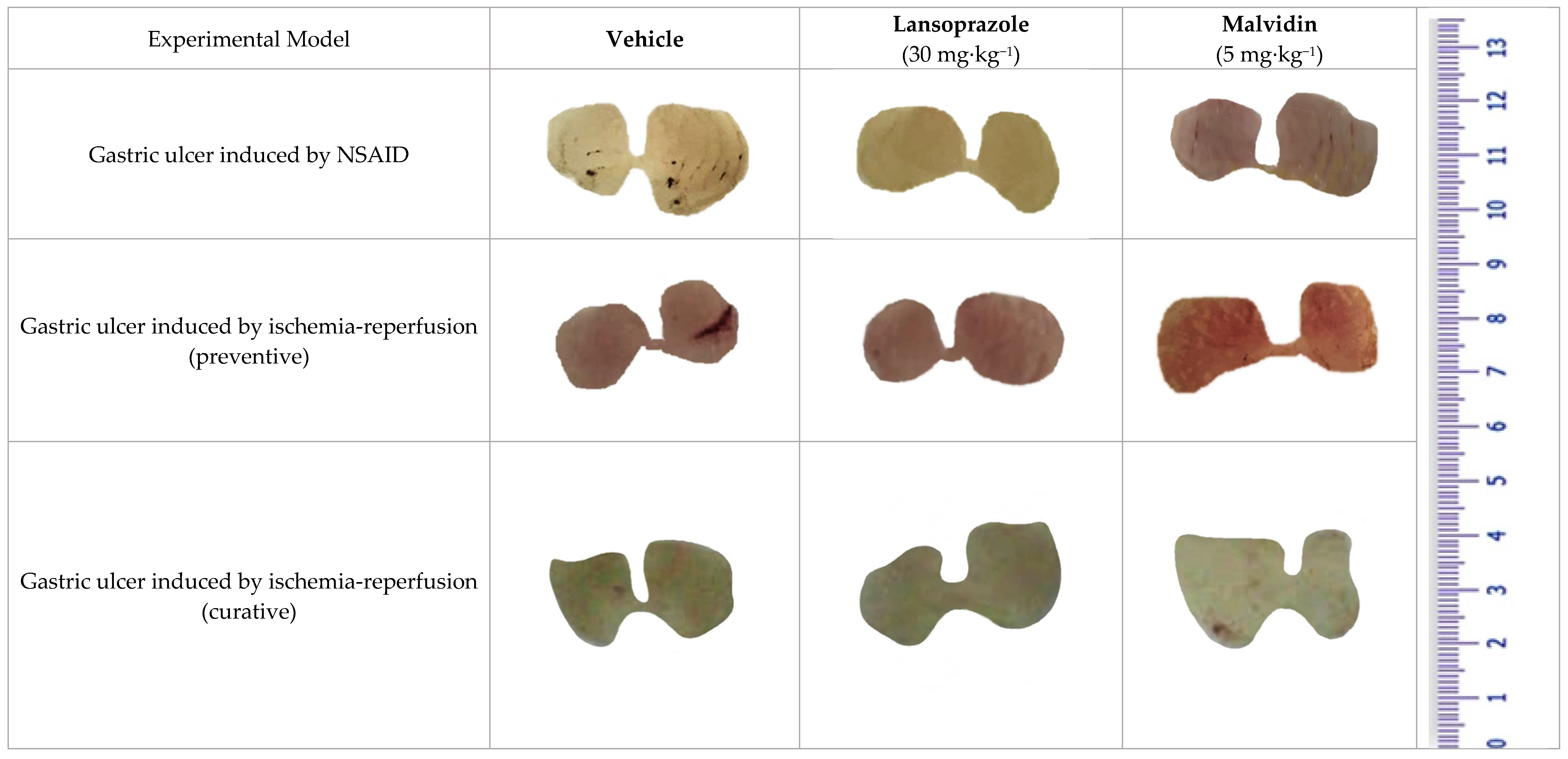
3.2. Malvidin Exerts a Protective Effect in the Indomethacin-Induced Gastric Ulcer Model by Promoting a Reduction in Inflammatory and Oxidative Markers
3.3. Malvidin Did Not Protect the Stomach from Macroscopic Alterations Induced by Ischaemia-Reperfusion, but Modulated Antioxidant Enzymes in Both Preventive and Curative Models
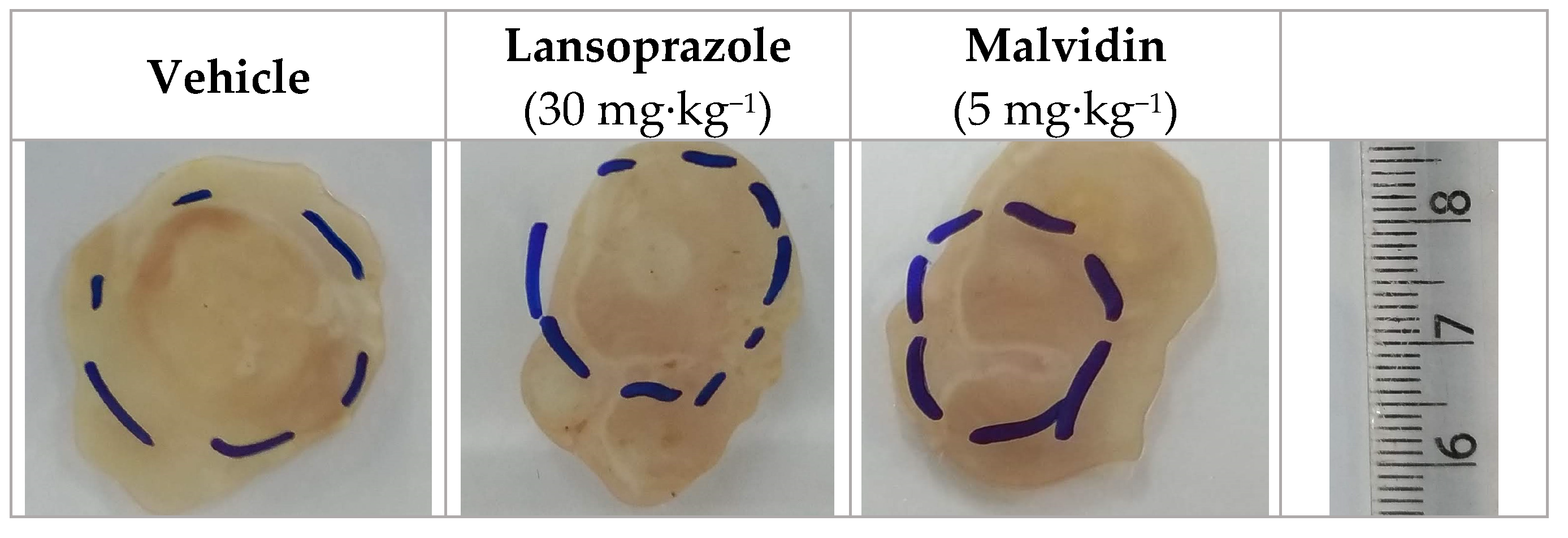
3.4. Malvidin Accelerates Gastric Ulcer Healing in Mice with Acetic Acid-Induced Gastric Ulcer by Modulating the Expression Levels of EGF, COX-1, and MMP-9
3.5. Malvidin Improves Antioxidant Defense in the Gastric Mucosa of Mice with Acetic acid-Induced Gastric Ulcer
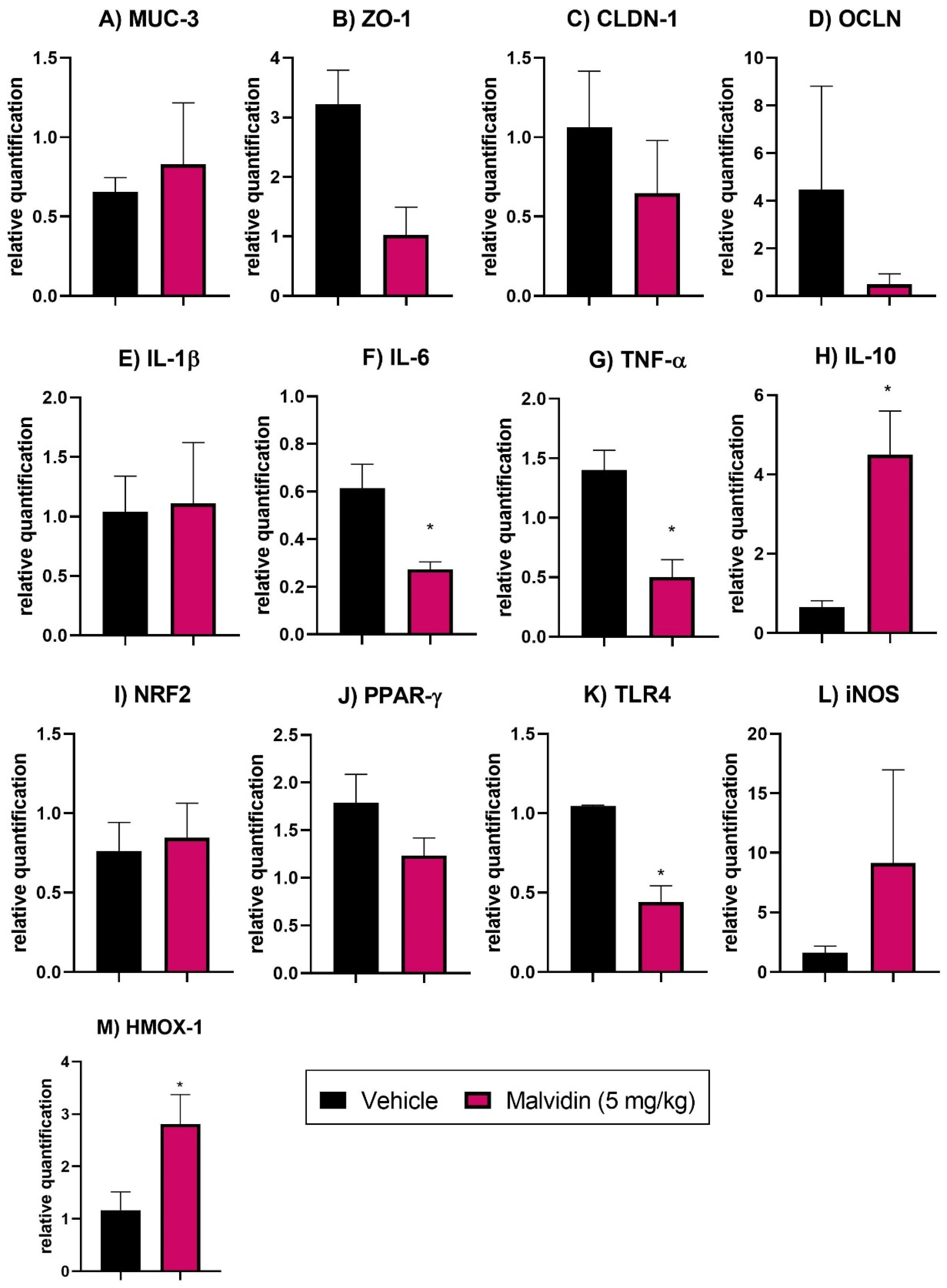
3.6. Anthocyanidins Ameliorate the Inflammatory Status and Oxidative Parameters in Mice with Polypharmacy-Induced Duodenal Ulcer
3.7. Malvidin Does Not Prevent Expression Profile Changes in Tight Junction Factors in the Duodenum of Mice with Polypharmacy-Induced Duodenal Ulcer
3.8. Malvidin Downregulates Genes Related to Inflammation, Oxidative Stress, and Immune System Activation in the Polypharmacy-Induced Duodenal Ulcer Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanas, A.; Chan, F.K. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.; Chen, L.; Wong, I.C.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Trifan, A.; Stanciu, C.; Girleanu, I.; Stoica, O.C.; Singeap, A.M.; Maxim, R.; Chiriac, S.A.; Ciobica, A.; Boiculese, L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 6500–6515. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Syer, S.; Denou, E.; de Palma, G.; Vong, L.; McKnight, W.; Jury, J.; Bolla, M.; Bercik, P.; Collins, S.M.; et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 2011, 141, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [Green Version]
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003, 51, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Konczak, I.; Zhang, W. Anthocyanins-More Than Nature’s Colours. J. Biomed. Biotechnol. 2004, 2004, 239–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, D.; Desideri, G.; Ferri, C. Flavonoids: Antioxidants against atherosclerosis. Nutrients 2010, 2, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Toma, W.; Hiruma-Lima, C.A.; Guerrero, R.O.; Souza Brito, A.R.M. Preliminary studies of Mammea americana L. (Guttiferae) bark/latex extract point to an effective antiulcer effect on gastric ulcer models in mice. Phytomedicine 2005, 12, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Nezamis, J.; Lancaster, C.; Hanchar, A. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 1979, 77, 433–443. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Martins, J.L.R.; de Oliveira, D.R.; Florentino, I.F.; da Silva, D.P.B.; dos Santos, F.C.A.; Costa, E.A. Effect of allantoin on experimentally induced gastric ulcers: Pathways of gastroprotection. Eur. J. Pharmacol. 2018, 821, 68–78. [Google Scholar] [CrossRef]
- Guidobono, F.; Pagani, F.; Ticozzi, C.; Sibilia, V.; Pecile, A.; Netti, C. Protection by amylin of gastric erosions induced by indomethacin or ethanol in rats. Br. J. Pharmacol. 1997, 120, 581–586. [Google Scholar] [CrossRef]
- Tamaddonfard, E.; Erfanparast, A.; Farshid, A.A.; Imani, M.; Mirzakhani, N.; Salighedar, R.; Tamaddonfard, S. Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer. Life Sci. 2019, 224, 88–94. [Google Scholar] [CrossRef]
- Ueda, S.; Yoshikawa, T.; Takahashi, S.; Ichikawa, H.; Yasuda, M.; Oyamada, H.; Tanigawa, T.; Sugino, S.; Kondo, M. Role of free radicals and lipid peroxidation in gastric mucosal injury induced by ischemia-reperfusion in rats. Scand. J. Gastroenterol. 1989, 24 (Suppl. 162), 55–58. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Drozdowicz, D.; Kwiecieñ, S.; Pajdo, R.; Bielanski, W.; Hahn, E.G. Role of gastric acid secretion in progression of acute gastric erosions induced by ischemia-reperfusion into gastric ulcers. Eur. J. Pharmacol. 2000, 398, 147–158. [Google Scholar] [CrossRef]
- Ohara, R.; Périco, L.L.; Rodrigues, V.P.; Bueno, G.; Zanatta, A.C.; dos Santos, L.C.; Vilegas, W.; Constatino, F.B.; Justulin, L.A.; Hiruma-Lima, C.A.; et al. Terminalia catappa L. infusion accelerates the healing process of gastric ischemia-reperfusion injury in rats. J. Ethnopharmacol. 2020, 256, 112793. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Roth, J.L.; Pfeiffer, C.J. A method for experimental, penetrating gastric and duodenal ulcers in rats. Observations on normal healing. Am. J. Dig. Dis. 1971, 16, 277–284. [Google Scholar] [CrossRef]
- Arunachalam, K.; Damazo, A.S.; Pavan, E.; Oliveira, D.M.; de Freitas Figueiredo, F.; Machado, M.T.M.; Balogun, S.O.; Soares, I.M.; dos Santos Barbosa, R.; da Costa Alvim, T.; et al. Cochlospermum regium (Mart. ex Schrank) Pilg.: Evaluation of chemical profile, gastroprotective activity and mechanism of action of hydroethanolic extract of its xylopodium in acute and chronic experimental models. J. Ethnopharmacol. 2019, 233, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Magierowska, K.; Bakalarz, D.; Wójcik, D.; Chmura, A.; Hubalewska-Mazgaj, M.; Licholai, S.; Korbut, E.; Kwiecien, S.; Sliwowski, Z.; Ginter, G.; et al. Time-dependent course of gastric ulcer healing and molecular markers profile modulated by increased gastric mucosal content of carbon monoxide released from its pharmacological donor. Biochem. Pharmacol. 2019, 163, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Vinnakota, R.R. The Time Course of Adaptation of Intestinal Nutrient Uptake in Mice Is Independent of Age. J. Nutr. 1995, 125, 2172–2182. [Google Scholar] [CrossRef]
- Blackler, R.; Syer, S.; Bolla, M.; Ongini, E.; Wallace, J.L. Gastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defence. PLoS ONE 2012, 7, e35196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Faure, P.; Lafond, J.-L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems; Birkhäuser: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gomes, A.É.I.; Stuchi, L.P.; Siqueira, N.M.G.; Henrique, J.B.; Vicentini, R.; Ribeiro, M.L.; Darrieux, M.; Ferraz, L.F.C. Selection and validation of reference genes for gene expression studies in Klebsiella pneumoniae using Reverse Transcription Quantitative real-time PCR. Sci. Rep. 2018, 8, 9001. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.W.; Miranda, J.; Teixeira, L.; Aiastui, A.; Matheu, A.; Gambero, A.; Portillo, M.P.; Ribeiro, M.L. Yerba Mate Stimulates Mitochondrial Biogenesis and Thermogenesis in High-Fat-Diet-Induced Obese Mice. Mol. Nutr. Food. Res. 2018, 62, 1800142. [Google Scholar] [CrossRef]
- Guslandi, M. Effects of ethanol on the gastric mucosa. Dig. Dis. 1987, 5, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Trier, J.S.; Brown, A.; Schnoor, J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology 1985, 88, 228–236. [Google Scholar] [CrossRef]
- Wallace, J.L.; Ma, L. Inflammatory mediators in gastrointestinal defense and injury. Exp. Biol. Med. 2001, 226, 1003–1015. [Google Scholar] [CrossRef]
- Wei, H.; Li, H.; Wan, S.P.; Zeng, Q.T.; Cheng, L.X.; Jiang, L.L.; Peng, Y.D. Cardioprotective effects of malvidin against isoproterenol-induced myocardial infarction in rats: A mechanistic study. Med. Sci. Monit. 2017, 23, 2007–2016. [Google Scholar] [CrossRef] [Green Version]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Selim, H.M. Nebivolol prevents indomethacin-induced gastric ulcer in rats. J. Immunotoxicol. 2016, 13, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, R.; Ceconi, C.; Curello, S.; Cargnoni, A.; Pasini, E.; Visioli, O. The occurrence of oxidative stress during reperfusion in experimental animals and men. Cardiovasc. Drugs Ther. 1991, 5 (Suppl. 2), 277–287. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Lu, C.C.; Yen, G.C. Phytochemicals enhance antioxidant enzyme expression to protect against NSAID-induced oxidative damage of the gastrointestinal mucosa. Mol. Nutr. Food Res. 2017, 61, 1600659. [Google Scholar] [CrossRef]
- Petruk, G.; Illiano, A.; Del Giudice, R.; Raiola, A.; Amoresano, A.; Rigano, M.M.; Piccoli, R.; Monti, D.M. Malvidin and cyanidin derivatives from açai fruit (Euterpe oleracea Mart.) counteract UV-A-induced oxidative stress in immortalized fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 172, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, X.; Han, X.; Xu, L.; Yin, L.; Qi, Y.; Zhao, Y.; Xu, Y.; Wang, C.; Peng, J.; et al. Dioscin attenuates gastric ischemia/reperfusion injury through the down-regulation of PKC/ERK1/2 signaling via PKCα and PKCβ2 inhibition. Chem. Biol. Interact. 2016, 258, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Gesslbauer, B.; Bochkov, V. Biochemical targets of drugs mitigating oxidative stress via redox-independent mechanisms. Biochem. Soc. Trans. 2017, 45, 1225–1252. [Google Scholar] [CrossRef]
- Ornellas, F.M.; Ornellas, D.S.; Martini, S.V.; Castiglione, R.C.; Ventura, G.M.; Rocco, P.R.; Gutfilen, B.; de Souza, S.A.; Takiya, C.M.; Morales, M.M.; et al. Bone marrow-derived mononuclear cell therapy accelerates renal ischemia-reperfusion injury recovery by modulating inflammatory, antioxidant and apoptotic related molecules. Cell. Physiol. Biochem. 2017, 41, 1736–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, S.; Amagase, K. An overview of acetic acid ulcer models—The history and state of the art of peptic ulcer research. Biol. Pharm. Bull. 2005, 28, 1321–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, K.; Swarnakar, S. Chronic gastric ulceration causes matrix metalloproteinases-9 and -3 augmentation: Alleviation by melatonin. Biochimie 2012, 94, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Zhao, J.R.; Ren, X.Y.; Xie, J.P.; Ma, Q.Z.; Rong, Q.H. Increased expression of matrix metalloproteinase-9 associated with gastric ulcer recurrence. World J. Gastroenterol. 2013, 19, 4590–4595. [Google Scholar] [CrossRef]
- Tarnawski, A.S. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. 2005, 50 (Suppl. 1), S24–S33. [Google Scholar] [CrossRef] [PubMed]
- DeFoneska, A.; Kaunitz, J.D. Gastroduodenal mucosal defense. Curr. Opin. Gastroenterol. 2010, 26, 604–610. [Google Scholar] [CrossRef]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.-H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiecien, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar]
- Da Silva, L.M.; Pezzini, B.C.; Somensi, L.B.; Mariano, L.N.B.; Mariott, M.; Boeing, T.; dos Santos, A.C.; Longo, B.; Cechinel-Filho, V.; de Souza, P.; et al. Hesperidin, a citrus flavanone glycoside, accelerates the gastric healing process of acetic acid-induced ulcer in rats. Chem. Biol. Interact. 2019, 308, 45–50. [Google Scholar] [CrossRef]
- Lanas, A.; Sopeña, F. Nonsteroidal Anti-Inflammatory Drugs and Lower Gastrointestinal Complications. Gastroenterol. Clin. N. Am. 2009, 38, 333–352. [Google Scholar] [CrossRef]
- Syer, S.D.; Blackler, R.W.; Martin, R.; de Palma, G.; Rossi, L.; Verdu, E.; Bercik, P.; Surette, M.G.; Aucouturier, A.; Langella, P.; et al. NSAID enteropathy and bacteria: A complicated relationship. J. Gastroenterol. 2015, 50, 387–393. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Eisen, G.M.; Lewis, B.; Gralnek, I.M.; Zlotnick, S.; Fort, J.G. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin. Gastroenterol. Hepatol. 2005, 3, 133–141. [Google Scholar] [CrossRef]
- Pavlidis, P.; Bjarnason, I. Aspirin Induced Adverse Effects on the Small and Large Intestine. Curr. Pharm. Des. 2015, 21, 5089–5093. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar]
- De Medina, F.S.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The Good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Magierowska, K.; Magierowski, M.; Hubalewska-Mazgaj, M.; Adamski, J.; Surmiak, M.; Sliwowski, Z.; Kwiecien, S.; Brzozowski, T. Carbon monoxide (CO) released from tricarbonyldichlororuthenium (II) dimer (CORM-2) in gastroprotection against experimental ethanol-induced gastric damage. PLoS ONE 2015, 10, e0140493. [Google Scholar]
- Magierowska, K.; Wojcik, D.; Chmura, A.; Bakalarz, D.; Wierdak, M.; Kwiecien, S.; Sliwowski, Z.; Brzozowski, T.; Magierowski, M. Alterations in Gastric Mucosal Expression of Calcitonin Gene-Related Peptides, Vanilloid Receptors, and Heme Oxygenase-1 Mediate Gastroprotective Action of Carbon Monoxide against Ethanol-Induced Gastric Mucosal Lesions. Int. J. Mol. Sci. 2018, 19, 2960. [Google Scholar] [CrossRef]

| Gene | Sequence 5′-3′ | GenBank Number |
|---|---|---|
| MMP-2 | 5′-GGACAGTGACACCACGTGAC-3′ | 17,390 |
| 5′-TGACACAGCCTTCTCCTCCT-3′ | ||
| MMP-9 | 5′-CGTCGTGATCCCCACTTACT-3′ | 17,395 |
| 5′-AACACACAGGGTTTGCCTTC-3′ | ||
| CASP-3 | 5′-GGCCGTGTTTCTGTTTTGTT-3′ | 12,367 |
| 5′-TTGAGGTAGCTGCATGTGG-3′ | ||
| COX-1 | 5′-AGGGTGTCTGTGTCCGCTTT-3′ | 19,224 |
| 5′-GTTGGGGACTGGAGTCTTGC-3′ | ||
| COX-2 | 5′-CCCCAAAGATAGCATCTGGA-3′ | 19,225 |
| 5′-TGCAGAATTGAAAGCCCTCT-3′ | ||
| EGF | 5′-AGGCATCAAGCACGGTAGGT-3′ | 13,645 |
| 5′-AGCAAGCACACCCCGTAAGT-3′ | ||
| iNOS | 5′-TGGTGGTGACAAGCACATTT-3′ | 18,126 |
| 5′-AAGGCCAAACACAGCATACC-3′ | ||
| B-actin | 5′-ACGAGGCCCAGAGCAAGAG-3′ | 11,461 |
| 5′-GGTGTGGTGCCAGATCTTCTC’-3′ | ||
| ZO-1 | 5′-CTGTGGGTTCCGTTTTGAGT-3′ | 21,872 |
| 5′-CAGAAGGCCAAAGACTCCAG-3′ | ||
| CLND | 5′-AAAATCCCTGACGGGGTATC-3′ | 12,737 |
| 5′-GGCGTTTCTGGATGTTGTCT-3′ | ||
| OCLD | 5′-CTCACGGAAACCAGAGAAGC-3′ | 18,260 |
| 5′-GCATTTCTGGTGGACAAGGT-3′ | ||
| IL-1β | 5′-CCCAAGCAATACCCAAAGAA-3′ | 16,176 |
| 5′-TACCAGTTGGGGAACTCTGC-3′ | ||
| TNF-α | 5′-TAGCCAGGAGGGAGAACAGA-3′ | 21,926 |
| 5′-TTTCTGGAGGGAGATGTGG-3′ | ||
| IL-6 | 5′-TCTCTGGGAAATCGTGGAA-3′ | 16,193 |
| 5′-TTCTGCAAGTGCATCATCG-3′ | ||
| IL-10 | 5′-AAAAGGTGCCACCCTGAAGA-3′ | 16,153 |
| 5′-GATGTGGTGGGACCAACCTT-3′ | ||
| NRF2 | 5′-CCCAGGGTTTGAAAAGTGAA-3′ | 18,024 |
| 5′-GCTGGAAAGTGAAGGCAGTC-3′ | ||
| HMOX-1 | 5′-CGATCTCAAGCAAGCCCTAC-3′ | 15,368 |
| 5′-TTGGTGAGTTCCTCCTTGCT-3′ | ||
| TLR4 | 5′-AGAAAATGCCAGGATGATGC-3′ | 21,898 |
| 5′-AGGGATTCAAGCTTCCTGGT-3′ | ||
| PPAR-γ | 5′-CCCTGGTGTCCCAACTCTTA-3′ | 19,016 |
| 5′-GTGCAACAGAAGAGCCATCA-3′ |
| Experimental Model | Treatment (p.o.) | Dose (mg/kg) | Ulcerative Lesion (mm2) | Gastric Lesion Inhibition (%) | MPO (Unit of MPO/g) | GSH (nmol/g) | CAT (Unit of CAT/g) | SOD (Unit of SOD/g) |
|---|---|---|---|---|---|---|---|---|
| Gastric ulcer induced by absolute ethanol | Vehicle | - | 243.0 ± 68.0 | - | 29.3 ± 4.3 | 827.3 ± 85.1 | 38.3 ± 6.3 | 16.6 ± 3.0 |
| Lansoprazole | 30 | 50.7 ± 25.8 | 79.1 * | 16.2 ± 1.5 * | 962.2 ± 153.2 * | 61.1 ± 6.1 * | 14.9 ± 1.6 | |
| Malvidin | 5 | 25.3 ± 8.8 | 89.6 ** | 14.1 ± 1.0 * | 627.5 ± 77.7 | 48.3 ± 4.2 | 10.2 ± 1.7 | |
| Naive | - | - | - | 16.8 ± 1.6 * | 979.5 ± 79.1 ** | 87.9 ± 5.0 ** | 19.3 ± 1.9 * | |
| Gastric ulcer induced by NSAID | Vehicle | - | 27.1 ± 4.8 | - | 20.7 ± 2.0 | 99.2 ± 10.4 | 36.8 ± 4.5 | 10.3 ± 1.2 |
| Lansoprazole | 30 | 1.7 ± 0.6 | 93.7 **** | 15.2 ± 0.9 * | 122.2 ± 12.3 | 44.3 ±5.7 | 13.3 ± 2.7 | |
| Malvidin | 5 | 9.8 ±3.3 | 63.8 ** | 13.3 ± 1.0 * | 174.6 ± 5.8 ** | 50.8 ±2.2 | 16.2 ± 1.6 | |
| Naive | - | - | 10.4 ± 0.7 ** | 142.0 ± 13.1 * | 56.6 ± 6.9 * | 13.5 ± 1.1 |
| Experimental Model Ischemia/Reperfusion | Treatment (p.o.) | Dose (mg/kg) | Ulcerative Lesion (mm2) | Gastric Lesion Inhibition (%) | MPO (Unit of MPO/g) | GSH (nmol/g) | CAT (Unit of CAT/g) | SOD (Unit of SOD/g) |
|---|---|---|---|---|---|---|---|---|
| Preventive treatment | Vehicle | - | 30.3 ± 6.2 | - | 16.3 ± 1.4 | 58.4 ± 7.5 | 18.2 ± 3.3 | 3.4 ± 0.2 |
| Lansoprazole | 30 | 2.4 ± 1.4 | 92.1 * | 13.4 ± 1.6 | 32.4 ± 7.2 | 7.5 ± 0.4 | 2.5 ± 0.6 | |
| Malvidin | 5 | 633.6 ± 77.9 | - | 15.8 ± 1.2 | 231.3 ± 87.0 * | 52.3 ± 12.4 *** | 13.8 ± 3.1 *** | |
| Naive | - | - | - | 12.2 ± 0.2 | 231.8 ± 73.1 * | 29.8 ± 7.2 *** | 14.5 ± 2.8 *** | |
| 6 days of post-ischemia-reperfusion treatment | Vehicle | - | 5.93 ± 0.025 | - | 29.87 ± 6.36 | 48.05 ± 20.55 | 53.10 ± 6.03 | 11.64 ± 0.69 |
| Lansoprazole | 30 | 0.38 ± 0.12 | 99.94 | 17.08 ± 2.65 * | 59.69 ± 3.73 | 55.25 ± 7.97 | 9.99 ± 0.32 | |
| Malvidin | 5 | 19.23 ± 4.10 | - | 15.63 ± 0.76 * | 95.65 ± 12.58 * | 109.7 ± 12.96 * | 14.02 ± 1.04 | |
| Sham | - | - | - | 11.95 ± 1.17 * | 129.3 ± 0.00 * | 63.71 ± 12.93 | 15.16 ± 1.16 * |
| Experimental Model | Treatment (p.o.) | Dose (mg/kg) | Ulcerative Lesion (mm2) | Gastric Lesion Reduction (%) | GSH (nmol/g) | CAT (Unit of CAT/g) | SOD (Unit of SOD/g) |
|---|---|---|---|---|---|---|---|
| Acetic acid-induced gastric ulcer | Vehicle | - | 7.1 ± 0.6 | - | 53.8 ± 6.7 | 26.7 ± 3.8 | 6.1 ± 0.6 |
| Lansoprazole | 30 | 4.8 ± 0.5 | 32.4 * | 72.05 ± 13.2 | 59.7 ± 18.2 | 7.2 ± 1.6 | |
| Malvidin | 5 | 4.9 ± 0.4 | 31.0 * | 70.4 ± 17.4 | 35.5 ± 4.7 | 11.8 ± 1.5 * | |
| Sham | - | - | - | 67.7 ± 14.8 | 33.2 ± 2.9 | 8.4 ± 1.8 |
| Experimental Model | Treatment (p.o.) | Dose (mg/kg) | MPO (Unit of MPO/g) | GSH (nmol/g) | CAT (Unit of CAT/g) | SOD (Unit of SOD/g) | IL-10 (pg/mL) | IL-6 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Polypharmacy-induced duodenal ulcer | Vehicle | - | 95.3 ± 19.4 | 734.9 ± 74.2 | 45.6 ± 6.0 | 12.3 ± 1.4 | 1791.0 ± 350.0 | 495.8 ± 167.7 | 6377.0 ± 1128.0 | 1433.0 ± 447.8 |
| Malvidin | 5 | 36.9 ± 8.0 ** | 765.0 ± 66.4 | 84.8 ± 6.6 ** | 9.7 ± 2.0 | 1801.0 ± 326.1 | 193.2 ± 40.8 * | 6056.0 ± 861.2 | 565.3 ± 91.1 * | |
| Naïve | - | 27.3 ± 9.4 ** | 910.7 ± 50.6 | 84.0 ± 4.1 ** | 16.7 ± 0.5 | 1799.0 ± 494.7 | 223.4 ± 33.4 | 5595.0 ± 674.0 | 649.6 ± 112.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagundes, F.L.; Pereira, Q.C.; Zarricueta, M.L.; dos Santos, R.d.C. Malvidin Protects against and Repairs Peptic Ulcers in Mice by Alleviating Oxidative Stress and Inflammation. Nutrients 2021, 13, 3312. https://doi.org/10.3390/nu13103312
Fagundes FL, Pereira QC, Zarricueta ML, dos Santos RdC. Malvidin Protects against and Repairs Peptic Ulcers in Mice by Alleviating Oxidative Stress and Inflammation. Nutrients. 2021; 13(10):3312. https://doi.org/10.3390/nu13103312
Chicago/Turabian StyleFagundes, Felipe Leonardo, Quélita Cristina Pereira, Melina Luzzi Zarricueta, and Raquel de Cássia dos Santos. 2021. "Malvidin Protects against and Repairs Peptic Ulcers in Mice by Alleviating Oxidative Stress and Inflammation" Nutrients 13, no. 10: 3312. https://doi.org/10.3390/nu13103312
APA StyleFagundes, F. L., Pereira, Q. C., Zarricueta, M. L., & dos Santos, R. d. C. (2021). Malvidin Protects against and Repairs Peptic Ulcers in Mice by Alleviating Oxidative Stress and Inflammation. Nutrients, 13(10), 3312. https://doi.org/10.3390/nu13103312






