PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling
Abstract
:1. Introduction
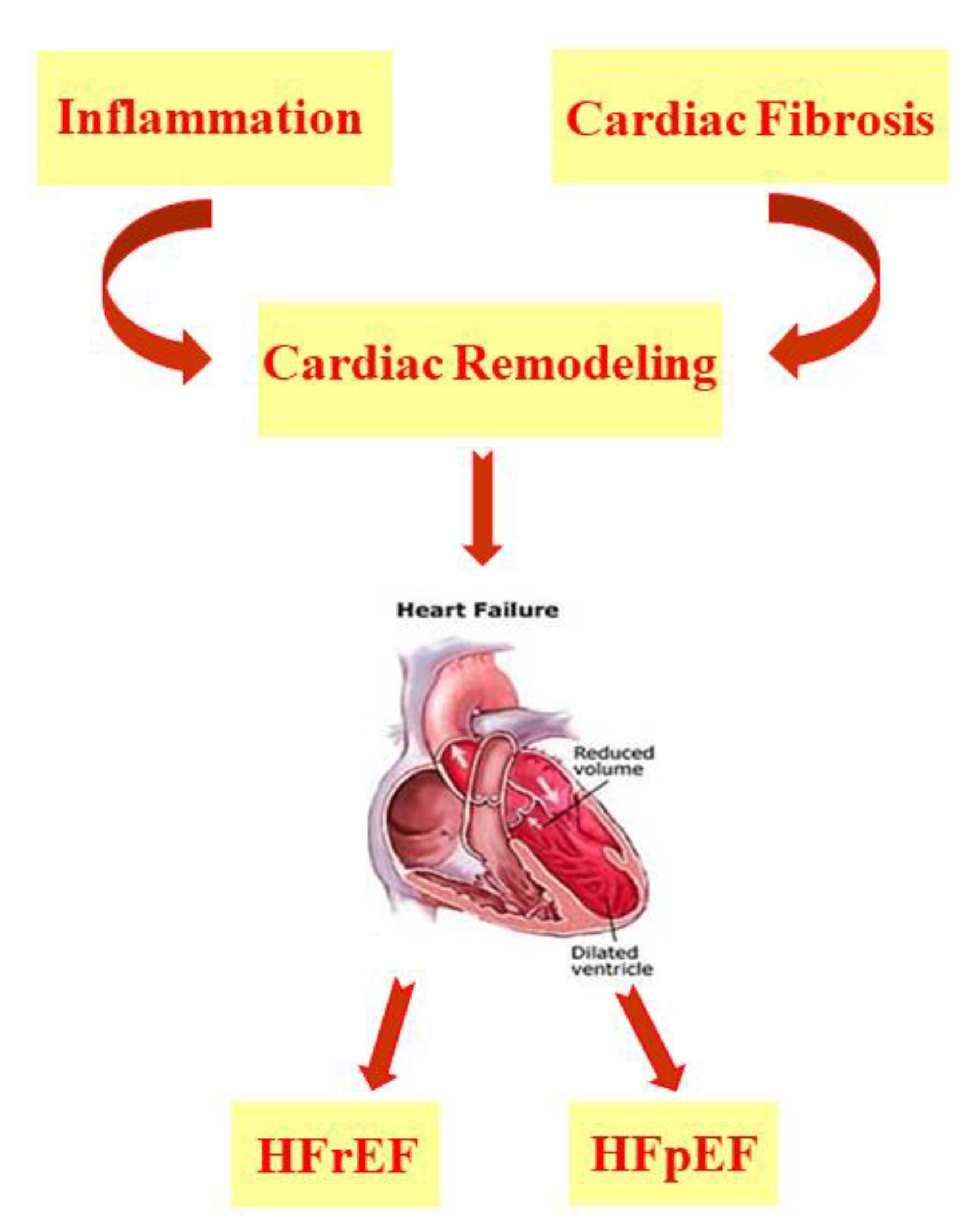
2. Heart Failure and PUFAs
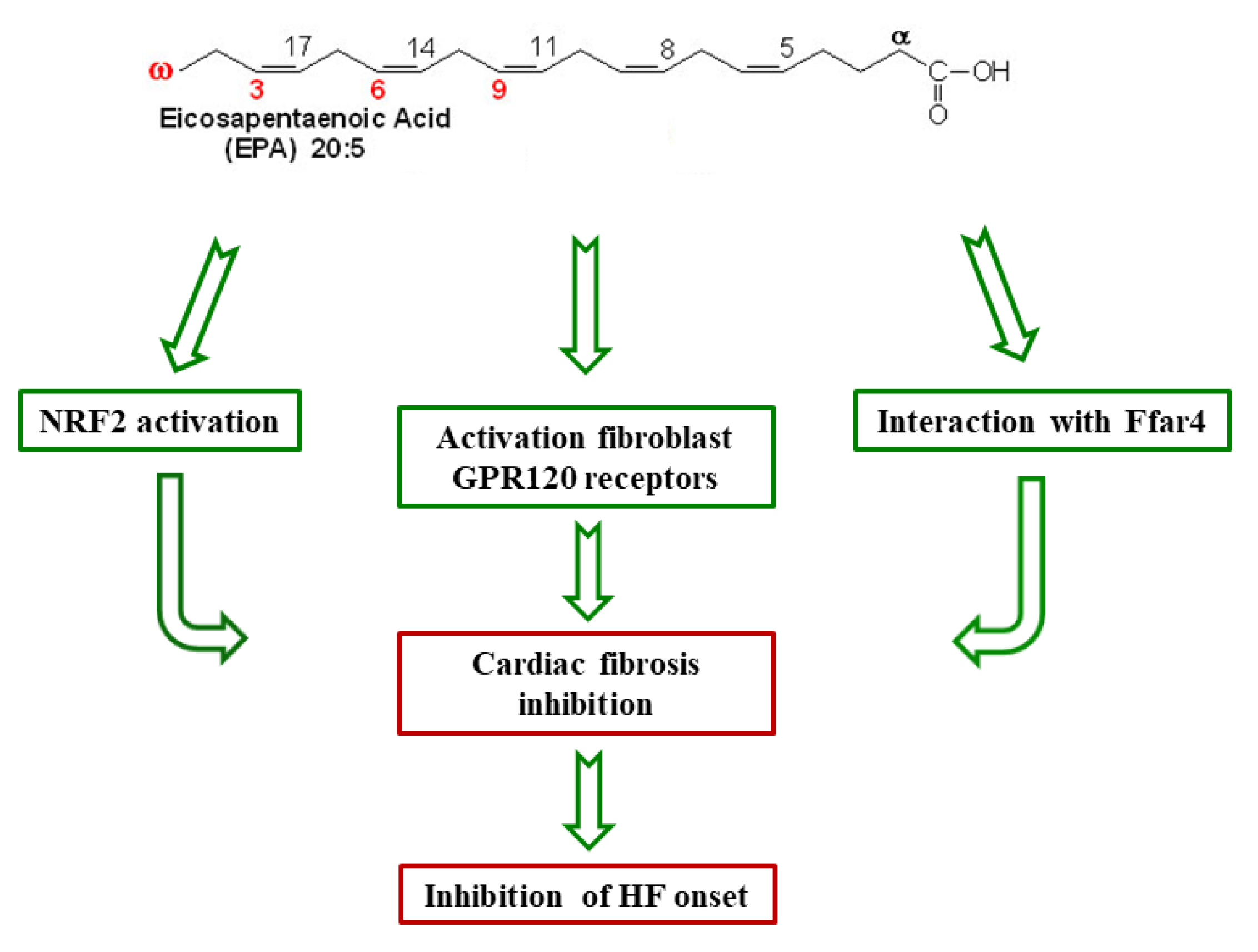
2.1. Cardioprotective Mechanisms of n-3 PUFAs
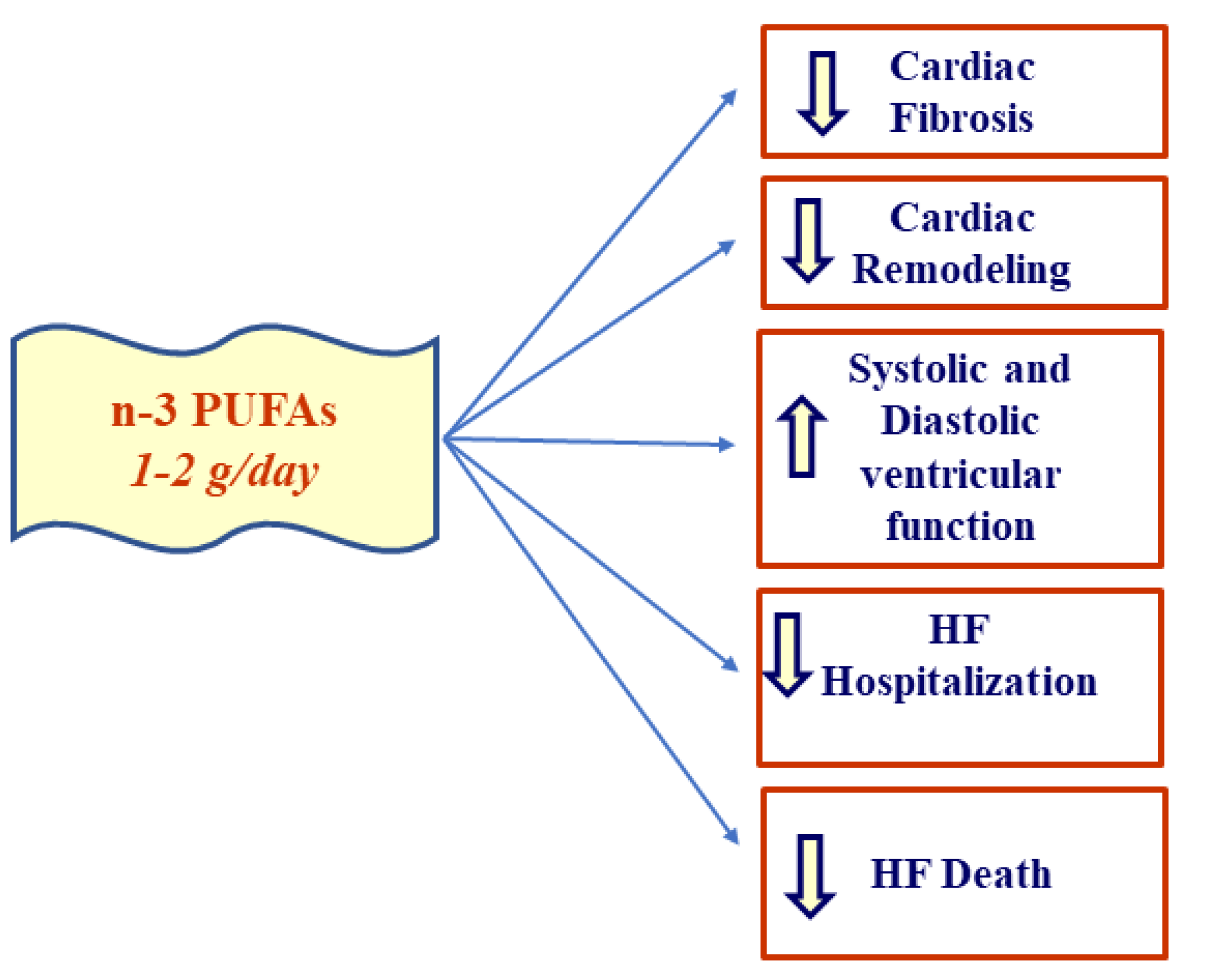
2.2. Effect of EPA/DHA Supplementation in HF
2.3. PUFAs in HFrEF and HFpEF
2.4. EPA/AA Ratio and HF
2.5. PUFAs and ADHF
2.6. Effect of PUFAs in HF Development after MI
2.7. n-3 PUFAs and Depression in HF Patients
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chia, N.; Fulcher, J.; Keech, A. Beta-blocker, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, nitrate-hydralazine, diuretics, aldosterone antagonist, ivabradine, devices and digoxin (BANDAID2): An evidence-based mnemonic for the treatment of systolic heart failure. Intern. Med. J. 2016, 46, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail. Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological Basis for Nutraceutical Supplementation in Heart Failure: A Comprehensive Review. Nutrients 2021, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, A.M.; Boyd, C.M.; Manemann, S.M.; Dunlay, S.M.; Gerber, Y.; Killian, J.M.; Weston, S.A.; Roger, V.L. Risk Factors for Heart Failure in the Community: Differences by Age and Ejection Fraction. Am. J. Med. 2020, 133, e237–e248. [Google Scholar] [CrossRef]
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular Dysfunction and Phenotypic Derangement in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Bhattacharya, S.; Estep, J.; Faiman, C. Diabetes and Heart Failure: A Marriage of Inconvenience. Clin. Geriatr. Med. 2020, 36, 447–455. [Google Scholar] [CrossRef]
- Rao, V.N.; Fudim, M.; Mentz, R.J.; Michos, E.D.; Felker, G.M. Regional adiposity and heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 1540–1550. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Florio, M.C.; Magenta, A.; Beji, S.; Lakatta, E.G.; Capogrossi, M.C. Aging, MicroRNAs, and Heart Failure. Curr. Probl. Cardiol. 2020, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Katsi, V.; Georgiopoulos, G.; Laina, A.; Koutli, E.; Parissis, J.; Tsioufis, C.; Nihoyannopoulos, P.; Tousoulis, D. Left ventricular ejection fraction as therapeutic target: Is it the ideal marker? Heart Fail. Rev. 2017, 22, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef]
- Juni, R.P.; Kuster, D.W.D.; Goebel, M.; Helmes, M.; Musters, R.J.P.; van der Velden, J.; Koolwijk, P.; Paulus, W.J.; van Hinsbergh, V.W.M. Cardiac Microvascular Endothelial Enhancement of Cardiomyocyte Function Is Impaired by Inflammation and Restored by Empagliflozin. JACC Basic Transl. Sci. 2019, 4, 575–591. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Saracini, S.; Fontana, D.; Finamore, P.; Giua, R.; Padovini, L.; Incalzi, R.A.; Maccarrone, M. Resolution of inflammation is altered in chronic heart failure and entails a dysfunctional responsiveness of T lymphocytes. FASEB J. 2019, 33, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.E.; Gao, S.; Sarhene, M.; Coffie, J.W.; Linhua, D.; Bao, X.; Jing, Z.; Li, S.; Guo, R.; Su, J.; et al. Macrophage Activities in Myocardial Infarction and Heart Failure. Cardiol. Res. Pract. 2020, 2020, 4375127. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz-Bahaghighat, H.; Darwesh, A.M.; Sosnowski, D.K.; Seubert, J.M. Mitochondrial Dysfunction and Inflammaging in Heart Failure: Novel Roles of CYP-Derived Epoxylipids. Cells 2020, 9, 1565. [Google Scholar] [CrossRef]
- Gortan Cappellari, G.; Aleksova, A.; Dal Ferro, M.; Cannatà, A.; Semolic, A.; Zanetti, M.; Springer, J.; Anker, S.D.; Giacca, M.; Sinagra, G.; et al. Preserved Skeletal Muscle Mitochondrial Function, Redox State, Inflammation and Mass in Obese Mice with Chronic Heart Failure. Nutrients 2020, 12, 3393. [Google Scholar] [CrossRef] [PubMed]
- Cortassa, S.; Juhaszova, M.; Aon, M.A.; Zorov, D.B.; Sollott, S.J. Mitochondrial Ca2+, redox environment and ROS emission in heart failure: Two sides of the same coin? J. Mol. Cell. Cardiol. 2021, 151, 113–125. [Google Scholar] [CrossRef]
- Dey, S.; DeMazumder, D.; Sidor, A.; Foster, D.B.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018, 123, 356–371. [Google Scholar] [CrossRef]
- Dietl, A.; Maack, C. Targeting Mitochondrial Calcium Handling and Reactive Oxygen Species in Heart Failure. Curr. Heart Fail. Rep. 2017, 14, 338–349. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Santhosh Kumar, T.R.; Kartha, C.C. Mitochondrial membrane transporters and metabolic switch in heart failure. Heart Fail. Rev. 2019, 24, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N. Targeting the Mitochondria in Heart Failure: A Translational Perspective. JACC Basic Transl. Sci. 2020, 5, 88–106. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Xu, H.X.; Cui, S.M.; Zhang, Y.M.; Ren, J. Mitochondrial Ca2+ regulation in the etiology of heart failure: Physiological and pathophysiological implications. Acta Pharmacol. Sin. 2020, 41, 1301–1309. [Google Scholar] [CrossRef]
- Kiyuna, L.A.; Albuquerque, R.P.E.; Chen, C.H.; Mochly Rosen, D.; Ferreira, J.C.B. Targeting mitochondrial dysfunction and oxidative stress in heart failure: Challenges and opportunities. Free Radic. Biol. Med. 2018, 129, 155–168. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Tham, Y.K.; Bernardo, B.C.; Huynh, K.; Ooi, J.Y.Y.; Gao, X.M.; Kiriazis, H.; Giles, C.; Meikle, P.J.; McMullen, J.R. Lipidomic Profiles of the Heart and Circulation in Response to Exercise versus Cardiac Pathology: A Resource of Potential Biomarkers and Drug Targets. Cell Rep. 2018, 24, 2757–2772. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Rivero, J.; Couto, G.K.; Paula, S.M.; Fontes, M.T.; Rossoni, L.V. Enhanced sympathetic neurotransduction in the superior mesenteric artery in a rat model of heart failure: Role of noradrenaline and ATP. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H563–H574. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Abraham, W.T.; Lindenfeld, J.; Bristow, M.; Carson, P.E.; Felker, G.M.; Fonarow, G.C.; Greene, S.J.; Psotka, M.A.; Solomon, S.D.; et al. Treatment of HF in an Era of Multiple Therapies: Statement From the HF Collaboratory. JACC Heart Fail. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Al-Gobari, M.; Al-Aqeel, S.; Gueyffier, F.; Burnand, B. Effectiveness of drug interventions to prevent sudden cardiac death in patients with heart failure and reduced ejection fraction: An overview of systematic reviews. BMJ Open. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossignol, P.; Hernandez, A.F.; Solomon, S.D.; Zannad, F. Heart failure drug treatment. Lancet. 2019, 393, 1034–1044. [Google Scholar] [CrossRef]
- Rosano, G.; Quek, D.; Martínez, F. Sodium-Glucose Co-transporter 2 Inhibitors in Heart Failure: Recent Data and Implications for Practice. Card. Fail. Rev. 2020, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Kuwahara, K. Sodium-Glucose Cotransporter-2 inhibitors are potential therapeutic agents for treatment of non-diabetic heart failure patients. J. Cardiol. 2020, 76, 123–131. [Google Scholar] [CrossRef]
- Grubić Rotkvić, P.; Cigrovski Berković, M.; Bulj, N.; Rotkvić, L.; Ćelap, I. Sodium-glucose cotransporter 2 inhibitors’ mechanisms of action in heart failure. World J. Diabetes 2020, 11, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.A.; Cooper, M.E. Contemporary Management of Heart Failure in Patients with Diabetes. Diabetes Care. 2020, 43, 2895–2903. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef]
- Nair, N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540. [Google Scholar]
- Von Haehling, S.; Arzt, M.; Doehner, W.; Edelmann, F.; Evertz, R.; Ebner, N.; Herrmann-Lingen, C.; Garfias Macedo, T.; Koziolek, M.; Noutsias, M.; et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: Evidence from clinical trials. Eur. J. Heart Fail. 2021, 23, 92–113. [Google Scholar] [CrossRef] [PubMed]
- Wintrich, J.; Kindermann, I.; Ukena, C.; Selejan, S.; Werner, C.; Maack, C.; Laufs, U.; Tschöpe, C.; Anker, S.D.; Lam, C.S.P.; et al. Therapeutic approaches in heart failure with preserved ejection fraction: Past, present, and future. Clin. Res. Cardiol. 2020, 109, 1079–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of heart failure patients: Practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 157–174. [Google Scholar] [CrossRef]
- Rahman, A.; Jafry, S.; Jeejeebhoy, K.; Nagpal, A.D.; Pisani, B.; Agarwala, R. Malnutrition and Cachexia in Heart Failure. JPEN J. Parenter. Enteral. Nutr. 2016, 40, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Ganguly, R.; Hasanally, D.; Stamenkovic, A.; Maddaford, T.G.; Chaudhary, R.; Pierce, G.N.; Ravandi, A. Alpha linolenic acid decreases apoptosis and oxidized phospholipids in cardiomyocytes during ischemia/reperfusion. Mol. Cell. Biochem. 2018, 437, 163–175. [Google Scholar] [CrossRef]
- Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The Protective effect of Cynara Cardunculus extract in diet-induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients 2020, 12, 1435. [Google Scholar] [CrossRef]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of natural antioxidants in the development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef] [Green Version]
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039. [Google Scholar] [CrossRef] [Green Version]
- Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Mollace, V. The effect of bergamot polyphenolic fraction in patients with non alcoholic liver steato-hepatitis and metabolic syndrome. PharmaNutrition 2016, 4S, S27–S31. [Google Scholar] [CrossRef]
- Aarsetoey, H.; Grundt, H.; Nygaard, O.; Nilsen, D.W. The role of long-chained marine N-3 polyunsaturated Fatty acids in cardiovascular disease. Cardiol. Res. Pract. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Landa-Juárez, A.Y.; Ortiz, M.I.; Castañeda-Hernández, G.; Chávez-Piña, A.E. Participation of potassium channels in the antinociceptive effect of docosahexaenoic acid in the rat formalin test. Eur. J. Pharmacol. 2016, 793, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kotwani, A. Omega-3 fatty acids in prevention of diabetic retinopathy. J. Pharm. Pharmacol. 2017, 69, 946–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, U.; Yang, M.; Kurian, D.; Mohan, C. Omega-3 Fatty Acids in Rheumatic Diseases: A Critical Review. J. Clin. Rheumatol. 2017, 23, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Messamore, E.; Almeida, D.M.; Jandacek, R.J.; McNamara, R.K. Polyunsaturated fatty acids and recurrent mood disorders: Phenomenology, mechanisms, and clinical application. Prog. Lipid Res. 2017, 66, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jayedi, A.; Shab-Bidar, S. Fish Consumption and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Adv. Nutr. 2020, 11, 1123–1133. [Google Scholar] [CrossRef]
- Napoli, C.; Benincasa, G.; Donatelli, F.; Ambrosio, G. Precision medicine in distinct heart failure phenotypes: Focus on clinical epigenetics. Am. Heart J. 2020, 224, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Nascimento Gomes, R.; Calviello, G. Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review. Int. J. Mol. Sci. 2017, 18, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 4025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef]
- O’Keefe, E.L.; Harris, W.S.; DiNicolantonio, J.J.; Elagizi, A.; Milani, R.V.; Lavie, C.J.; O’Keefe, J.H. Sea Change for Marine Omega-3s: Randomized Trials Show Fish Oil Reduces Cardiovascular Events. Mayo Clin. Proc. 2019, 94, 2524–2533. [Google Scholar] [CrossRef] [Green Version]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [PubMed]
- Hopper, I.; Connell, C.; Briffa, T.; De Pasquale, C.G.; Driscoll, A.; Kistler, P.M.; Macdonald, P.S.; Sindone, A.; Thomas, L.; Atherton, J.J. Nutraceuticals in Patients With Heart Failure: A Systematic Review. J. Card. Fail. 2020, 26, 166–179. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beam, J.; Botta, A.; Ye, J.; Soliman, H.; Matier, B.J.; Forrest, M.; MacLeod, K.M.; Ghosh, S. Excess Linoleic Acid Increases Collagen I/III Ratio and “Stiffens” the Heart Muscle Following High Fat Diets. J. Biol. Chem. 2015, 290, 23371–23384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.J.; Kim, E.J.; Lee, C.H. Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation. Antioxidants 2020, 9, 1–27. [Google Scholar]
- McCarty, M.F. Nutraceutical, Dietary, and Lifestyle Options for Prevention and Treatment of Ventricular Hypertrophy and Heart Failure. Int. J. Mol. Sci. 2021, 22, 3321. [Google Scholar] [CrossRef]
- Oikonomou, E.; Vogiatzi, G.; Karlis, D.; Siasos, G.; Chrysohoou, C.; Zografos, T.; Lazaros, G.; Tsalamandris, S.; Mourouzis, K.; Georgiopoulos, G.; et al. Effects of omega-3 polyunsaturated fatty acids on fibrosis, endothelial function and myocardial performance, in ischemic heart failure patients. Clin. Nutr. 2019, 38, 1188–1197. [Google Scholar] [CrossRef]
- Sakamoto, A.; Saotome, M.; Hasan, P.; Satoh, T.; Ohtani, H.; Urushida, T.; Katoh, H.; Satoh, H.; Hayashi, H. Eicosapentaenoic acid ameliorates palmitate-induced lipotoxicity via the AMP kinase/dynamin-related protein-1 signaling pathway in differentiated H9c2 myocytes. Exp. Cell Res. 2017, 351, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Wurm, R.; Schrutka, L.; Hammer, A.; Moertl, D.; Berger, R.; Pavo, N.; Lang, I.M.; Goliasch, G.; Huelsmann, M.; Distelmaier, K. Polyunsaturated fatty acids supplementation impairs anti-oxidant high-density lipoprotein function in heart failure. Eur. J. Clin. Invest. 2018, 48, 1–6. [Google Scholar] [CrossRef]
- Toko, H.; Morita, H.; Katakura, M.; Hashimoto, M.; Ko, T.; Bujo, S.; Adachi, Y.; Ueda, K.; Murakami, H.; Ishizuka, M.; et al. Omega-3 fatty acid prevents the development of heart failure by changing fatty acid composition in the heart. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Nowacki, D.; Martynowicz, H.; Skoczyńska, A.; Wojakowska, A.; Turczyn, B.; Bobak, Ł.; Trziszka, T.; Szuba, A. Lecithin derived from ω-3 PUFA fortified eggs decreases blood pressure in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; von Haehling, S.; Vinereanu, D.; Bielecka-Dabrowa, A.; Sahebkar, A.; Toth, P.P.; Reiner, Ž.; Wong, N.D.; Mikhailidis, D.P.; et al. International Lipid Expert Panel. Nutraceutical support in heart failure: A position paper of the International Lipid Expert Panel (ILEP). Nutr. Res. Rev. 2020, 33, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Metallinos, G.; Georgiopoulos, G.; Mendrinos, D.; Papanikolaou, A.; Magkas, N.; Pitsavos, C.; Vyssoulis, G.; Stefanadis, C.; Tousoulis, D. Short term omega-3 polyunsaturated fatty acid supplementation induces favorable changes in right ventricle function and diastolic filling pressure in patients with chronic heart failure; A randomized clinical trial. Vascul. Pharmacol. 2016, 79, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, B.; Huang, J. The Role of Omega-3 Polyunsaturated Fatty Acids in Heart Failure: A Meta-Analysis of Randomised Controlled Trials. Nutrients. 2016, 9, 18. [Google Scholar] [CrossRef]
- Berliner, D.; Mattern, S.; Wellige, M.; Malsch, C.; Güder, G.; Brenner, S.; Morbach, C.; Deubner, N.; Breunig, M.; Kiefl, R.; et al. The omega-3 index in patients with heart failure: A prospective cohort study. Prostaglandins Leukot. Essent. Fatty Acids. 2019, 140, 34–41. [Google Scholar] [CrossRef]
- Kones, R.; Howell, S.; Rumana, U. n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: Principles, Practices, Pitfalls, and Promises - A Contemporary Review. Med. Princ. Pract. 2017, 26, 497–508. [Google Scholar] [CrossRef]
- Block, R.C.; Liu, L.; Herrington, D.M.; Huang, S.; Tsai, M.Y.; O’Connell, T.D.; Shearer, G.C. Predicting Risk for Incident Heart Failure With Omega-3 Fatty Acids: From MESA. JACC Heart Fail. 2019, 7, 651–661. [Google Scholar] [CrossRef]
- Li, H.; Duan, Y.; Chen, B.; Zhao, Y.; Su, W.; Wang, S.; Wu, J.; Lu, L. New pharmacological treatments for heart failure with reduced ejection fraction (HFrEF): A Bayesian network meta-analysis. Medicine 2020, 99, 1–14. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar]
- Carbone, S.; Canada, J.M.; Buckley, L.F.; Trankle, C.R.; Billingsley, H.E.; Dixon, D.L.; Mauro, A.G.; Dessie, S.; Kadariya, D.; Mezzaroma, E.; et al. Dietary Fat, Sugar Consumption, and Cardiorespiratory Fitness in Patients With Heart Failure With Preserved Ejection Fraction. JACC Basic Transl. Sci. 2017, 2, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Miyoshi, T.; Takaishi, A.; Kishinoue, T.; Yasuhara, K.; Tanimoto, M.; Nakano, Y.; Onishi, N.; Ueeda, M.; Ito, H. High Plasma Docosahexaenoic Acid Associated to Better Prognoses of Patients with Acute Decompensated Heart Failure with Preserved Ejection Fraction. Nutrients 2021, 13, 371. [Google Scholar] [CrossRef]
- Watanabe, S.; Yoshihisa, A.; Kanno, Y.; Takiguchi, M.; Yokokawa, T.; Sato, A.; Miura, S.; Shimizu, T.; Abe, S.; Sato, T.; et al. Associations With Eicosapentaenoic Acid to Arachidonic Acid Ratio and Mortality in Hospitalized Heart Failure Patients. J. Card. Fail. 2016, 22, 962–969. [Google Scholar] [CrossRef] [Green Version]
- Kanoh, M.; Inai, K.; Shinohara, T.; Tomimatsu, H.; Nakanishi, T. Clinical implications of eicosapentaenoic acid/arachidonic acid ratio (EPA/AA) in adult patients with congenital heart disease. Heart Vessels. 2017, 32, 1513–1522. [Google Scholar] [CrossRef]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Honda, Y.; Sugano, Y.; Nishimura, K.; Nakai, M.; Honda, S.; Iwakami, N.; Okada, A.; Asaumi, Y.; Aiba, T.; et al. Circulating Omega-6, But Not Omega-3 Polyunsaturated Fatty Acids, Are Associated with Clinical Outcomes in Patients with Acute Decompensated Heart Failure. PLoS ONE 2016, 11, e0165841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses. Nutrients. 2017, 9, 956. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Decreased circulating dihomo-gamma-linolenic acid levels are associated with total mortality in patients with acute cardiovascular disease and acute decompensated heart failure. Lipids Health Dis. 2017, 16, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. OMEMI Investigators. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction: A Randomized, Controlled Trial. Circulation. 2021, 143, 528–539. [Google Scholar] [CrossRef]
- Hussain, A.; Al Rifai, M.; Mahtta, D.; Liu, J.; Jain, V.; Virani, S.S. Highlights from Studies Presented at the American Heart Association Scientific Session 2020: Navigating New Roads in Prevention. Curr. Atheroscler. Rep. 2021, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laake, K.; Seljeflot, I.; Schmidt, E.B.; Myhre, P.; Tveit, A.; Norseth, J.; Arnesen, H.; Solheim, S. Galectin-3, a marker of cardiac remodeling, is inversely related to serum levels of marine omega-3 fatty acids. A cross-sectional study. J.R.S.M. Cardiovasc. Dis. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Heydari, B.; Ge, Y.; Kaneko, K.; Abdullah, S.; Harris, W.S.; Jerosch-Herold, M.; Kwong, R.Y. Insulin Resistance Modifies the Effects of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction (from the OMEGA-REMODEL Randomized Clinical Trial). Am. J. Cardiol. 2020, 125, 678–684. [Google Scholar] [CrossRef]
- Halade, G.V.; Kain, V.; Dillion, C.; Beasley, M.; Dudenbostel, T.; Oparil, S.; Limdi, N.A. Race-based and sex-based differences in bioactive lipid mediators after myocardial infarction. ESC Heart Fail. 2020, 7, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Takamura, M.; Kurokawa, K.; Ootsuji, H.; Inoue, O.; Okada, H.; Nomura, A.; Kaneko, S.; Usui, S. Long-Term Administration of Eicosapentaenoic Acid Improves Post-Myocardial Infarction Cardiac Remodeling in Mice by Regulating Macrophage Polarization. J. Am. Heart Assoc. 2017, 6, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbolli, M.; Fiuzat, M.; Cani, D.; O’Connor, C.M. Depression and heart failure: The lonely comorbidity. Eur. J. Heart Fail. 2020, 22, 2007–2017. [Google Scholar] [CrossRef]
- Jiang, W.; Whellan, D.J.; Adams, K.F.; Babyak, M.A.; Boyle, S.H.; Wilson, J.L.; Patel, C.B.; Rogers, J.G.; Harris, W.S.; O’Connor, C.M. Long-Chain Omega-3 Fatty Acid Supplements in Depressed Heart Failure Patients: Results of the OCEAN Trial. JACC Heart Fail. 2018, 6, 833–843. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppedisano, F.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Macrì, R.; Carresi, C.; Maiuolo, J.; Serra, M.; Cardamone, A.; et al. PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling. Nutrients 2021, 13, 2965. https://doi.org/10.3390/nu13092965
Oppedisano F, Mollace R, Tavernese A, Gliozzi M, Musolino V, Macrì R, Carresi C, Maiuolo J, Serra M, Cardamone A, et al. PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling. Nutrients. 2021; 13(9):2965. https://doi.org/10.3390/nu13092965
Chicago/Turabian StyleOppedisano, Francesca, Rocco Mollace, Annamaria Tavernese, Micaela Gliozzi, Vincenzo Musolino, Roberta Macrì, Cristina Carresi, Jessica Maiuolo, Maria Serra, Antonio Cardamone, and et al. 2021. "PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling" Nutrients 13, no. 9: 2965. https://doi.org/10.3390/nu13092965
APA StyleOppedisano, F., Mollace, R., Tavernese, A., Gliozzi, M., Musolino, V., Macrì, R., Carresi, C., Maiuolo, J., Serra, M., Cardamone, A., Volterrani, M., & Mollace, V. (2021). PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling. Nutrients, 13(9), 2965. https://doi.org/10.3390/nu13092965







