Growth, Dietary Intake, and Vitamin D Receptor (VDR) Promoter Genotype in Indonesian School-Age Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Intake Assessment
2.3. Sun Exposure Measurements
2.4. Genotyping Procedure
2.5. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indonesia Ministry of Health. Basic Health Reasearch 2018; Indonesia Ministry of Health: Jakarta, Indonesia, 2018.
- World Health Organization. Nutrition Lanscape Information System (NLiS) Country Profile Indicators: Interpretation Guide; WHO Press: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization. Childhood Stunting: Context, Causes and Consequences; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Butte, N.F. Energy Requirements of Infants and Children. Protein Energy Requir. Infancy Child. 2006, 58, 19–37. [Google Scholar]
- Uauy, R. Keynote: Rethinking Protein. Food Nutr. Bull. 2013, 34, 228–231. [Google Scholar] [CrossRef]
- Semba, R.D.; Shardell, M.; Sakr Ashour, F.A.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child Stunting Is Associated with Low Circulating Essential Amino Acids. EBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Setiawan, N. Perkembangan Konsumsi Protein Hewani di Indonesia: Analisis Hasil Survey Sosial Ekonomi Nasional 2002–2005 (The Trend of Animal Protein Consumption in Indonesia: Data Analysis of 2002–2005 National Socio Economic Survey). J. Ilmu Ternak Univ. Padjadjaran 2006, 6. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Schmidt Rio-Valle, J.; González-Jiménez, E.; Rueda-Medina, B. A Cross-Sectional Study of the Association of VDR Gene, Calcium Intake, and Heel Ultrasound Measures in Early Adulthood. Calcif. Tissue Int. 2016, 98, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Esterle, L.; Jehan, F.; Sabatier, J.P.; Garabedian, M. Higher Milk Requirements for Bone Mineral Accrual in Adolescent Girls Bearing Specific Caucasian Genotypes in the VDR Promoter. J. Bone Miner. Res. 2009, 24, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Jehan, F.; Voloc, A.; Esterle, L.; Walrant-Debray, O.; Nguyen, T.-M.; Garabedian, M. Growth, Calcium Status and Vitamin D Receptor (VDR) Promoter Genotype in European Children with Normal or Low Calcium Intake. J. Steroid Biochem. Mol. Biol. 2010, 121, 117–120. [Google Scholar] [CrossRef] [PubMed]
- D’Alésio, A.; Garabédian, M.; Sabatier, J.P.; Guaydier-Souquières, G.; Marcelli, C.; Lemaçon, A.; Walrant-Debray, O.; Jehan, F. Two Single-Nucleotide Polymorphisms in the Human Vitamin D Receptor Promoter Change Protein–DNA Complex Formation and Are Associated with Height and Vitamin D Status in Adolescent Girls. Hum. Mol. Genet. 2005, 14, 3539–3548. [Google Scholar] [CrossRef]
- Arai, H.; Miyamoto, K.-I.; Yoshida, M.; Yamamoto, H.; Taketani, Y.; Morita, K.; Kubota, M.; Yoshida, S.; Ikeda, M.; Watabe, F.; et al. The Polymorphism in the Caudal-Related Homeodomain Protein Cdx-2 Binding Element in the Human Vitamin D Receptor Gene. J. Bone Miner. Res. 2001, 16, 1256–1264. [Google Scholar] [CrossRef]
- Guo, Y.; Jamison, D.C. The Distribution of SNPs in Human Gene Regulatory Regions. BMC Genom. 2005, 6, 140. [Google Scholar] [CrossRef]
- De Vooght, K.M.K.; van Wijk, R.; van Solinge, W.W. Management of Gene Promoter Mutations in Molecular Diagnostics. Clin. Chem. 2009, 55, 698–708. [Google Scholar] [CrossRef]
- Nguyễn Công, K.; Hà Thị Anh, D. Vietnam Food Ingredients Table; Medical Publishing House: Beijing, China, 2007. [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: Fdc.nal.usda.gov (accessed on 20 August 2019).
- Hanwell, H.E.C.; Vieth, R.; Cole, D.E.C.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun Exposure Questionnaire Predicts Circulating 25-Hydroxyvitamin D Concentrations in Caucasian Hospital Workers in Southern Italy. J. Steroid Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. TaqMan SNP Genotyping Assay User Guide; Thermo Fisher Scientific: Waltham, MA, USA, 2017. [Google Scholar]
- Slatkin, M. Linkage Disequilibrium—Understanding the Evolutionary Past and Mapping the Medical Future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef]
- Fang, Y.; van Meurs, J.B.J.; d’Alesio, A.; Jhamai, M.; Zhao, H.; Rivadeneira, F.; Hofman, A.; van Leeuwen, J.P.T.; Jehan, F.; Pols, H.A.P.; et al. Promoter and 3′-Untranslated-Region Haplotypes in the Vitamin D Receptor Gene Predispose to Osteoporotic Fracture: The Rotterdam Study. Am. J. Hum. Genet. 2005, 77, 807–823. [Google Scholar] [CrossRef]
- Choi, S.K.; Park, M.S.; Song, J.K.; Yoon, K.S.; Yoon, K.L.; Shim, K.S. Association of Polymorphisms in the Vitamin D Receptor Promoter with Idiopathic Short Stature. J. Korean Med. Sci. 2013, 28, 1329. [Google Scholar] [CrossRef]
- Tessema, M.; Gunaratna, N.S.; Brouwer, I.D.; Donato, K.; Cohen, J.L.; McConnell, M.; Belachew, T.; Belayneh, D.; De Groote, H. Associations among High-Quality Protein and Energy Intake, Serum Transthyretin, Serum Amino Acids and Linear Growth of Children in Ethiopia. Nutrients 2018, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Nuss, E.T.; Tanumihardjo, S.A. Quality Protein Maize for Africa: Closing the Protein Inadequacy Gap in Vulnerable Populations. Adv. Nutr. 2011, 2, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A. Protein and Micronutrient Intakes Are Associated with Child Growth and Morbidity from Infancy to Adulthood in the Philippines. J. Nutr. 2016, 146, 133–141. [Google Scholar] [CrossRef]
- Chowdhury, R.; Taneja, S.; Kvestad, I.; Hysing, M.; Bhandari, N.; Strand, T.A. Vitamin D Status in Early Childhood Is Not Associated with Cognitive Development and Linear Growth at 6–9 Years of Age in North Indian Children: A Cohort Study. Nutr. J. 2020, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.L.; Czepielewski, M.A. The Importance for Growth of Dietary Intake of Calcium and Vitamin D. J. Pediatr. 2008, 84, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Liang, L. Height and Height Z-Score Are Related to Calcium Absorption in Five- to Fifteen-Year-Old Girls. J. Clin. Endocrinol. Metab. 2005, 90, 5077–5081. [Google Scholar] [CrossRef][Green Version]
- Maalouf, N.M.; Moe, O.W.; Adams-Huet, B.; Sakhaee, K. Hypercalciuria Associated with High Dietary Protein Intake Is Not Due to Acid Load. J. Clin. Endocrinol. Metab. 2011, 96, 3733–3740. [Google Scholar] [CrossRef]
- Baron, J.; Sävendahl, L.; De Luca, F.; Dauber, A.; Phillip, M.; Wit, J.M.; Nilsson, O. Short and Tall Stature: A New Paradigm Emerges. Nat. Rev. Endocrinol. 2015, 11, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Wu, K.Y.; Auyeung, V.; Chen, Q.; Gruppuso, P.A.; Phornphutkul, C. Leucine Restriction Inhibits Chondrocyte Proliferation and Differentiation through Mechanisms Both Dependent and Independent of MTOR Signaling. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1374–E1382. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Fleet, J.C.; Schoch, R.D. Molecular Mechanisms for Regulation of Intestinal Calcium Absorption by Vitamin D and Other Factors. Crit. Rev. Clin. Lab. Sci. 2010, 47, 181–195. [Google Scholar] [CrossRef]
- Setiati, S.; Oemardi, M.; Sutrisna, B. The Role of Ultraviolet-B from Sun Exposure on Vitamin D3 and Parathyroid Hormone Level in Elderly Women in Indonesia. Asian J. Gerontol. Geriatr. 2007, 2, 126–132. [Google Scholar]
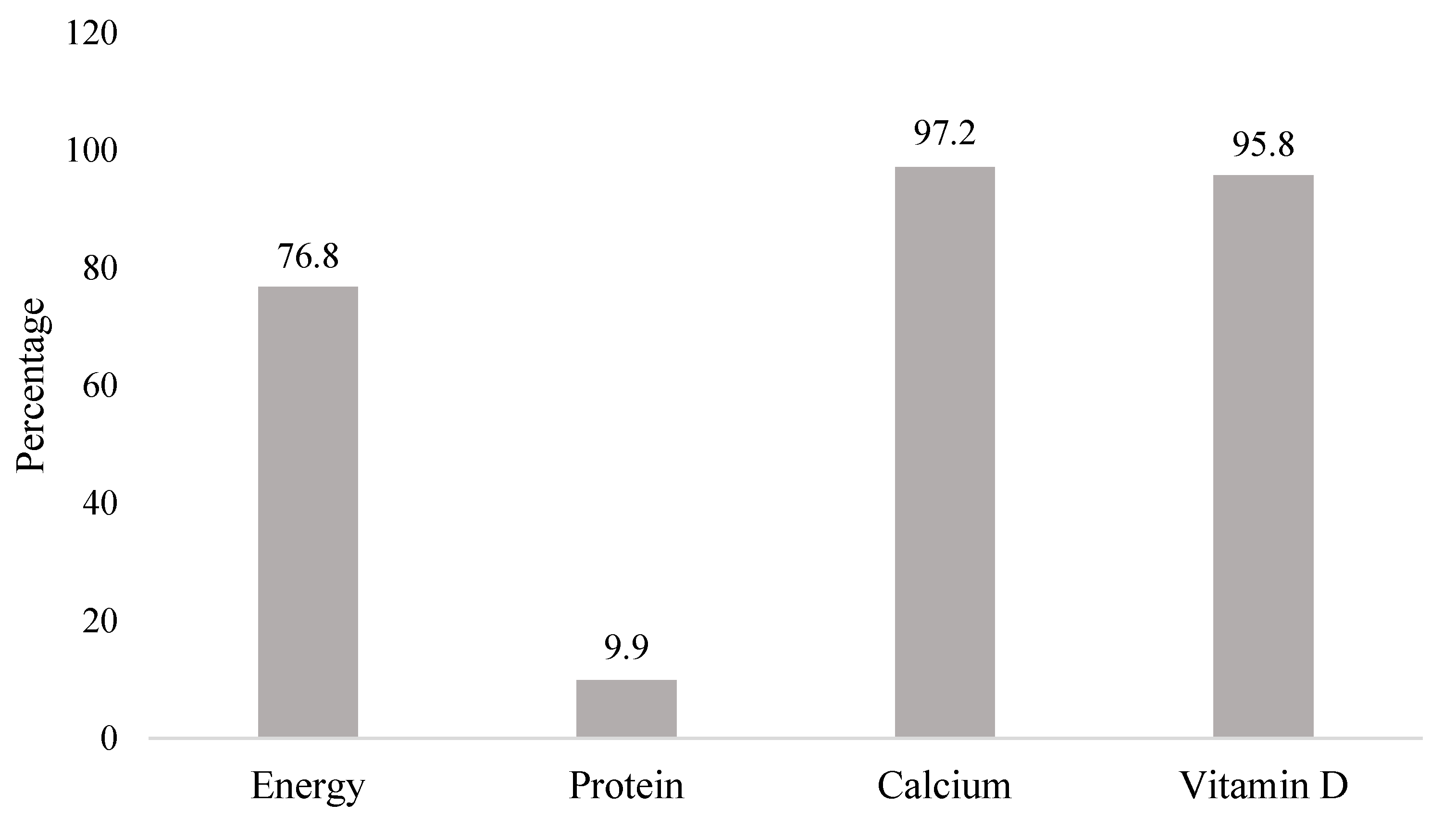
| Factors | n (%) |
|---|---|
| Age (year) * | 9 (8–10) |
| Sex | |
| Boy | 85 (59.9) |
| Mother Education Level | |
| ≤9 years | 100 (70.4) |
| > 9 years | 42 (29.6) |
| Father Education Level | |
| ≤9 years | 98 (69) |
| >9 years | 44 (31) |
| Father Occupation | |
| Government employee | 3 (2.1) |
| Private employee | 20 (14.1) |
| Trader | 41 (28.9) |
| Laborer | 76 (53.5) |
| Not working | 2 (1.4) |
| Mother Occupation | |
| Working | 62 (43.7) |
| Not Working | 80 (56.3) |
| History of Infectious Diseases | |
| a. Diarrhea b. Malaria c. Helminths Respiratory Infection | 21 (14.8) 0 (0) 3 (2.1) 64 (45.1) |
| Sun exposure score (7 days) * | 33.5 (2–56) |
| SNPs | Variant T Allele Frequency (%) | Wild-Type Homozygote (TT) n (%) | Heterozygote (CT) n (%) | Mutant Homozygote (CC) n (%) |
|---|---|---|---|---|
| rs11568820 | 40.85 | 27 (19) | 62 (43.7) | 53 (37.3) |
| rs4516035 | 95.42 | 129 (90.8) | 13 (9.2) | 0 (0) |
| Nutrient | Mean ± SD Median (min–max) | Indonesian RDA (AKG, 2019) | EAR |
|---|---|---|---|
| Energy (Kcal) | 1390 ± 355 | 1650 * | N/A |
| Protein (g/day) | 39 (19–111) | 40 * | N/A |
| Calcium (mg/day) | 328.22 (118.68–1010.55) | 1000 | 800 |
| Vitamin D (mcg/day) | 2.21 (0.09–17.77) | 15 | 10 |
| SNPs | n | Height-for-Age Z-Score (HAZ) | |
|---|---|---|---|
| Mean ± SD | p Value a | ||
| rs4516035 b | 0.868 | ||
| C | 13 | −1.04 ± 0.87 | |
| T | 129 | −0.99 ± 1.12 | |
| rs11568820 b | 0.706 | ||
| C | 115 | −1.01 ± 1.12 | |
| T | 27 | −0.92 ± 1.02 | |
| Nutrient Intake | Height-for-Age Z-Score (HAZ) | |
|---|---|---|
| r | p Value | |
| Energy (kcal/day) | 0.183 | 0.030 a |
| Protein (g/day) | 0.203 | 0.016 b |
| Calcium (mg/day) | 0.021 | 0.804 b |
| Vitamin D (mcg/day) | 0.018 | 0.829 b |
| Variables 1 | rs11568820 | rs4516035 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Model a | Final Model b | Full Model a | Final Model b | |||||||||||||
| B | 95% CI | p Value | Adj. R2 | B | 95% CI | p Value | Adj. R2 | B | 95% CI | p Value | Adj. R2 | B | 95% CI | p Value | Adj. R2 | |
| (Constant) | −0.951 | −1.386–(−0.516) | < 0.001 | 0.003 | −0.949 | −1.208–(−0.691) | < 0.001 | 0.002 | −1.000 | −1.720–(−0.279) | 0.007 | 0.002 | −0.949 | −1.208–(−0.691) | < 0.001 | 0.002 |
| VDR gene 2 | 0.109 | −0.365–0.583 | 0.650 | N/A | N/A | N/A | 0.064 | −0.575–0.703 | 0.844 | N/A | N/A | N/A | ||||
| Calcium Intake (mg/day) | 0.000 | −0.001–0.001 | 0.942 | N/A | N/A | N/A | 0.000 | −0.001–0.001 | 0.972 | N/A | N/A | N/A | ||||
| Vitamin D Intake (mcg/day) | −0.015 | −0.081–0.052 | 0.666 | −0.014 | −0.072–0.044 | 0.641 | −0.014 | −0.080–0.053 | 0.687 | −0.014 | −0.072–0.044 | 0.641 | ||||
| Variables 1 | rs11568820 | rs4516035 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Model a | Final Model b | Full Model a | Final Model b | |||||||||||||
| B | 95% CI | p Value | Adj. R2 | B | 95% CI | p Value | Adj. R2 | B | 95% CI | p Value | Adj. R2 | B | 95% CI | p Value | Adj. R2 | |
| (Constant) | −1.903 | −2.742–(−1.064) | < 0.001 | 0.061 | −1.811 | −2.403–(−1.220) | < 0.001 | 0.089 | −1.783 | −2.764–(−0.802) | < 0.001 | 0.059 | −1.811 | −2.403–(−1.220) | < 0.001 | 0.089 |
| VDR gene 2 | 0.156 | −0.308–0.621 | 0.507 | N/A | N/A | N/A | −0.095 | −0.725–0.535 | 0.766 | N/A | N/A | N/A | ||||
| Energy Intake (kcal/day) | 0.000 | −0.001–0.001 | 0.833 | N/A | N/A | N/A | 0.000 | −0.001–0.001 | 0.923 | N/A | N/A | N/A | ||||
| Protein Intake (g/day) | 0.032 | 0.004–0.060 | 0.027 * | 0.034 | 0.015–0.052 | <0.001 * | 0.033 | 0.004–0.061 | 0.024 * | 0.034 | 0.015–0.052 | <0.001 * | ||||
| Calcium Intake (mg/day) | −0.002 | −0.003–0.000 | 0.022 * | −0.002 | −0.003–0.000 | 0.013 * | −0.002 | −0.003–0.000 | 0.026 * | −0.002 | −0.003–0.000 | 0.013 * | ||||
| Vitamin D Intake (mcg/day) | 0.005 | −0.060–0.070 | 0.879 | N/A | N/A | N/A | 0.008 | −0.058– 0.073 | 0.811 | N/A | N/A | N/A | ||||
| Sun Exposure Score | 0.000 | −0.013–0.013 | 0.962 | N/A | N/A | N/A | 0.000 | −0.013–0.013 | 0.976 | N/A | N/A | N/A | ||||
| Father Education 3 | 0.243 | −0.213–0.699 | 0.294 | 0.327 | −0.054–0.708 | 0.092 | 0.268 | −0.193–0.729 | 0.252 | 0.327 | −0.054–0.708 | 0.092 | ||||
| Mother Education 3 | 0.149 | −0.304–0.602 | 0.516 | N/A | N/A | N/A | 0.149 | −0.304–0.602 | 0.517 | N/A | N/A | N/A | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelin, T.C.; Bardosono, S.; Shinta, D.; Fahmida, U. Growth, Dietary Intake, and Vitamin D Receptor (VDR) Promoter Genotype in Indonesian School-Age Children. Nutrients 2021, 13, 2904. https://doi.org/10.3390/nu13092904
Angelin TC, Bardosono S, Shinta D, Fahmida U. Growth, Dietary Intake, and Vitamin D Receptor (VDR) Promoter Genotype in Indonesian School-Age Children. Nutrients. 2021; 13(9):2904. https://doi.org/10.3390/nu13092904
Chicago/Turabian StyleAngelin, Tiffany Cornelia, Saptawati Bardosono, Dewi Shinta, and Umi Fahmida. 2021. "Growth, Dietary Intake, and Vitamin D Receptor (VDR) Promoter Genotype in Indonesian School-Age Children" Nutrients 13, no. 9: 2904. https://doi.org/10.3390/nu13092904
APA StyleAngelin, T. C., Bardosono, S., Shinta, D., & Fahmida, U. (2021). Growth, Dietary Intake, and Vitamin D Receptor (VDR) Promoter Genotype in Indonesian School-Age Children. Nutrients, 13(9), 2904. https://doi.org/10.3390/nu13092904






