Prehabilitation for Bariatric Surgery: A Randomized, Controlled Trial Protocol and Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
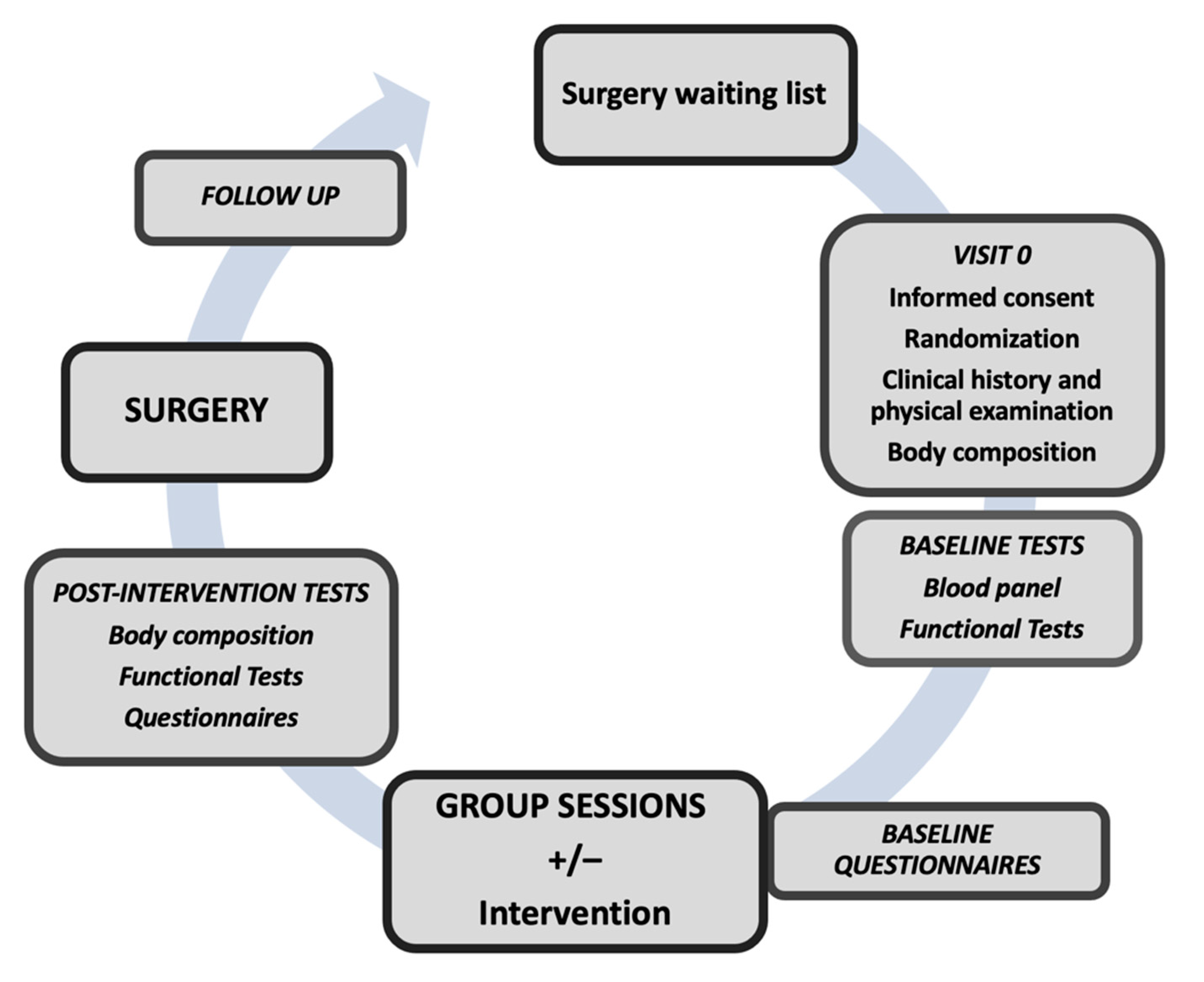
2.1. Study Design
2.2. Study Population
2.3. Randomization and Allocation Masking
2.4. Intervention Masking
2.5. Standard Treatment
2.6. Intervention (Prehabilitation)
2.6.1. Physical Conditioning
2.6.2. Minimal Preoperative Respiratory Physiotherapy Intervention
- Incentive spirometry: The purpose of incentive spirometry is to facilitate a sustained, maximal inspiration (SMI). An SMI is a slow, deep inspiration from the Functional Residual Capacity up to the total lung capacity, followed by 4–8 s breath-hold, mimicking natural sighing. We use Coach 2® (Portex®, Smith-Medicals, Minneapolis, MN, USA) 500–4000 mL devices which provide visual cues to the patients that the desired flow or volume has been achieved (Figure 2). This serves as a motivation and a goal for the patient to try to meet, by repeating the maximal effort frequently. We recommend four repetitions of this maneuver both standing and sitting. Patients are encouraged to use the device twice daily.
- Respiratory exercises: Participants are taught how to perform specific respiratory exercises (breathing with pursed lips, quick and slow expiratory techniques, in order to manage secretions, and assisted cough with the protection of the surgical wound).
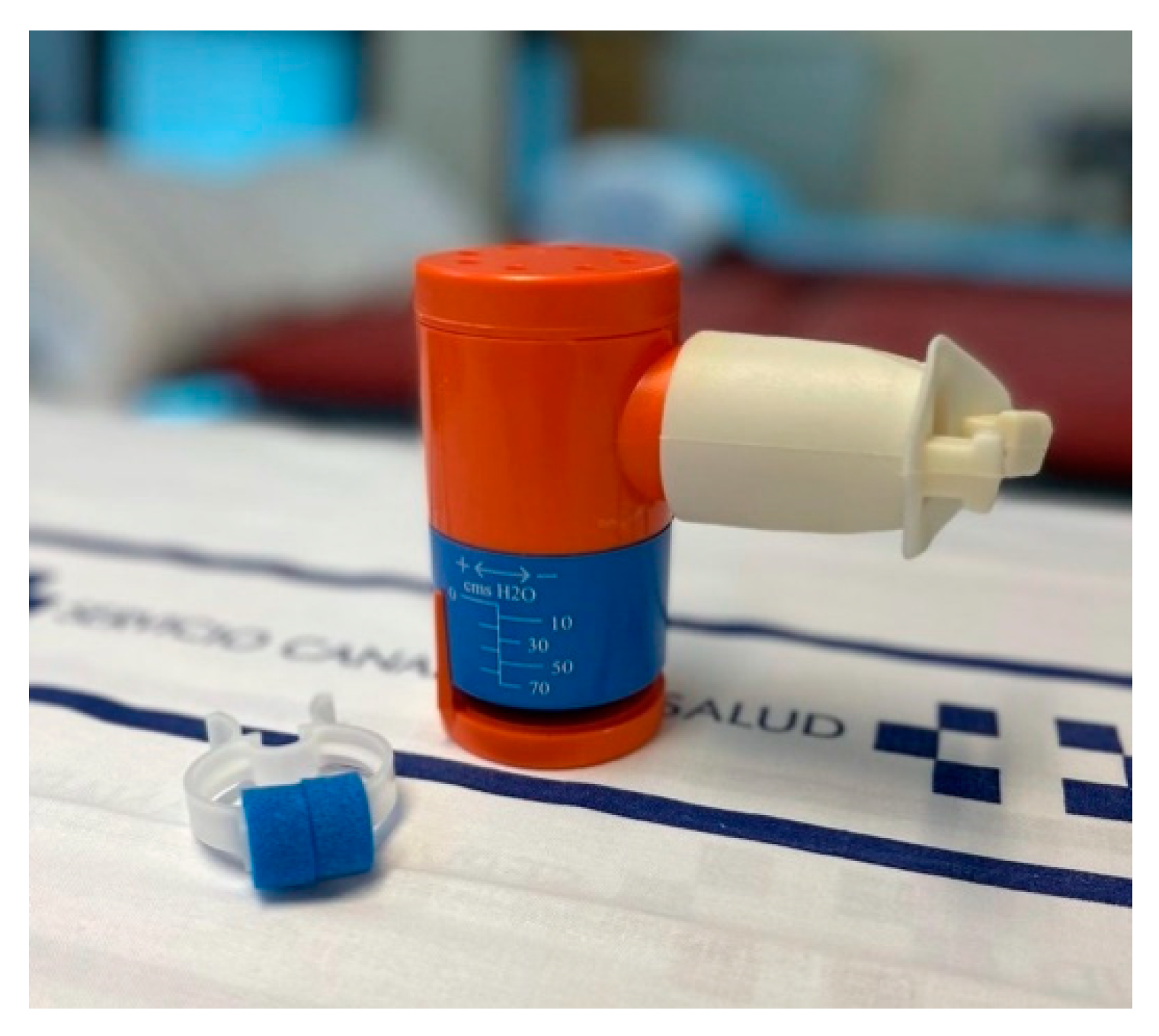
- Inspiratory muscle training: This is performed using an inspiratory valve (0–70 cm H2O) (Orygen Inspiratory Valve, Forumed S.L., Gerona, Spain) (see Figure 3) [46]. Training starts at 30% of the maximal inspiratory pressure (MIP) with progressive 10 cm H2O increments from session to session, according to the participant’s tolerance to the exercises. Patients are advised to repeat these exercises for 10 min twice daily, at 2 min intervals.
2.7. Outcomes
2.8. Clinical History and Blood Tests
2.9. Body Composition
2.10. Questionnaires
2.11. Functional Tests
- Six-minute walk test (6MWT): This is a cheap and easy to perform, submaximal stress test. Its aim is to cover the maximal distance in 6 min, walking on a flat surface, following a standardized protocol [55]. The patient is not coached nor stimulated to walk quickly, and he/she can stop and rest as needed. Blood pressure (beginning and end), heart rate (every minute), oxygen saturation (every minute), and self-perceived exertion (Borg, beginning and end). The percentage of the theoretical 6MWT distance (6MWD) is calculated using Enright’s regression equation [56].
- Handgrip strength (HGS) is measured in both hands with a classical, analogic hand dynamometre (0–90 kg, 0–200 Lb) (JAMAR™ Hydraulic Hand Dynamometer, Preston, Jackson, Missouri, EEUU) following the protocol described by the American Society of Hand Therapists [57], with the patient seated, back and feet resting, shoulders adducted, elbow at 90°, and forearm and wrist in a neutral position. Three 3-s alternating measurements are performed on each hand and the average is calculated and the dominant hand is registered.
- Pulmonary function tests are performed according to the European Respiratory Society (ERS), American Thoracic Society (ATS) and Spanish Society of Pneumology and Thoracic Surgery (SEPAR) technical standards [58,59], by trained staff with a MasterScreen, Jaeger, Germany. Forced spirometry is performed under basal conditions, to assess respiratory capacity, maximal inspiratory pressure, and maximal expiratory pressure are measured at baseline. Plethysmography is used to measure pulmonary volumes, residual volume and functional residual capacity. Pulmonary diffusion (DLCO) is assessed by gas dilution. Baseline arterial gasometry is also performed.
2.12. Adherence to the Intervention and Physical Activity
2.13. Monitoring and Follow-Up
2.14. Surgery
2.15. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailly, L.; Schiavo, L.; Sebastianelli, L.; Fabre, R.; Morisot, A.; Pradier, C.; Iannelli, A. Preventive effect of bariatric surgery on type 2 diabetes onset in morbidly obese inpatients: A national French survey between 2008 and 2016 on 328,509 morbidly obese patients. Surg. Obes. Relat. Dis. 2019, 15, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, G.S.; Småstuen, M.C.; Sandbu, R.; Nordstrand, N.; Hofsø, D.; Lindberg, M.; Hertel, J.K.; Hjelmesæth, J. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA J. Am. Med. Assoc. 2018, 319, 291–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.K.; Cha, Y.M. Cardiovascular benefits of bariatric surgery. Trends Cardiovasc. Med. 2016, 26, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.R.; Baggesen, L.M.; Richelsen, B.; Thomsen, R.W. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: A Danish population-based matched cohort study. Diabetologia 2019, 62, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nieuwenhove, Y.; Dambrauskas, Z.; Campillo-Soto, A.; Van Dielen, F.; Wiezer, R.; Janssen, I.; Kramer, M.; Thorell, A. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch. Surg. 2011, 146, 1300–1305. [Google Scholar] [CrossRef] [Green Version]
- Schauer, P.R.; Nor Hanipah, Z.; Rubino, F. Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world’s leading diabetes organizations. Clevel. Clin. J. Med. 2017, 84, S47–S56. [Google Scholar] [CrossRef]
- Sjöström, L.; Narbro, K.; Sjöström, D.; Karason, K.; Bo, L.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [Green Version]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.H.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—3-Year Outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef] [Green Version]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; Nanni, G.; Pomp, A.; Castagneto, M.; Ghirlanda, G.; et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 2012, 366, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, C.A.; Ikeoka, D.; Santucci, E.V.; Santos, R.N.; Damiani, L.P.; Bueno, P.T.; Oliveira, J.D.; Torreglosa, C.R.; Bersch-Ferreira, A.C.; Miranda, T.A.; et al. Effects of bariatric surgery versus medical therapy on the 24-hour ambulatory blood pressure and the prevalence of resistant hypertension: The GATEWAY randomized clinical trial. Hypertension 2019, 73, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Pradhan, A.; Khan, M.A.; Anderson, S.G.; Keavney, B.D.; Myint, P.K.; Mamas, M.A.; Loke, Y.K. Bariatric surgery and its impact on cardiovascular disease and mortality: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, T.; Hurley, D.L.; McMahon, M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient-2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Sociaty fo. Obesity 2013, 21, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Anderin, C.; Gustafsson, U.O.; Heijbel, N.; Thorell, A. Weight Loss before Bariatric Surgery and Postoperative Complications: Data from the Scandinavian Obesity Registry (SOReg). Ann. Surg. 2015, 261, 909–913. [Google Scholar] [CrossRef]
- Cassie, S.; Menezes, C.; Birch, D.W.; Shi, X.; Karmali, S. Effect of preoperative weight loss in bariatric surgical patients: A systematic review. Surg. Obes. Relat. Dis. 2011, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.L.; Dixon, J.B.; Marks, P.; Strauss, B.J.; O’Brien, P.E. Preoperative weight loss with a very-low-energy diet: Quantitation of changes in liver and abdominal fat by serial imaging. Am. J. Clin. Nutr. 2006, 84, 304–311. [Google Scholar] [CrossRef]
- Edholm, D.; Kullberg, J.; Haenni, A.; Karlsson, F.A.; Ahlström, A.; Hedberg, J.; Ahlström, H.; Sundbom, M. Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes. Surg. 2011, 21, 345–350. [Google Scholar] [CrossRef]
- Roman, M.; Monaghan, A.; Serraino, G.F.; Miller, D.; Pathak, S.; Lai, F.; Zaccardi, F.; Ghanchi, A.; Khunti, K.; Davies, M.J.; et al. Meta-analysis of the influence of lifestyle changes for preoperative weight loss on surgical outcomes. Br. J. Surg. 2019, 106, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.; Jayaraman, L.; Punhani, D.; Chowbey, P. Enhanced Recovery after Bariatric Surgery in the Severely Obese, Morbidly Obese, Super-Morbidly Obese and Super-Super Morbidly Obese Using Evidence-Based Clinical Pathways: A Comparative Study. Obes. Surg. 2017, 27, 560–568. [Google Scholar] [CrossRef]
- Livhits, M.; Mercado, C.; Yermilov, I.; Parikh, J.A.; Dutson, E.; Mehran, A.; Ko, C.Y.; Gibbons, M.M. Does weight loss immediately before bariatric surgery improve outcomes: A systematic review. Surg. Obes. Relat. Dis. 2009, 5, 713–721. [Google Scholar] [CrossRef]
- Gerber, P.; Anderin, C.; Gustafsson, U.O.; Thorell, A. Weight loss before gastric bypass and postoperative weight change: Data from the Scandinavian Obesity Registry (SOReg). Surg. Obes. Relat. Dis. 2016, 12, 556–562. [Google Scholar] [CrossRef]
- Thorell, A.; MacCormick, A.D.; Awad, S.; Reynolds, N.; Roulin, D.; Demartines, N.; Vignaud, M.; Alvarez, A.; Singh, P.M.; Lobo, D.N. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J. Surg. 2016, 40, 2065–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dronkers, J.J.; Lamberts, H.; Reutelingsperger, I.M.M.D.; Naber, R.H.; Dronkers-Landman, C.M.; Veldman, A.; Van Meeteren, N.L.U. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: A randomized controlled pilot study. Clin. Rehabil. 2010, 24, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Coats, V.; Maltais, F.; Simard, S.; Fréchette, É.; Tremblay, L.; Ribeiro, F.; Saey, D. Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Can. Respir. J. 2013, 20, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.S.; Stein, B.; Charlebois, P.; Feldman, L.S.; et al. Prehabilitation versus Rehabilitation. A randomized control trial in patients undergoing colorectal resection cancer. Anesthesiology 2014, 121, 937–947. [Google Scholar] [CrossRef]
- Li, C.; Carli, F.; Lee, L.; Charlebois, P.; Stein, B.; Liberman, A.S.; Kaneva, P.; Augustin, B.; Wongyingsinn, M.; Gamsa, A.; et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: A pilot study. Surg. Endosc. 2013, 27, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.T.; Petersen, A.K.; Jensen, J.B.; Laustsen, S.; Borre, M. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: A prospective randomized controlled trial. Scand. J. Urol. 2015, 49, 133–141. [Google Scholar] [CrossRef]
- Carli, F.; Zavorsky, G.S. Optimizing functional exercise capacity in the elderly surgical population. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 23–32. [Google Scholar] [CrossRef]
- Topp, R.; Swank, A.M.; Quesada, P.M.; Nyland, J.; Malkani, A. The Effect of Prehabilitation Exercise on Strength and Functioning After Total Knee Arthroplasty. PM R 2009, 1, 729–735. [Google Scholar] [CrossRef]
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.W.; Jack, S. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: A blinded interventional pilot study. Br. J. Anaesth. 2015, 114, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Dunne, D.F.J.; Jack, S.; Jones, R.P.; Jones, L.; Lythgoe, D.T.; Malik, H.Z.; Poston, G.J.; Palmer, D.H.; Fenwick, S.W. Randomized clinical trial of prehabilitation before planned liver resection. Br. J. Surg. 2016, 103, 504–512. [Google Scholar] [CrossRef]
- Minnella, E.M.; Bousquet-Dion, G.; Awasthi, R.; Scheede-Bergdahl, C.; Carli, F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: A five-year research experience. Acta Oncol. 2017, 56, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Sebio García, R.; Yáñez-Brage, M.I.; Giménez Moolhuyzen, E.; Salorio Riobo, M.; Lista Paz, A.; Borro Mate, J.M. Preoperative exercise training prevents functional decline after lung resection surgery: A randomized, single-blind controlled trial. Clin. Rehabil. 2017, 31, 1057–1067. [Google Scholar] [CrossRef]
- Chen, B.P.; Awasthi, R.; Sweet, S.N.; Minnella, E.M.; Bergdahl, A.; Santa Mina, D.; Carli, F.; Scheede-Bergdahl, C. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Support. Care Cancer 2017, 25, 33–40. [Google Scholar] [CrossRef]
- Huang, G.H.; Ismail, H.; Murnane, A.; Kim, P.; Riedel, B. Structured exercise program prior to major cancer surgery improves cardiopulmonary fitness: A retrospective cohort study. Support. Care Cancer 2016, 24, 2277–2285. [Google Scholar] [CrossRef]
- Carli, F.; Charlebois, P.; Stein, B.; Feldman, L.; Zavorsky, G.; Kim, D.J.; Scott, S.; Mayo, N.E. Randomized clinical trial of prehabilitation in colorectal surgery. Br. J. Surg. 2010, 97, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Fenton, T.R.; Sajobi, T.T.; Minnella, E.M.; Awasthi, R.; Loiselle, S.È.; Liberman, A.S.; Stein, B.; Charlebois, P.; Carli, F. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: A pooled analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.B.; Bernardi, K.; Olavarria, O.A.; Dhanani, N.; Shah, P.; Holihan, J.L.; Ko, T.C.; Kao, L.S.; Liang, M.K. Prehabilitation among Patients Undergoing Non-Bariatric Abdominal Surgery: A Systematic Review. J. Am. Coll. Surg. 2020, 231, 480–489. [Google Scholar] [CrossRef]
- Waldburger, R.; Wilms, B.; Ernst, B.; Thurnheer, M.; Schultes, B. Cardio-respiratory fitness is independently associated with cardio-metabolic risk markers in severely obese women. Exp. Clin. Endocrinol. Diabetes 2014, 122, 190–194. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Gallagher, M.J.; DeJong, A.T.; Sandberg, K.R.; Trivax, J.E.; Alexander, D.; Kasturi, G.; Jafri, S.M.A.; Krause, K.R.; Chengelis, D.L.; et al. Cardiorespiratory fitness and short-term complications after bariatric surgery. Chest 2006, 130, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management. Obes. Facts 2017, 10, 597–632. [Google Scholar] [CrossRef]
- Casali, C.C.C.; Pereira, A.P.M.; Martinez, J.A.B.; De Souza, H.C.D.; Gastaldi, A.C. Effects of inspiratory muscle training on muscular and pulmonary function after bariatric surgery in obese patients. Obes. Surg. 2011, 21, 1389–1394. [Google Scholar] [CrossRef]
- Pazzianotto-Forti, E.M.; Munno, C.M.d.C.; Merino, D.F.B.; da Rocha, M.R.S.; de Mori, T.A.; Rasera, I., Jr. Effects of inspiratory exercise with linear and nonlinear load on respiratory variables post-bariatric surgery. Respir. Care 2019, 64, 1516–1522. [Google Scholar] [CrossRef]
- Carver, T.E.; Mayo, N.; Andersen, R.E.; Zavorsky, G.S. Pilot investigation to evaluate changes in exercise capacity following a prehabilitation intervention among seriously obese patients awaiting bariatric surgery. Can. J. Diabetes 2011, 35, 149. [Google Scholar] [CrossRef]
- Lecube, A.; Monereo, S.; Rubio, M.Á.; Martínez-de-Icaya, P.; Martí, A.; Salvador, J.; Masmiquel, L.; Goday, A.; Bellido, D.; Lurbe, E.; et al. Prevención, diagnóstico y tratamiento de la obesidad. Posicionamiento de la Sociedad Española para el Estudio de la Obesidad de 2016. Endocrinol. Diabetes Nutr. 2017, 64, 15–22. [Google Scholar] [CrossRef]
- Dronkers, J.; Veldman, A.; Hoberg, E.; van der Waal, C.; van Meeteren, N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: A randomized controlled pilot study. Clin. Rehabil. 2008, 22, 134–142. [Google Scholar] [CrossRef]
- Riddle, M.C.; Bakris, G.; Blonde, L.; Boulton, A.J.M.; D’Alessio, D.; Greene, E.L.; Hood, K.K.; Hu, F.B.; Kahn, S.E.; Kaul, S.; et al. Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [Green Version]
- Kotler, D.P.; Burastero, S.; Wang, J.; Pierson, R.N. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: Effects of race, sex, and disease. Am. J. Clin. Nutr. 1996, 64. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, G.; Garin, O.; Pardo, Y.; Vilagut, G.; Pont, À.; Suárez, M.; Neira, M.; Rajmil, L.; Gorostiza, I.; Ramallo-Fariña, Y.; et al. Validity of the EQ–5D–5L and reference norms for the Spanish population. Qual. Life Res. 2018, 27, 2337–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.Á.; Corella, D.; Salas-salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Garner, D.M.; Elosua, P.; López-Jáuregui, A.; Sánchez-Sánchez, F. EDI-Inventario de Trastornos de la Conducta Alimentaria-3; Editions Tea: Madrid, Spain; ISBN 9788471748812.
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short screener is valid for assessing mediterranean diet adherence among older spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Stern, A.F. The Hospital Anxiety and Depression Scale. Occup. Med. 2014, 64, 393–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmen Terol-Cantero, M.; Cabrera-Perona, V.; Martín-Aragón, M. Revisión de estudios de la Escala de Ansiedad y Depresión Hospitalaria (HAD) en muestras españolas. Ann. Psychol. 2015, 31, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; MacIntyre, N.R.; McKay, R.T.; Johnson, D.; Wanger, J.S.; Zeballos, R.J. American Thoracic Society ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef] [Green Version]
- MacDermid, J.; Solomon, G.; Valdes, K.; Therapists of ASHT. ASHT Clinical Assessment Recommendations, 3rd ed.; ASHT: Mount Laurel, NJ, USA, 2015; ISBN 9780692525159. [Google Scholar]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; Macintyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rio, F.; Calle, M.; Burgos, F.; Casan, P.; del Campo, F.; Galdiz, J.B.; Giner, J.; Gonzalez-Mangado, N.; Ortega, F.; Puente Maestu, L. Espirometria. Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Disorders. International Classification of Sleep Disorders (ICSD-3); American Academy of Sleep Disorders: Darien, IL, USA, 2014; ISBN 9780991543410. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Mantilla Toloza, S.C.; Gómez-Conesa, A. El Cuestionario Internacional de Actividad Física. Un instrumento adecuado en el seguimiento de la actividad física poblacional. Rev. Iberoam. Fisioter. Kinesiol. 2007, 10, 48–52. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Marcon, E.R.; Baglioni, S.; Bittencourt, L.; Lopes, C.L.N.; Neumann, C.R.; Trindade, M.R.M. What Is the Best Treatment before Bariatric Surgery? Exercise, Exercise and Group Therapy, or Conventional Waiting: A Randomized Controlled Trial. Obes. Surg. 2017, 27, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Hennis, P.J.; Meale, P.M.; Hurst, R.A.; O’Doherty, A.F.; Otto, J.; Kuper, M.; Harper, N.; Sufi, P.A.; Heath, D.; Montgomery, H.E.; et al. Cardiopulmonary exercise testing predicts postoperative outcome in patients undergoing gastric bypass surgery. Br. J. Anaesth. 2012, 109, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Baillot, A.; Mampuya, W.M.; Dionne, I.J.; Comeau, E.; Méziat-Burdin, A.; Langlois, M.F. Impacts of Supervised Exercise Training in Addition to Interdisciplinary Lifestyle Management in Subjects Awaiting Bariatric Surgery: A Randomized Controlled Study. Obes. Surg. 2016, 26, 2602–2610. [Google Scholar] [CrossRef]
- Baillot, A.; Vallée, C.A.; Mampuya, W.M.; Dionne, I.J.; Comeau, E.; Méziat-Burdin, A.; Langlois, M.F. Effects of a Pre-surgery Supervised Exercise Training 1 Year After Bariatric Surgery: A Randomized Controlled Study. Obes. Surg. 2018, 28, 955–962. [Google Scholar] [CrossRef]
- Marc-Hernández, A.; Ruiz-Tovar, J.; Aracil, A.; Guillén, S.; Moya-Ramón, M. Impact of Exercise on Body Composition and Cardiometabolic Risk Factors in Patients Awaiting Bariatric Surgery. Obes. Surg. 2019, 29, 3891–3900. [Google Scholar] [CrossRef]
- Bond, D.S.; Thomas, J.G.; Vithiananthan, S.; Unick, J.; Webster, J.; Roye, G.D.; Ryder, B.A.; Sax, H.C. Intervention-related increases in preoperative physical activity are maintained 6-months after Bariatric surgery: Results from the bari-active trial. Int. J. Obes. 2017, 41, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Bond, D.S.; Thomas, G.; Vithiananthan, S.; Webster, J.; Unick, J.; Ryder, B.; Pohl, D. Changes in enjoyment, self-efficacy, and motivation during a randomized trial to promote habitual physical activity adoption in bariatric surgery patients. Surg. Obes. Relat. Dis. 2016, 12, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Camolas, J.; Santos, O.; Moreira, P.; do Carmo, I. INDIVIDUO: Results from a patient-centered lifestyle intervention for obesity surgery candidates. Obes. Res. Clin. Pract. 2017, 11, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Kalarchian, M.A.; Marcus, M.D.; Courcoulas, A.P.; Cheng, Y.; Levine, M.D. Preoperative lifestyle intervention in bariatric surgery: Initial results from a randomized, controlled trial. Obesity 2013, 21, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Kalarchian, M.A.; Marcus, M.D.; Courcoulas, A.P.; Cheng, Y.; Levine, M.D. Preoperative lifestyle intervention in bariatric surgery: A randomized clinical trial. Surg. Obes. Relat. Dis. 2016, 12, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Lier, H.; Biringer, E.; Stubhaug, B.; Tangen, T. The impact of preoperative counseling on postoperative treatment adherence in bariatric surgery patients: A randomized controlled trial. Patient Educ. Couns. 2012, 87, 336–342. [Google Scholar] [CrossRef]

| Session | Description | Contents |
|---|---|---|
| Session 1 | Welcome and introduction | Introduction Questionnaires completion Group dynamics activity |
| Medical aspects 1 (Endocrinologist) | Obesity and its complications Treatment: drugs, intragastric baloon How to prepare for surgery: healthy lifestyle, weight-loss, cardiovascular risk factors. Evidence Bariatric surgery: procedures. Benefits and risks. What to expect from surgery Physical activity. How to monitor | |
| Session 2 | Nutrition 1 (Dietician) | Food groups The Mediterranean diet as example of a healthy diet. Portion size Calories |
| Session 3 | Psychoeducation 1 (Psychologist) | I am not fat. Differences between trait and state Weight regain and how to deal with it. The importance of cognitive-behavioural changes Personality and food Behavioural strategies with food: the importance of how and what to eat How to create a habit. Setting realistic goals |
| Session 4 | Nutrition 2 (Dietician) | How to do the shopping Reading food labels |
| Session 5 | Psychoeducation 2 (Psychologist) | Cognitive restructuring: identifying, analysing and modifying negative and irrational thoughts associated with eating behaviours How to say NO Self-control and travelling, celebrations, and eating out |
| Session 6 | Nutrition 3 (Dietician) | Unnecessary foods Sweeteners Miraculous diets Myths |
| Session 7 | Psychoeducation 3 (Psychologist) | Self-esteem and self-trust Strategies to reduce stress and anxiety Progressive relaxation technique |
| Session 8 | Medical aspects 2 (Endocrinologist) | Postoperative diet. Protein sources. How to enrich food. Vitamin supplementation. Postoperative follow-up Weight regain When to go to the emergency room Changes in drug treatments after surgery. Long-term complications. Frequent problems after surgery: heartburn, reflux, dumping, diarrhoea. Skin flaps Pregnancy |
| Intervention (n= 7) | Control (n= 8) | |
|---|---|---|
| Age (years) | 38 ± 10 | 41 ±10 |
| Sex (n) Women/Men | 7/0 | 7/1 |
| Weight (kg) | 112.9 ±19.5 | 135.9 ±23.2 |
| BMI (kg/m2) | 44.7 ± 4.5 | 48.5 ± 6.7 |
| Waist circumference (cm) | 130.4 ± 12.1 | 138.0 ± 11.4 |
| FM (%) | 47.7 ± 4.1 | 52.4 ± 3.8 |
| PA (°) | 6.6 ± 0.6 | 6.0 ± 0.6 |
| 6MWD (m) | 447.1 ± 83.5 | 433.5 ± 37.4 |
| 6MWD (%) | 86.1 ± 16.1 | 90.8 ± 12.5 |
| HGS Right Hand (kg) | 24.0 ± 7.7 | 29.7 ± 9.1 |
| HGS Left Hand (kg) | 24.1 ± 2.6 | 26.2 ± 8.6 |
| IPAQ 2 | ||
| Low | 1 (20) | 1 (16.6) |
| Moderate | 2 (40) | 5 (83.3) |
| High | 2 (40) | 0 (0) |
| NA | 2 | 2 |
| HbA1c (%) | 5.9 ± 1.7 | 5.5 ± 0.4 |
| Diabetes mellitus 2 | 3 (42.9%) | 1 (12.5%) |
| Hypertension 2 | 3 (42.9%) | 2 (25.0%) |
| HDL-cholesterol (mg/dL) | 47 ± 10 | 41 ± 7 |
| LDL-cholesterol (mg/dL) | 114 ± 46 | 105 ± 25 |
| Dyslipidaemia 2 | 2 (28.6%) | 2 (25%) |
| NAFLD 2 | 1 (14.3%) | 2 (25%) |
| Ferriman-Gallwey Score | 0 ± 1 | 1 ± 1 |
| Infertility 2 | 3 (42.9) | 2 (25%) |
| Chronic venous insufficiency 2 | 2 (28.6) | 2 (25%) |
| OSA 2 | 3 (75) | 3 (50) |
| Function limiting osteoarthritis 2 | 2 (28.6) | 4 (50%) |
| Gastroesophageal reflux/Hiatal hernia 2 | 1 (14.3) | 1 (12.5%) |
| Bronchial asthma 2 | 2 (28.6) | 3 (37.5%) |
| Intracranial hypertension 2 | 1 (14.3) | 0 (0%) |
| EQ-5D-5L score | 57 ± 23 | 47 ± 24 |
| MEDAS | ||
| High | 1 (20) | 7 (100) |
| Low | 4 (80) | 0 (0) |
| NA | 2 | 1 |
| EDI-3 score | 168 ± 27 | 198 ± 49 |
| HADS score | 16 (7) | 16 (5) |
| HADS 2 | ||
| Normal | 3 (60) | 1 (20) |
| Probable case | 2 (40) | 5 (80) |
| NA | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Delgado, Y.; López-Madrazo-Hernández, M.J.; Alvarado-Martel, D.; Miranda-Calderín, G.; Ugarte-Lopetegui, A.; González-Medina, R.A.; Hernández-Lázaro, A.; Zamora, G.; Pérez-Martín, N.; Sánchez-Hernández, R.M.; et al. Prehabilitation for Bariatric Surgery: A Randomized, Controlled Trial Protocol and Pilot Study. Nutrients 2021, 13, 2903. https://doi.org/10.3390/nu13092903
García-Delgado Y, López-Madrazo-Hernández MJ, Alvarado-Martel D, Miranda-Calderín G, Ugarte-Lopetegui A, González-Medina RA, Hernández-Lázaro A, Zamora G, Pérez-Martín N, Sánchez-Hernández RM, et al. Prehabilitation for Bariatric Surgery: A Randomized, Controlled Trial Protocol and Pilot Study. Nutrients. 2021; 13(9):2903. https://doi.org/10.3390/nu13092903
Chicago/Turabian StyleGarcía-Delgado, Yaiza, María José López-Madrazo-Hernández, Dácil Alvarado-Martel, Guillermo Miranda-Calderín, Arantza Ugarte-Lopetegui, Raúl Alberto González-Medina, Alba Hernández-Lázaro, Garlene Zamora, Nuria Pérez-Martín, Rosa María Sánchez-Hernández, and et al. 2021. "Prehabilitation for Bariatric Surgery: A Randomized, Controlled Trial Protocol and Pilot Study" Nutrients 13, no. 9: 2903. https://doi.org/10.3390/nu13092903
APA StyleGarcía-Delgado, Y., López-Madrazo-Hernández, M. J., Alvarado-Martel, D., Miranda-Calderín, G., Ugarte-Lopetegui, A., González-Medina, R. A., Hernández-Lázaro, A., Zamora, G., Pérez-Martín, N., Sánchez-Hernández, R. M., Ibarra-González, A., Bengoa-Dolón, M., Mendoza-Vega, C. T., Appelvik-González, S. M., Caballero-Díaz, Y., Hernández-Hernández, J. R., & Wägner, A. M. (2021). Prehabilitation for Bariatric Surgery: A Randomized, Controlled Trial Protocol and Pilot Study. Nutrients, 13(9), 2903. https://doi.org/10.3390/nu13092903






